Abstract
Renal inflammatory lesions rarely present as unilateral solitary mass. These types of lesions are often misdiagnosed as renal tumors that lead to unnecessary nephrectomy. So far there are very few comprehensive studies about both their clinical features and pathologic characteristics of these inflammatory pseudotumors. Six renal inflammatory pseudotumors were identified in atotal of 1195 radical or partial nephrectomy cases retrospectively reviewed in our institution. The inflammatory lesions included xanthogranulomatous pyelonephritis (2 cases), renal tuberculosis (1 case), renal malakoplakia (1 case), IgG4-related tubulointerstitial nephritis (1 case), and renal Wegener’s granulomatosis (1 case). All patients underwent radical nephrectomy because they had the unilateral renal solitary mass by the imaging examination. Following the postoperative pathological diagnosis, these patients received anti-inflammatory therapy according to their specific etiology. In conclusion, the attention must be paid for rare renal inflammatory pseudotumors that may mimic tumors since these diseases require a total different treatment strategy from neoplastic diseases.
Keywords: Kidney, inflammatory pseudotumor, renal tumor, clinicopathological features, differential diagnosis
Introduction
Renal inflammatory diseases include glomerular, tubular-interstitial, and renal vascular lesions. Clinically, these inflammatory diseases (such as primary or secondary glomerulonephritis, pyelonephritis, ANCA associated vasculitis, etc.) mostly involve bilateral kidneys with diffuse pathological change. However, it is worth mentioning that a few types of inflammatory pseudotumors may present as unilateral solitary renal mass on the radiograph, which may be misdiagnosed as renal tumors and undergo unnecessary surgical treatment. However, comprehensive studies on clinical features and pathologic characteristics of unilateral renal inflammatory pseudotumors are rare. In this article, we retrospectively reviewed the clinical and pathological features of these renal inflammatory Lesion in combination with the relevant literature review, especially highlighted their diagnosis and differential diagnosis. We hope to provide further knowledge and draw more attention of this type of lesion to avoid misdiagosis and mistreatment.
Materials and methods
Patient selection
A total 1195 cases of renal (including renal pelvic) neoplasm which were treated by radical or partial nephrectomy from March 2006 to August 2015 in the department of pathology at Xijing Hospital were collected retrospectively. Among them, there were six cases of renal inflammatory lesions. These patients underwent radical nephrectomy and were followed up for a mean of 49 months (from 36 to 63 months). The chart reviews were approved by an Institutional Review Board at Xijing Hospital.
Clinical and pathological features
Clinical features studied included patient age, gender, manifestations, treatment, and follow-up data. The patients with a palpable flank or abdominal mass, discomfort, gross hematuria, fever, night sweating, weight loss, and fatigue were considered symptomatic at presentation.Meanwhile, the imaging data of six cases of renal inflammatory lesions were retrospectively reviewed.
Histopathological features were assessed by a review of hematoxylin and eosin (H&E), histochemical and immunohistochemical (IHC) stained sections of nephrectomy specimens. The specimen for each case was fixed in 10% buffered formalin, embedded in paraffin, and subsequently sectioned at 4.0 μm for hematoxylin & eosin (H&E) staining and histochemical and immunohistochemical studies. According to the histological changes of each case, corresponding histochemical and/or IHC staining was performed. The histochemical staining included Masson trichrome, periodic acid-schiff (PAS), Ziehl-Neelsen acid-fast, and hexamine silver stains. The IHC staining was performed with MaxVision™ HRP-Polymer anti-mouse IHC kit (Maixin Biotech. Co., Ltd, Fuzhou, Fujian, China). Replacement of the primary antibodies by PBS served as a negative control. All of the controls yielded satisfactory results. The detailed information of the antibodies and technical specifications were shown in Table 1.
Table 1.
