Abstract
The application of Doxorubicin (DOX) in the chemotherapy of cervical cancer is seriously hampered by the side effects of DOX, especially the cardiotoxicity and nephrotoxicity. Naringin (NIN), a bioflavonoid found in citrus fruit extract, has demonstrated a marked ability to inhibit preclinical models of cancer cell growth and tumor formation. Moreover, its other pharmacological effects are also widely explored. In this study, the antitumor activities of DOX, NIN and their combination were tested in vitro and in vivo. In addition, the attenuation of NIN toward DOX was also explored. Our data demonstrated that the combined application of DOX and NIN more effectively inhibited the cell proliferation than single agent in vitro. In the in vivo antitumor test, the combined application of DOX and NIN more effectively inhibited the growth of HeLa cervical tumor and promoted cell apoptosis. More importantly, the body weight loss and cardiotoxicity, nephrotoxicity and hepatotoxicity were obviously attenuated compared to the DOX treatment group. Taken together, all the results indicated that the combined application of DOX and NIN could inhibit the growth of HeLa cervical cancer cells and attenuate the toxicity of DOX, which suggested NIN could be used as a combined agent for the prevention and treatment of cervical cancer.
Keywords: Antitumor, doxorubicin, flavonoid, naringin, toxicity
Introduction
Cervical carcinoma as the second most common female cancer has been an important world health problem for women [1]. It is generally accepted that radical surgery or radiotherapy is the standard treatment for the patients with early-stage. Meanwhile, for those patients with advanced cervical cancer, chemotherapy or neoadjuvant chemotherapy is always the first choice [2]. However, the chemotherapy drugs always cause various side effects, which seriously hampered the clinical use of chemotherapy. Doxorubicin (DOX), as an anthracycline drug, is widely used for the treatment of various cancers, including the cervical carcinoma, in clinical chemotherapy. However, the toxic effects of DOX such as cardiotoxicity can seriously endanger the patient’s condition [3]. DOX has been reported to be metabolically activated to a free radical state and then interacts with molecular oxygen to generate superoxide radicals [4]. The formation of DOX-iron complexes through oxygen free radicals is considered to play a crucial role in DOX-induced toxicity [5]. As a solution, antioxidants were used to combat the toxicity induced by DOX without influencing its antitumor activity [6].
Fortunately, flavonoids are a class of naturally occurring compounds, which have excellent iron chelating and radical scavenging properties [7]. Therefore, flavonoids maybe an effective modulator for the DOX induced toxicity. Naringin (NIN) is one kind of flavonoids extracted from grapefruit or other citrus fruits. Moreover, studies have proved that NIN possessed a variety of biological and pharmacological properties such as antioxidant activities, cholesterol-lowering, superoxide scavenging, antiatherogenic, anti-inflammatory and antitumor [8-12]. It has also been reported that NIN could protect cells against the iron and radiation-induced oxidative stress [13]. In addition, NIN was proved could alleviate cardiotoxicity caused by DOX in mice by inhibiting PARP activity and reducing the DNA adduct formation, scavenging of free radicals, increasing oxidant status and reduced lipid peroxidation [14]. Especially point out that NIN could inhibit the proliferation of human cervical cancer (SiHa) cells through cell cycle arrest and inducing cell apoptosis [15].
In view of the above conclusions, the present study soughs to explore the antitumor efficiency of combined DOX and NIN toward the HeLa cervical cancer cells and attenuation of NIN toward DOX induced. The results demonstrated that combined application of DOX and NIN improved the antitumor efficiency compared to single agent and the body weight loss and the organs damage caused by DOX was attenuated by NIN obviously.
Materials and methods
Materials
Naringin (NIN) and MTT were purchased from Sigma-Aldrich (St. Louis, mO, USA). Doxorubicin (DOX) was purchased from Zhejiang Hisun Pharmaceutical Co., Ltd. (Taizhou, P. R. China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Gibco-BRL (Grand Island, NY, USA). Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) kit was purchased from Roche (Mannheim, Germany). Ki-67 and poly (ADP-ribose) polymerase-1 (PARP-1) antibodies were obtained from Abcam Company (Cambridge, UK). 96-well tissue culture polystyrene (TCP) plates were obtained from Corning Costar Co. (Cambridge, MA, USA).
Culture and treatments
HeLa cell, a human cervical cancer line, was obtained from the American Type Culture Collection. The cells were cultured in DmEm medium supplemented with 10% FBS at 37°C.
The anticancer effects of DOX (a final concentration from 1.6 to 10.0 μg mL-1) and NIN (a final concentration from 200 to 2000 μmol L-1) were tested by a MTT assay on HeLa cells. In brief, the cells were seeded on a 96-well plate at a density of 7.0 × 103 cells in 180.0 μL of DMEM and incubated at 37°C for 24 h. And then, 20.0 μL of DOX and NIN at different concentrations were added to each well and cultured for another 24 h, respectively. Subsequently, 20.0 μL of MTT at a concentration of 5.0 mg mL-1 was added to each well. After incubation for another 4 h, the medium was carefully removed, and 150.0 μL of dimethyl sulfoxide (DMSO) was added. After vibration for 5 min, the absorbance of medium was determined at 490 nm using a Bio-Rad 680 microplate reader. In addition, we also tested the cytotoxicities of half maximal inhibitory concentrations (IC50) of DOX and NIN added different concentrations of NIN and DOX following the above protocol, respectively. The cell viability was calculated as Formula (1).
Cell Viability (%) = A sample/A control × 100 (1)
In Formula (1), A sample and A control represented the absorbances of sample and control wells, respectively.
In vivo antitumor efficacy assay
The 5-6 week old female athymic nude mice were obtained from the Experimental Animal Center of Jilin University (Changchun, P. R. China). All animals received careful care under the guidelines approved by the Institutional Animal Care and Use Committee of Jilin University.
A number of 3.0 × 106 HeLa cells in 0.1 mL of PBS were subcutaneously injected into the right flank of mice for the construction of tumor-bearing model. When the tumors grew to about 100 mm3, mice were randomly divided into 4 groups (n = 8 for each group): normal saline (NS) as a control, DOX, NIN and DOX+NIN groups. Subsequently, mice were treated 5 times with a 3-day interval at a DOX dose of 5.0 mg and NIN dose of 20 mg per kg body weight (mg (kg BW)-1) in 0.2 mL of PBS by tail veil injection, respectively. The tumor sizes and body weights of mice were measured once-every-other-day. The antitumor efficacy of different groups was evaluated through the tumor volume (V) as calculated by Formula (2).
V (mm3) = L × S 2/2 (2)
In Formula (2), L and S (mm) were the largest and smallest diameters of tumor, respectively. The tumor inhibition ratio was calculated using Formula (3).
Tumor inhibition rate (%) = (V control - V sample)/V control × 100 (3)
In Formula (3), V control and V sample represented the tumor volumes of control and sample groups, respectively.
Histopathological analyses
Four days after the last treatment, all the mice were sacrificed. And then, tumors and major organs (i.e., heart, liver, spleen, lung, and kidney) were isolated and fixed in 4% (W/V) PBS-buffered paraformaldehyde overnight, and then embedded in paraffin. The paraffin-embedded tumor and organ tissues were stained with hematoxylin and eosin (H&E). The histological alterations of tumors and different organs were observed by a microscope (Nikon Eclipse Ti, Optical Apparatus Co., Ardmore, PA, USA). The relative necrotic area (%) of tumor tissues was calculated by Formula (4).
Relative necrotic area (%) = Necrotic area in tumor section/Total area of tumor section × 100 (4)
In situ cell apoptosis detections
In order to observe the in situ cell apoptosis of tumor tissue, TUNEL assay was performed according to manufacturer’s protocol on the TUNEL kit. The fluorescence microimages of TUNEL-labeled tumor tissues were measured by confocal laser scanning microscopy (CLSM; Carl Zeiss, LSM 780, Jena, Germany). The area of apoptotic cells in tumor tissues were counted in three randomly selected areas photographed by CLSM under 100 × magnifications. When for statistical analysis, the area of total observation field was defined as “100%”. These data were measured with ImageJ software (National Institutes of Health, Bethesda, Maryland). The relative apoptosis area (%) of tumor tissue section was calculated by Formula (5).