Panel of antibodies used in the present study
| Antigen | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| pan-cytokeratin | AE1/AE3 | Ready-to-use antibody | 10 min proteinase | Maixin Biotech. Co., Ltd., Fuzhou, China |
| CD38 | 38C03 | Ready-to-use antibody | Heat PH6.0 citric acid | Maixin Biotech. Co., Ltd., Fuzhou, China |
| CD138 | MI15 | Ready-to-use antibody | Heat PH6.0 citric acid | Maixin Biotech. Co., Ltd., Fuzhou, China |
| CD68 | PG-M1 | Ready-to-use antibody | Heat PH6.0 citric acid | Gene Tech, Shanghai, China |
| CD163 | 10D6 | 1:100 | Heat PH6.0 citric acid | Maixin Biotech. Co., Ltd., Fuzhou, China |
| IgG(R) | / | 1:300 | Heat PH6.0 citric acid | Zhongshan Biotech. Co., Ltd., Beijing, China |
| IgG4 | HP6025 | Ready-to-use antibody | Pressure cooker, PH9.0 EDTA | Maixin Biotech. Co., Ltd., Fuzhou, China |
Results
Clinical features
A total of 1195 cases diagnosed with renal (including renal pelvis) tumors and treated with radical or partial nephrectomy were collected. Among them, there were 1012 cases (85%) of renal cell carcinoma, 60 cases (5%) of angiomyolipoma, 58 cases (5%) of urothelial carcinoma, 24 cases (2%) of oncocytoma, and 35 cases (3%) of other types of renal benign and malignant tumors (e.g., Wilm’s tumor, metanephric adenoma, inflammatory myofibroblastoma, etc.), and 6 cases (0.5%) of renal inflammatory lesions.
Those inflammatory lesions included xanthogranulomatous pyelonephritis (XGPN, 2 cases), renal tuberculosis (1 case), renal malakoplakia (1 case), IgG4 associated tubule-interstitial nephritis (IgG4-TIN, 1 case), and renal Wegener granulomatosis (1 case). The imaging data were shown in Figure 1. The clinical data and pathological diagnosis of these patients were summarized in Table 2. In general, the patients included 2 male and 4 female, with an age range from 14 to 55 years old (median 34.5 years old). All of six patients underwent radical nephrectomy because a unilateral solitary renal mass by the imaging examination was concerning for renal tumor. The lesions mainly located or involved the middle-portion of the kidney. All of the patients were symptomatic at presentation except case 5 whose renal lesion was found accidentally.After nephrectomy, his serum IgG4 was detected to be elevated at 150 mg/dl and multiple organs (bilateral submandibular glands, lymph nodes at the left renal hilum, bladder and ureter) were involved, consistent with IgG4-TIN; Case 6 was found to have serum cytoplasmic anti-neutrophil cytoplasmic antibodies (C-ANCA) (3+) and elevated proteinase 3 (PR3) at 206RU, supporting the diagnosis of renal Wegener’s granulomatosis. After the postoperative pathological analysis, all six patients underwent appropriate treatment according to their specific etiology for inflammation.
Figure 1.
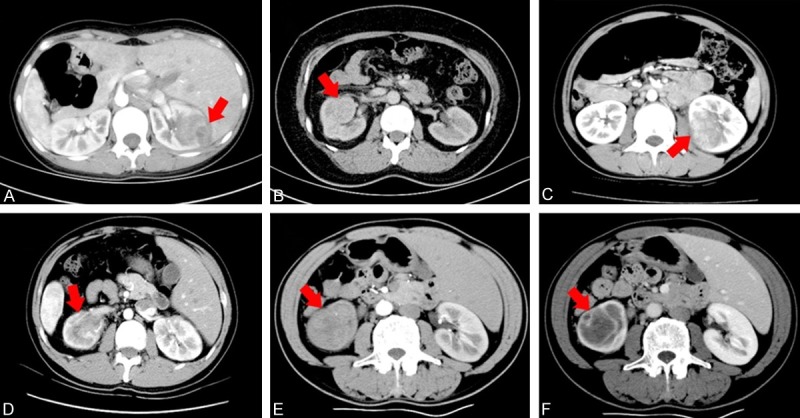
The computerized tomography (CT) finding of the patients with renal inflammatory lesions presenting as masses. (A) Case 1, the patient with xanthogranulomatous pyelonephritis exhibiting an ill-defined, round, heterogeneously enhancing mass in the right kidney by contrast-enhanced CT scaning. (B) Case 3, contrasted tomographic image of the patient with renal malakoplakia showing a well-defined mass in the mid portion of left kidney, which mainly located at the renal pelvis and involving in renal medulla. (C) Case 4, the patient with renal tuberculosis displaying an ill-defined heterogeneously enhancing mass lesion in the mid and lower pole of the right kidney. (D) Case 5, the patient with IgG4-related renal tubular-interstitial nephritis revealing an ill-defined hypodense mass in the left kidney by contrast-enhanced CT scaning. Case 6, the patient with renal Wegener granulomatous presenting an ill-defined hypodense space-occupying lesion (E) with focally heterogeneously annular enhancement sign (F) in the mid and lower pole of the left kidney. (Red arrow showing the renal mass).