Relative apoptosis area (%) = Apoptosis area in tumor section/Total area of tumor section × 100 (5)
Immunohistochemical assays
The immunohistochemistries of Ki-67 and PARP-1 expressions were performed on the paraffin-embedded tumor tissue section. The Ki-67 staining was performed with primary antibody (i.e., rabbit anti-mouse monoclonal antibody Ki-67) and secondary antibody, that is, goat anti-rabbit IgG fluorescein isothiocyanate (FITC)-conjugated F (ab’), through the labeled streptavidin-biotin immunoperoxidase technique. The PARP-1 activity was measured according to the routine methods: (1) antigen retrieval, (2) serum closing, (3) primary antibody addition, and (4) second antibody addition. In order to quantify the Ki-67 and PARP-1 expressions, the average of tumor tissue with fluorescence for each sample was also counted under 200 × magnifications for statistical analysis with the area of total observation field as “100%”. The relative positive area (%) of tumor tissue section was calculated by Formula (6).
Relative positive area (%) = Positive area in tumor section/Total area of tumor section × 100 (6)
Statistical analysis
All experiments were carried out at least three times and expressed as means ± standard deviation (SD). Data were analyzed for statistical significance using SPSS (Version 13.0, SPSS Inc., Chicago, IL, USA). *P < 0.001 was considered statistically highly significant.
Results and discussion
In vitro antitumor efficacy measurement
DOX, a widely used small molecule anthracycline antitumor agent used in clinic, is known to work through the intercalation and inhibition of macromolecular biosynthesis of DNA [16]. The toxicity profiles of DOX and NIN were evaluated regarding the viability of HeLa cells. The cellular proliferation inhibition capabilities of DOX, NIN and their mixture were performed. As shown in Figure 1A and 1B, single DOX and NIN both showed obvious proliferation inhibitory with the increase in drug concentration. The half maximal inhibitory concentrations (IC50s) of DOX and NIN were calculated to be 1 μg mL-1 and 1730 μmol L-1, respectively. Interestingly, when the half maximal inhibitory concentration (IC50) of DOX or NIN added to single agent, the mixtures both exhibited more effective proliferation inhibition effects on HeLa cells than single agent (Figure 1C and 1D). This phenomenon indicated that the combined application of DOX and NIN could inhibit the growth of HeLa cells more effectively in vitro. The result verified the potential advantage of combing DOX and NIN as a potential strategy for antitumor in vivo.
Figure 1.
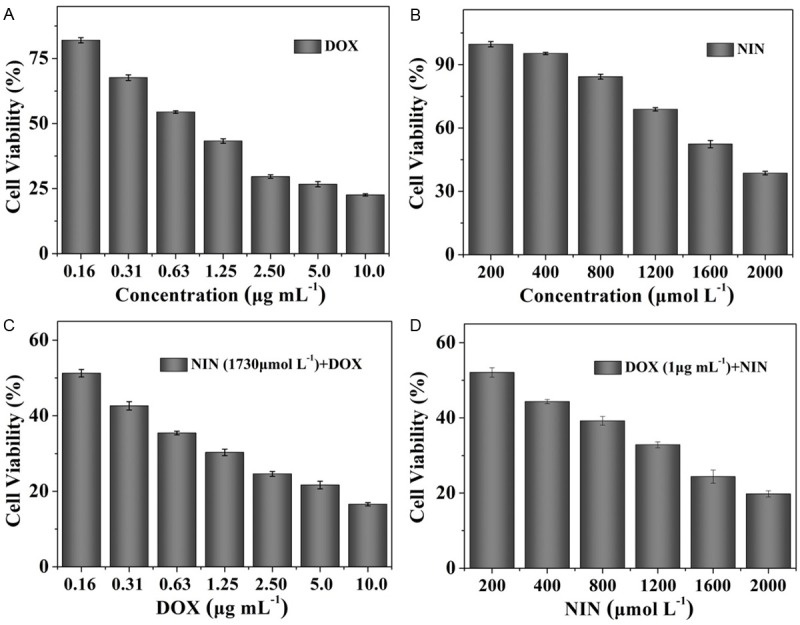
Relative cell viabilities of DOX (A), NIN (B), NIN (IC50)+DOX (C) and DOX (IC50)+NIN (D) toward HeLa cells. The data were shown as mean ± SD (n = 4).
In vivo antitumor efficacy assay measurement
Taking the advantages of combining DOX and NIN in terms of the high antitumor effect in vitro, we then examined the antitumor efficacy of the mixture in vivo. The HeLa-bearing nude mouse models were treated every three days with NS, DOX, NIN or DOX+NIN, individually. The changes of tumor volumes of different groups were real-time monitored during the therapeutic process. The antitumor efficacies of various formulations were assessed. As shown in Figure 2A, DOX, NIN and DOX+NIN were all effective in retarding tumor growth compared to the NS control. The DOX and NIN groups showed similar tumor inhibition. The tumor inhibition rates of these two agents were 44% and 37%, respectively, which were shown in Figure 2B. As expected, the DOX+NIN group exhibited the most satisfactory antitumor efficiency and the tumor inhibition rate was 60%, which was significantly higher than that of DOX and NIN alone. The satisfactory antitumor efficacy of combing DOX and NIN should be related to the combined pesticide effect of these two agents, which was in accordance with the antitumor activity in vitro. This result strongly supported the potential combined application of DOX and NIN in antitumor activity.
Figure 2.
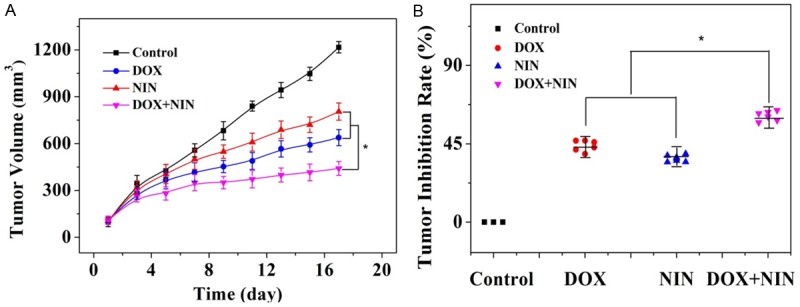
Evaluations of tumor volume (A) and tumor inhibition rate (B) of HeLa-bearing nude mice after treatment with DOX, NIN or DOX+NIN employing NS as a control. The data were represented as mean ± SD (n = 8; *P < 0.001).
The clinical use of DOX is greatly limited to its severe side effects, such as the serious cardiotoxicity and nephrotoxicity [17]. Interestingly, NIN as one kind of flavonoids has been widely explored and was proved to be capable of protecting mice against doxorubicin-induced cardiotoxicity [14]. Body weight loss is a significant indicator for assessing the DOX-induced toxicity toward mice. In order to verify the role of NIN in the antitumor activity and alleviating the toxicity of DOX, the changes of Body weight of different treatment groups were carefully recorded. As shown in Figure 3, the mice treated with DOX showed about 20% of body weight lost at the end of treatment. Moreover, the mice of this group appeared to be much weak than other groups, which indicated the serious systematic toxicity of DOX. As a control, the NIN treatment group showed relatively light decrease in body weight during the treatment observation period. As expected, the DOX+NIN group showed reduced weight loss compared to DOX treatment group while still reduced much more than NIN treatment group. This result confirmed that the NIN could reduce the systematic side effects of DOX at the same time of enhancing antitumor efficiency. This result also indicated the NIN could be used as a potential antitumor agent combined with other antitumor drugs.
Figure 3.
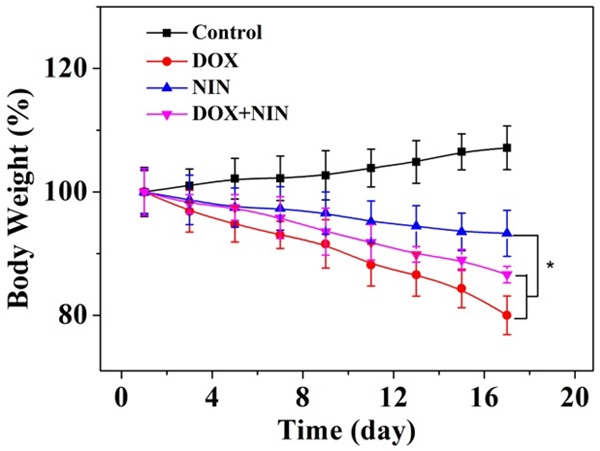
Evaluations of body weight loss of HeLa-bearing nude mice after treatment with DOX, NIN or DOX+NIN employing NS as a control. The data were represented as mean ± SD (n = 8; *P < 0.001).