Table 2.
Clinical data and pathological diagnosis of 6 cases of renal inflammatory disease
| Case | Sex | Age | Side | Clinical manifestations & history | CT examination | Pathologic diagnosis | Treatment | Follow-up duration (month) |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 14 | Right | Right flank pain; percussion tender over right renal region; fatigue; urinary bacterial quantitation: 115.10/µl | An ill-defined heterogeneously enhancing mass measuring 4 cm × 4.5 cm × 3 cm in the middle portion of kidney | Xanthogranulomatous pyelonephritis | Uninephrectomy & treatment with quinolones antibiotics | 46 months, disease-free survival |
| 2 | Female | 15 | Right | Right flank intermittent pain; fatigue | A heterogeneously enhancing mass measuring 2.7 cm × 2.5 cm × 2 cm in the middle portion of kidney | Xanthogranulomatous pyelonephritis | Uninephrectomy & treatment with quinolones antibiotics | 51 months, disease-free survival |
| 3 | Female | 43 | Left | Low-grade fever; discomfort; eight-year history of pyelonephritis | A regular shape and well-defined mass measuring 3.6 cm × 2.7 cm × 2.5 cm in the renal hilum | Malakoplakia | Uninephrectomy & treatment with quinolones antibiotics and cholinergic agonist | 36 months, disease-free survival |
| 4 | Female | 38 | Right | Right flank pain; low-grade fever; weight loss; fatigue; urinary frequency and urgency | An ill-defined hypodense mass measuring 4.6 cm × 3.4 cm × 3 cm in the middle and lower pole of kidney | Tuberculosis | Uninephrectomy & anti-tuberculostatic multidrug therapy | 63 months, disease-free survival |
| 5 | Male | 40 | Left | Proteinuria and renal mass by physical examination | An ill-defined mass measuring 5.7 cm × 3 cm × 3 cm in the middle and lower pole of kidney | IgG4-related tubulointerstitial nephritis | Uninephrectomy & treatment with steroid | 57 months, disease in remission |
| 6 | Male | 55 | Left | Gross hematuria; percussion tender over left renal region | An ill-defined hypodense mass (maximum diameter 4.2 cm) with annular enhancement in the middle and lower pole of shrunken left kidney; enlarged lymph nodes at the renal hilum | Wegener’s Granulomatosis | Uninephrectomy & treatment with steroid and immunosuppressive agents | 38 months, disease in remission |
Abbreviations: CT, computerized tomography.
Histopathologic and IHC findings
Case 1 and case 2 showed very similar morphology, which characteristically exhibited a large number of xanthomatous histiocytes with abundant foamy cytoplasm, mixed with neutrophils, lymphocytes and plasma cells (Figure 2A). The inflammatory infiltration diffusely or focally involved renal pelvis and parenchyma. In addition, a variable degree of renal tubular atrophy, lymphoid aggregates with germinal center formation, and marked proliferation of fibroblast cells were observed. The lesions showed strong and diffuse immunostain against CD68 and CD163 (Figure 2B), and negative staining for epithelial marker (pan-cytokeration), PAS, and hexamine silver. A diagnosis of XGPN was confirmed.
Figure 2.