Histological and immunohistochemical analyses
For further investigate the antitumor efficacy of different antitumor agents, the tumor tissues were dissected and sectioned for H&E pathology analysis. As shown in Figure 4, the tumor sections treated with NS showed large amount of stroma and the tumor cells exhibited more chromatin and binucleolates, clear spherical or spindle shape and large nucleus, which indicated a rapid tumor growth. Furthermore, the necrosis region was scarcely observed in the tumor tissue. On the contrary, after the treatment with different antitumor agents, extensive nuclear shrinkage and fragmentation and various degrees of tissue necrosis were observed. In order for a more intuitive illustration, a quantitative estimation of necrosis area was performed. As shown in Figure 5A, the necrosis area of DOX, NIN and DOX+NIN treatment groups were about 42%, 37% and 61%, respectively. This result was in accordance with the tumor inhibition ratio and quantitatively confirmed the effective tumor inhibitory effect of DOX+NIN.
Figure 4.
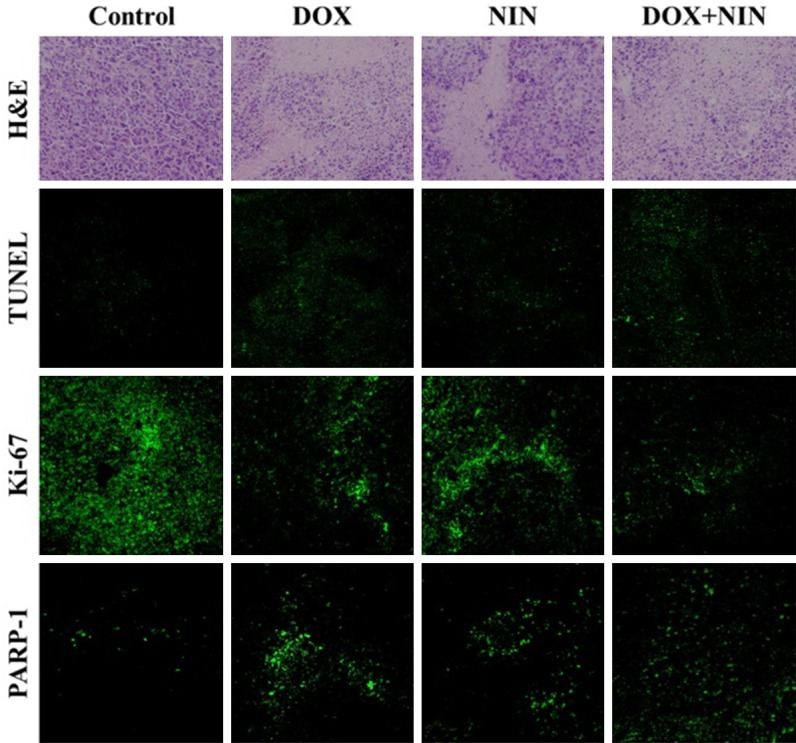
Ex vivo histopathological (i.e., H&E), in situ cell apoptosis (i.e., TUNEL), and immunohistochemical analyses (i.e., Ki-67 and PARP-1) of HeLa tumor sections after treatment with NS, DOX, NIN or DOX+NIN. Magnification: 200 × for H&E and immunohistochemistry, and 100 × for TUNEL.
Figure 5.