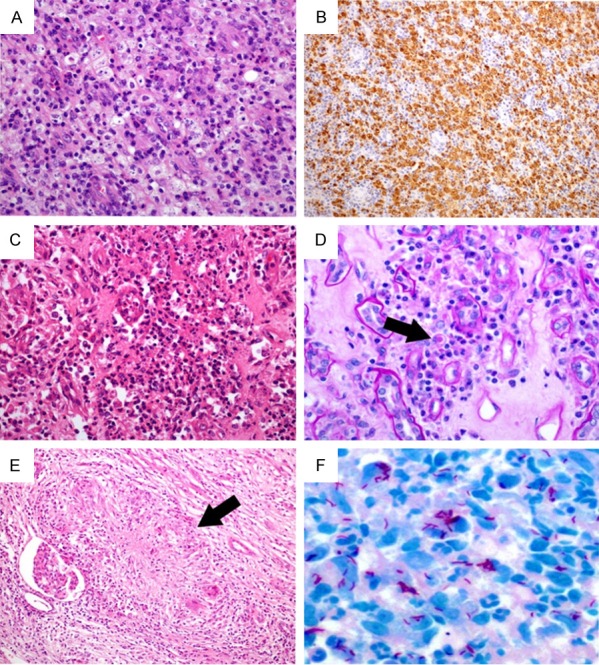
The histopathological changes of case 1, 3 and 4. In case 1, the inflammatory infiltration of xanthogranulomatous pyelonephritis showing variable numbers of foamy histiocytes (A: Hematoxylin & eosin, 200×) with strong and diffuse CD68 immunostaining (B: 100×). In case 3, renal malakoplakia exhibiting aggregates of macrophages, focal microabscesses (C: Hematoxylin & eosin, 200×), Michaelis-Gutmann body within the cytoplasm of the histiocyte (D: Black arrow, Periodic Acid Schiff staining, 400×). In case 4, renal tuberculosis presenting as epithelioid granuloma (E: Black arrow, hematoxylin & eosin, 100×) and Ziehl-Neelsen staining revealing acid fast bacilli (F: 600×).
Microscopic examination of case 3 showed large collections of plump macrohages with few lymphocytes and plasma cells in the renal pelvis and parenchyma on microscopic examination. Simultaneously, there were multifocal microabscess and necrosis (Figure 2C). Importantly, Michaelis-Gutmann (MG) bodies were identified within cytoplasm of histiocytes by PAS staining (Figure 2D). With regard to this characteristic change, the diagnosis of renal malakoplakia was rendered.
Case 4 revealed granulomatous interstitial nephritis with numerous granulomas and confluent granulomas. The characteristic structure of granuloma was the central area of acellular caseous necrosis, surrounded by epithelioid cells, Langhans giant cells and lymphocytes (Figure 2E). The acid-fast bacilli were identified by the Ziehl-Neelsen staining (Figure 2F). After further bacteriological and laboratory examinations, the patient was confirmed to have renal tuberculosis.
In case 5, morphologically, the lesion demonstrated fibrous background with storiform fibrosis, inflammatory infiltration with lymphoid germinal center formation, diffuse renal tubular atrophy, and glomerular sclerosis (Figure 3A). Noticeably, there was dense mature plasma cell infiltrate (Figure 3B). The IHC staining showed a dominant interstitial infiltrate of CD38- and CD138-positive plasma cells. There were a total of 50 IgG4-positive plasma cells/high-power field (HPF) and the ratio of IgG4-positive/IgG-positive plasma cells in tissues were 45% (Figure 3C). Physical examination showed that the patient had obviously enlargement of bilateral submandibular glands. Laboratory examination demonstrated a high-level serum IgG4 (150 mg/dl). In combination with the renal pathological changes and the laboratory results, a diagnosis of IgG4-TIN with the involvement of renal pelvis, perirenal fat and ureter was made.
Figure 3.
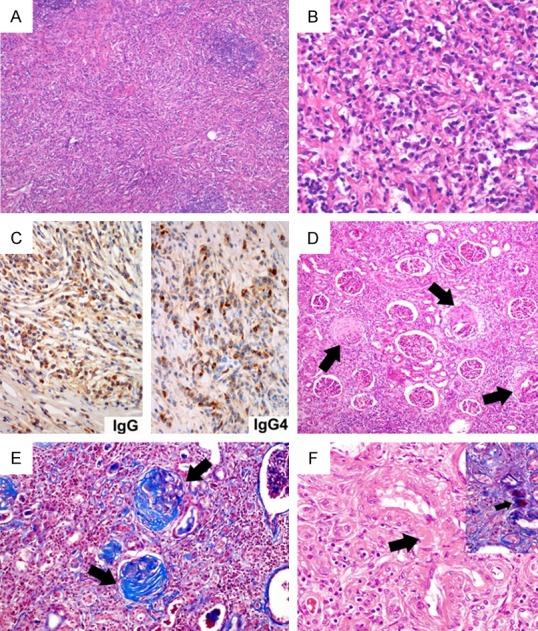
The histopathological changes of case 5 and 6. In case 5, IgG4-related renal tubular-interstitial nephritis demonstrating fibrous background with storiform fibrosis, inflammatory infiltration with lymphoid germinal center formation (A: Hematoxylin & eosin, 40×), and large collections of mature plasma cells (B: Hematoxylin & eosin, 400×) with densely IgG-positive and IgG4-positive immunostain (C: Immunohistochemistry, 400×). In case 6, the patient with renal Wegener granulomatous exhibiting the formation of fibro-cellular crescents (D: Black arrows, hematoxylin & eosin, 100×) that were highlighted by Masson trichrome staining (E: Black arrows, 200×), the fibrinoid necrotizing blood vessel (F: Black arrow, hematoxylin & eosin, 400×; the inset showing the same vessel by Masson trichrome staining).
In case 6, microscopically, the lesion presented extensive chronic inflammatory infiltration,formation of fibrous-cellular and fibrous crescents (Figure 3D), and multifocal fibrinoid necrosis of medium to small blood vessels, which was further highlighted by Masson trichrome staining (Figure 3E and 3F). Combining with his high titer of c-ANCA (3+) and the increasing level of PR3 (206RU), this patients was diagnosed with renal Wegener granulomatosis.
Discussion
The renal inflammatory diseases usually exhibit diffuse lesions involving bilateral kidneys, which can be easily distinguished from renal tumor by imaging examination. However, it should be noted that very few inflammatory diseases as in this article, such as XGPN, malakoplakia, tuberculosis, IgG4-TIN, and Wegener’s granulomatosis, may present as unilateral renal solitary mass mimicking tumors, and lead to unnecessary surgical treatment. Fully understanding of these diseases may help the accurate diagnosis and correct treatment.
XGPN is an uncommon granulomatous lesion involving renal parenchyma, but it is the most common renal inflammatory lesion mimicking tumor. XGPN is associated with long-term urinary tract obstruction and infection. The most common organisms are Escherichia coli and Proteus mirabilis [1]. The range of age at onset is 2 to 84 years, with female predominance. The manifestations include flank or abdominal pain, lower urinary tract symptoms, fever, palpable mass, gross hematuria, and weight loss [2]. Unlike the inflammatory lesions seen in a diffuse pattern, focal and segmental XGPN may be difficult to be differentiated form renal neoplasm [3]. It is reported that the examination with contrast-enhanced computer tomography (CT) is the most useful diagnostic method in delineating the characteristics and distribution of the renal lesions, which often reveals a well-defined localized renal mass with fluid-like attenuation [4]. Morphologically, XGPN is characterized by a granulomatous inflammatory infiltration mixed with fibrosis and cholesterol clefts in the background. These changes diffusely or focally involve renal parenchyma in stage I (nephric XGPN), the perirenal soft tissue in stage II (perinephric XGPN), and retroperitoneum in stage III (paranephric XGPN) [5]. The inflammatory infiltration is composed of a variable number of xanthomatous histiocytes, neutrophils, lymphocytes, plasma cells, and multinucleated giant cells. In addition, a variable degree of renal tubular atrophy, tubular dilatation and focal squamous metaplasia of the urothelium, microabscesses, and lymphoid aggregates with germinal center formation can be observed. In treatment, only the patients with diffuse or advanced-stage (stage II and III) diseasemay need to undergo the nephrectomy. Whereas, the patients with focal XGPN, even bilateral XGPN, still do not need surgery. Preoperative and postoperative broad-spectrum antibiotics and symptomatic management are key factors for successful management of this disease [3].