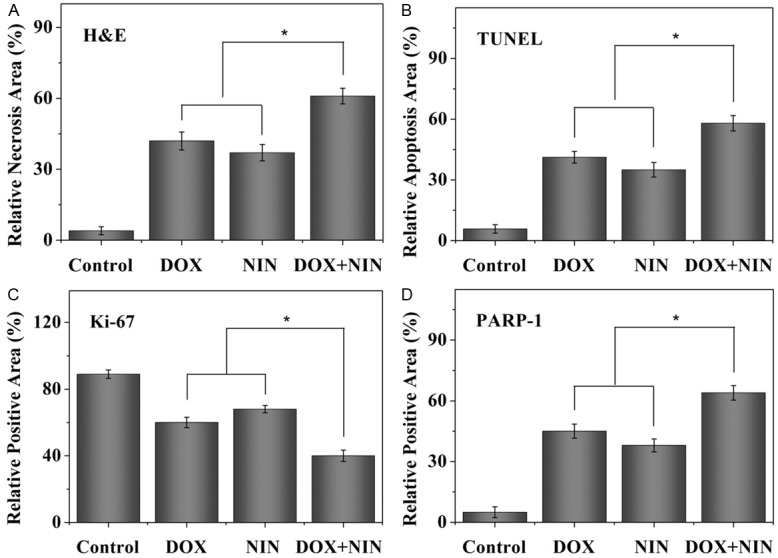
Relative necrotic area of tumor section from H&E (A), relative apoptosis area of tumor section from TUNEL (B), and relative positive area of tumor sections analyzed from Ki-67 and PARP-1 (C and D) after treatment with NS, DOX, NIN or DOX+NIN. Data were presented as mean ± SD (n = 4; *P < 0.001).
For further confirming the in situ cell apoptosis of tumor tissue caused by the combined use of DOX and NIN, the TUNEL assay was performed by detecting the DNA fragmentation, a marker of late apoptosis. The DNA fragmentation was stained as green by fluorescent probe in TUNEL assessment. The part of cell apoptosis was represented by the fluorescing area of TUNEL assessment. As shown in Figure 4, almost no cell apoptosis was detected in the NS group. On the contrary, varying degrees of apoptosis were observed in the different treatment groups. At the same tiome, the semiquantitative data of different groups were evaluated and displayed in Figure 5B. In detail, the DOX+NIN group exhibited the most relative apoptosis area, which was about 58.0%. In addition, the relative apoptosis areas of DOX and NIN groups were 41% and 35%, respectively. This result proved the combined application of DOX and NIN could improve the degree of apoptosis toward HeLa cells. All in all, both H&E and TUNEL results verified the excellent antitumor efficacy of combined DOX and NIN in vivo toward HeLa cells.
A nuclear protein, Ki-67, massively expressed in proliferating cells, has been diffusely employed as a marker of cell proliferation in tumor tissues [18]. We explored the expression of Ki-67 in different treatment groups. As shown in Figure 4, a weakest positive signal in nucleus was observed in the DOX+NIN treatment group compared to DOX- and NIN treatment groups. This result illustrated that the DOX+NIN effectively inhibited tumor cells proliferation in vivo. In order to further analyze Ki-67 expression in tumor tissues quantitatively, the parts of proliferating cells were counted carefully. As shown in Figure 5C, the percentages of proliferating cells of DOX, NIN and DOX+NIN groups were about 60%, 68% and 40.0%, respectively. This result prompted that the combined application of DOX and NIN effectively inhibited tumor cells proliferation in vivo.
PARP, an abundant DNA-binding enzyme, is widely used characteristic hallmark of apoptosisis in many types of cells [19,20]. In order to further confirm the tumor cells apoptosis in tumor tissues, the 25 kDa fragment of cleaved PARP-1 was performed by immunohistochemistry. As shown in Figure 4, a significantly intensive number of PARP-1 positive fluorescence signals were observed in the DOX+NIN treatment group, indicating that more cells underwent apoptosis. As shown in Figure 5D, the percentages of apoptotic cells of DOX, NIN and DOX+NIN groups were about 45%, 38% and 64%, respectively. This result further verified that the combined DOX and NIN could effectively inhibit the growth of tumor than single DOX or NIN in vivo.
In summary, all the evaluations of H&E, TUNEL, Ki-67, and PARP-1 verified the combined application of DOX and NIN exhibited more potent inhibition toward HeLa cells growth compared with single DOX or NIN in vivo.