Michaelis and Gutmann first described malakoplakia in 1902 [6]. The higher incidence is in female and the ratio between female and male is 4 to 1. The age at diagnosis ranges from 6 to 85 years [7]. Malakoplakia can affect any organ and system including the gastrointestinal system, bones, lungs, lymph nodes and skin, but the collecting system of the urinary tract is most frequently involved [8]. It is associated with urinary tract infections in the majority of cases. The renal malakoplakia most usually demonstrates multifocal lesions [6]. Escherichia coli and Proteus mirabilis are the most commonly identified etiologic agents. The findings also show that about 40% of patients have some form of immunosuppression (solid organ transplants, autoimmune diseases requiring steroid use or chemotherapy), chronic systemic diseases, malignancy, alcohol abuse, and poorly controlled diabetes mellitus [9,10]. Microscopically, renal malakoplakia usually exhibits large collections of plump macrophages with relatively few lymphocytes and plasma cells. The distinctive basophilic inclusions with surrounding clear halos known as MG bodies are found within the histiocytes. These MG bodies have a specific significance of diagnosis for malakoplakia and show the characteristic intense PAS, Von Kossaor iron staining. Ultrastructurally, MG bodies are composed of circular unstructured material with a central dense core around the film. The mechanism for the formation of MG bodies is unclear. It is proposed that the bacterium within cells can not be completely digested and persist in the phagolysosomes as a mineralized material due to the lysosome malfunction of the macrophage obstacles [11]. Apart from the characteristic of MG bodies, the diagnosis of malakoplakia must be kept in mind for patients presenting with a history of long-term recurrent renal infections or renal failure. These two features are also useful in differentiating XGPN and renal malakoplakia. There are few reports that renal malakoplakia mimicking tumors led to unnecessary nephrectomy [6,12,13]. The treatment of bilateral or multifocal malacoplakia most often are the use of antibiotics (e.g., quinolones and rifampin) and cholinergic agonist (e.g., bethanechol chloride). Surgical excision is one of the choice of treatment for unifocal lesion [6,13]. In our study, due to an eight years’ history of pyelonephritis in combination with MG bodies in the renal tissue, the patient was identified as renal malakoplakia and received treatment with both quinolones and bethanechol chloride. The patient was disease-free after 3 years postoperative follow-up.
Commonly, the patients with renal tuberculosis present with dysuria, haematuria, sterile pyuria, flank pain, recurrent urinary tract infections, and systemic symptoms. Although its radiographic appearance is variable and depends on the stage of infection, the cases of renal tuberculosis that present as well-defined parenchymal nodules known as pseudotumor type, are extremely rare [14,15]. Once diagnosed, the anti-tuberculostatic multidrug is the preferred treatment. The patient must receive this type of therapy even after surgery [15]. In the current study, the patient underwent anti-tuberculostatic multidrug therapy for 6 months and kept disease-free status after 63 months of follow-up.
Immunoglobulin IgG4-related disease (IgG4-RD) belongs to a systemic fibro-inflammatory disease with an unknown pathophysiological mechanism affecting multiple organs and tissues [16]. It is characterized by the elevated serum IgG4 levels and tumor-like infiltrations of IgG4 positive plasma cells most often accompanying with fibrotic abnormalities in multiple organs and tissues. The typical histological features of IgG4-RD are dense lymphoplasmacytic infiltrates, storiform fibrosis and obliterative phlebitis [17]. IgG4-TIN is the most common form of renal involvement, which characteristically exhibits a dominant interstitial infiltration of IgG4-positive plasma cells and storiform fibrosis. Based on consensus guideline on IgG4-TIN [18], the finding of >30 IgG4+ plasma cells in nephrectomy specimens or >10 IgG4+ plasma cells in renal biopsy tissues, and an IgG4+/IgG+ plasma cell ratio of >40%, has been proposed as its pathological diagnostic criteria.IgG4-TIN locates predominantly in the renal cortex. It rarely appears as an isolated, unilateral lesion nodule or mass mimicking a solid renal neoplasm, which can be quite challenge to make accurate preoperative diagnose [19].Correct recognition of IgG4-TIN will guide appropriate therapy because this disease is extremely responsive to steroid therapy. In accordance with increasing IgG4 positive plasma cells in other systemic diseases, alternative diagnosis such as XGPN, Wegener granulomatosis and sarcoidosis should be excluded before making the diagnosis of IgG4-TIN. In this regard, the number of IgG4 positive plasma cells and the ratio of IgG4-positive/IgG-positive plasma cellsare very important because none of the aforementioned differential diagnoses meet the criteria of IgG4-TIN. Furthermore, multicentric Castleman disease should also be considered rather than IgG4-TIN regardless of serum elevated IgG4 or the infiltration of IgG4 positive plasma cells in multiple organs if the patient has continuously elevated serum C-reactive protein level and partial steroid responsiveness [20].
Wegener granulomatosis is also known as granulomatosis with polyangiitis, which is a multi-systemic inflammatory disease characterized by involvement of the medium and small vasculitis in the respiratory tract, lung, kidney, etc. The common pathological features include fibrinoid necrosis of the medium and small blood vessels, neutrophil infiltration, and necrotizing granulomatous. Renal Wegener granulomatous often presents as a diffuse pauci-immune necrotizing crescentic glomerulonephritis involving bilateral kidneys [21]. Immunofluorescence staining for immunoglobulin (IgG, IgA and IgM) and complement (C3 and C4) is negative or only shows weak positive expression. The positive level of circulating anti-neutrophilic cytoplasmic antibodies (ANCA) is detected in 92% cases of patients [22]. Although Wegener granulomatosis presenting as solitary mass in unilateral kidney is very rare, it may be correctly diagnosed with a comprehensive consideration of the involvement of multiple organs, necrotizing vasculitis, granulomatous inflammation, crescentic glomerulonephritis, and the positive level of c-ANCA and elevated PR3. Therapeutically, the patients with Wegener granulomatosis often receive the treatment with steroid (such as methylprednisolone) and immunosuppressive agents (e.g, cyclophosphamide). Furthermore, some of them undergo the plasma exchange or rituximab therapy [21].
In conclusion, we reported here several renal inflammatory lesions mimicking unilateral solitary renal neoplasm, which are extremely rare. In addition to the above-mentioned lesions, it has been documented that similar changes can be found in the lesions of both histoplasma-associated renal inflammatory pseudotumour [23] and renal sarcoidosis [24]. Given the differences of required treatment and to avoid unnecessary nephrectomy, it is important to make correct diagnosis with sufficient understanding of the clinicopathological features of these diseases.
Acknowledgements
The authors thank Dr. Xiaohui Zhang form the department of pathology and cell biology at The University of South Florida College of Medicine for his constructive comments.
Disclosure of conflict of interest
None.
References
- 1.Korkes F, Favoretto RL, Broglio M, Silva CA, Castro MG, Perez MD. Xanthogranulomatous pyelonephritis: clinical experience with 41 cases. Urology. 2008;71:178–180. doi: 10.1016/j.urology.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Dwivedi US, Goyal NK, Saxena V, Acharya RL, Trivedi S, Singh PB, Vyas N, Datta B, Kumar A, Das S. Xanthogranulomatous pyelonephritis: our experience with review of published reports. ANZ J Surg. 2006;76:1007–1009. doi: 10.1111/j.1445-2197.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Parwani AV. Xanthogranulomatous pyelonephritis. Arch Pathol Lab Med. 2011;135:671–674. doi: 10.5858/2009-0769-RSR.1. [DOI] [PubMed] [Google Scholar]
- 4.Dell’Aprovitola N, Guarino S, Del Vecchio W, Camera L, Chiancone F, Imbimbo C, Salvatore M, Imbriaco M. Xanthogranulomatous pyelonephritis mimicking a renal cell carcinoma: a unique and challenging case. Acta Radiol Short Rep. 2014;3:2047981613513763. doi: 10.1177/2047981613513763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrickson RJ, Lutfiyya WL, Karrer FM, Furness PD 3rd, Mengshol S, Bensard DD. Xanthogranulomatous pyelonephritis. J Pediatr Surg. 2006;41:e15–17. doi: 10.1016/j.jpedsurg.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Wielenberg AJ, Demos TC, Rangachari B, Turk T. Malacoplakia presenting as a solitary renal mass. AJR Am J Roentgenol. 2004;183:1703–1705. doi: 10.2214/ajr.183.6.01831703. [DOI] [PubMed] [Google Scholar]