In vivo security assessment
The serious cardiotoxicity and nephrotoxicity greatly limited the use of DOX in clinic. So weakening the side effect of DOX is an urgent problem need to be solves, which is directly related to the survivals of patients. Therefore, the decreased systemic toxicity of DOX due to the combined application of NIN was further confirmed by the histopathological detections of the main organs (i.e., heart, liver, spleen, lung, and kidney). As shown in Figure 6, DOX caused various degrees of heart damage in the DOX treatment group. It could be seen that the myocardial fiber was damaged and apoptosis, varying numbers of inflammatory cell infiltrated. These damages were shown by the black arrows in Figure 6. Nevertheless, there was faint heart damage in the NIN treatment group. The myocardial cells lined in order, and sarcolemma kept integrity. On the contrary, the heart damage caused by DOX was strongly alleviated by combined application of NIN, which was clearly exhibited in the DOX+NIN treatment group. Most obviously, the crack of myocardium was strongly alleviated compared to the DOX treatment group. This result further illustrated that NIN could attenuate the side effects caused by DOX. In addition, the structural disturbance caused by DOX was also alleviated by NIN. The microregional necrosis of hepatocytes of DOX+NIN group was obviously weakened than DOX treatment group. Besides, the NIN caused faintly damage to the hepatocyte. Moreover, an obvious nephrotoxicity caused by free DOX was also alleviated by NIN. As shown in the pictures, the shriveled glomerular and unclear cell morphology in the DOX treatment grouo was lmost could not be seen in the DOX+NIN group. In addition, all groups exhibited no noticeably damages toward spleen and lung.
Figure 6.
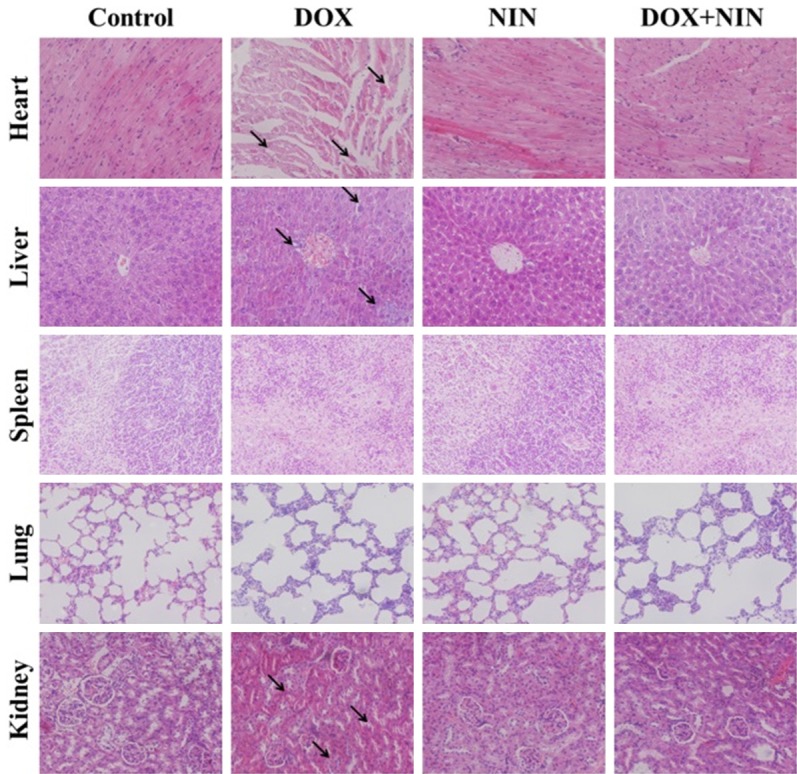
Histopathological analyses of visceral organ sections from HeLa-bearing nude mice after treatment with NS, DOX, NIN or DOX+NIN. The arrows pointed out a certain myocardial damage and fracture, the nucleus shrunk of liver tissue and nephrotoxicity judged from the shriveled glomerular and gathered nuclei. Magnification: 200 ×.
Conclusion
NIN as a functional bioflavonoid has been used in our work to verify its antitumor effect toward HeLa cells combined with DOX. In summary, the combined application of DOX and NIN showed obviously enhanced antitumor efficiency compared to single agent toward HeLa cells in vitro. Moreover, in the in vivo antitumor test, the DOX+NIN exhibited satisfactory behaviors. Apart from inhibiting tumor growth, NIN was also found to increase apoptosis in HeLa cells and alleviate the toxic side effects of DOX toward various organs of mice. These results are significant as they provide new insights in the further application of NIN in antitumor activity. Thus, our findings provide a framework for the further exploration of NIN combined with other chemotherapeutics. However, the exact mechanism of attenuation by NIN is not known, so more work needs to be done in the future. All in all, the NIN might be a potent chemotherapeutic agent for the treatment of human cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ren G, Zhao YP, Yang L, Fu CX. Anti-proliferative effect of clitocine from the mushroom Leucopaxillus giganteus on human cervical cancer HeLa cells by inducing apoptosis. Cancer Lett. 2008;262:190–200. doi: 10.1016/j.canlet.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Karimi G, Ramezani M, Abdi A. Protective effects of lycopene and tomato extract against doxorubicin‐induced cardiotoxicity. Phytother Res. 2005;19:912–914. doi: 10.1002/ptr.1746. [DOI] [PubMed] [Google Scholar]
- 4.Powis G. Free radical formation by antitumor quinones. Free Radic Biol Med. 1989;6:63–101. doi: 10.1016/0891-5849(89)90162-7. [DOI] [PubMed] [Google Scholar]
- 5.Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol. 1998;25(Suppl 10):10–4. [PubMed] [Google Scholar]
- 6.Singal P, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–936. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 7.Kaiserová H, Šimůnek T, Van Der Vijgh WJ, Bast A, Kvasničková E. Flavonoids as protectors against doxorubicin cardiotoxicity: role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim Biophys Acta. 2007;1772:1065–1074. doi: 10.1016/j.bbadis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol. 2010;48:626–632. doi: 10.1016/j.fct.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Nie YC, Wu H, Li PB, Luo YL, Long K, Xie LM, Shen JG, Su WW. Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J Med Food. 2012;15:894–900. doi: 10.1089/jmf.2012.2251. [DOI] [PubMed] [Google Scholar]
- 10.Xulu S, Oroma Owira PM. Naringin ameliorates atherogenic dyslipidemia but not hyperglycemia in rats with type 1 diabetes. J Cardiovasc Pharmacol. 2012;59:133–141. doi: 10.1097/FJC.0b013e31823827a4. [DOI] [PubMed] [Google Scholar]
- 11.Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, AbuBakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagetia GC, Reddy TK. Modulation of radiationinduced alteration in the antioxidant status of mice by naringin. Life Sci. 2005;77:780–794. doi: 10.1016/j.lfs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Jagetia GC, Reddy TK. Alleviation of iron induced oxidative stress by the grape fruit flavanone naringin in vitro. Chem Biol Interact. 2011;190:121–128. doi: 10.1016/j.cbi.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Jagetia GC, Reddy TK. The grape fruit flavonone naringin protects mice against doxorubicin-induced cardiotoxicity. Journal of Molecular Biochemistry. 2014:3. [Google Scholar]
- 15.Ramesh E, Alshatwi AA. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem Toxicol. 2013;51:97–105. doi: 10.1016/j.fct.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 17.Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol. 2014;728:107–118. doi: 10.1016/j.ejphar.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 18.Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology. 2006;48:674–682. doi: 10.1111/j.1365-2559.2006.02402.x. [DOI] [PubMed] [Google Scholar]
- 19.Fu C, Lin L, Shi H, Zheng D, Wang W, Gao S, Zhao Y, Tian H, Zhu X, Chen X. Hydrophobic poly (amino acid) modified PEI mediated delivery of rev-casp-3 for cancer therapy. Biomaterials. 2012;33:4589–4596. doi: 10.1016/j.biomaterials.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 20.Misra R, Sahoo SK. Coformulation of Doxorubicin and Curcumin in Poly(D,L-lactidecoglycolide) Nanoparticles Suppresses the Development of Multidrug Resistance in K562 Cells. Mol Pharm. 2011;8:852–866. doi: 10.1021/mp100455h. [DOI] [PubMed] [Google Scholar]