- 7.Crouch E, White V, Wright J, Churg A. Malakoplakia mimicking carcinoma metastatic to lung. Am J Surg Pathol. 1984;8:151–156. doi: 10.1097/00000478-198402000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med. 2007;131:297–300. doi: 10.5858/2007-131-297-MOTUT. [DOI] [PubMed] [Google Scholar]
- 9.Hegde S, Coulthard MG. End stage renal disease due to bilateral renal malakoplakia. Arch Dis Child. 2004;89:78–79. [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavo LC, Robert ME, Lamps LW, Lagarde SP, Jain D. Isolated gastric malakoplakia: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:e153–156. doi: 10.5858/2004-128-e153-IGMACR. [DOI] [PubMed] [Google Scholar]
- 11.Patnayak R, Reddy MK, Subramanian S, Jena A, Ravisankar G, Dandu RS. An unusual case of bilateral hydroureteronephrosis caused by uretero-vesico malakoplakia in a young male: a case report and review of the literature. Cases J. 2009;2:7527. doi: 10.1186/1757-1626-2-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayllon J, Verkarre V, Scotte F, Fournier L, Corréas JM, Mejean A, Teghom C, Oudard S. Renal malacoplakia: case report of a differential diagnosis for renal cell carcinoma. Am J Case Rep. 2012;13:38–40. doi: 10.12659/AJCR.882596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abolhasani M, Jafari AM, Asgari M, Salimi H. Renal malakoplakia presenting as a renal mass in a 55-year-old man: a case report. J Med Case Rep. 2012;6:379. doi: 10.1186/1752-1947-6-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta H, Kone K, Pandey S, Dorairajan LN, Kumar S. Tubercular mass mimicking a tumour in a horseshoe kidney: a unique presentation. Int Urol Nephrol. 2004;36:323–324. doi: 10.1007/s11255-004-0925-y. [DOI] [PubMed] [Google Scholar]
- 15.Panwar A, Ranjan R, Drall N, Mishra N. Pseudotumor presentation of renal tuberculosis mimicking renal cell carcinoma: a rare entity. Turk J Urol. 2016;42:206–209. doi: 10.5152/tud.2016.91129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 17.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Nakamura S, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 19.Cornell LD. IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:951–953. doi: 10.1038/ki.2010.342. [DOI] [PubMed] [Google Scholar]
- 20.Zoshima T, Yamada K, Hara S, Mizushima I, Yamagishi M, Harada K, Sato Y, Kawano M. Multicentric Castleman disease with tubulointerstitial nephritis mimicking IgG4-related disease: two case reports. Am J Surg Pathol. 2016;40:495–501. doi: 10.1097/PAS.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 21.Ward A, Konya C, Mark EJ, Rosen S. Granulomatosis with polyangiitis presenting as a renal tumor. Am J Surg Pathol. 2014;38:1444–1448. doi: 10.1097/PAS.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 22.Finkielman JD, Lee AS, Hummel AM, Viss MA, Jacob GL, Homburger HA, Peikert T, Hoffman GS, Merkel PA, Spiera R, St Clair EW, Davis JC Jr, McCune WJ, Tibbs AK, Ytterberg SR, Stone JH, Specks U WGET Research Group. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120:643, e649–614. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 23.den Bakker MA, Goemaere NN, Severin JA, Nouwen JL, Verhagen PC. Histoplasma-associated inflammatory pseudotumour of the kidney mimicking renal carcinoma. Virchows Arch. 2009;454:229–232. doi: 10.1007/s00428-008-0714-6. [DOI] [PubMed] [Google Scholar]
- 24.Duveau A, Sayegh J, Beloncle F, Moreau A, Subra JF, Augusto JF. Pseudotumours: an atypical presentation of renal sarcoidosis. QJM. 2013;106:947–949. doi: 10.1093/qjmed/hct108. [DOI] [PubMed] [Google Scholar]


