Abstract
This study aimed to re-evaluate the concordance between FISH and IHC for HER-2 status, and evaluate its clinicopathologic significance in breast cancer by the new guideline (2014) in China. We determined the HER-2 status in 589 cases of invasive breast cancer, the concordance and correlation between these two results and their relationship to cliniopathological characteristics were evaluated. The rate of HER-2 gene amplification identified by FISH was 31.07% (183/589). Concordance was detected in 93.67% with IHC 0/+, 32.26% in IHC 2+, and 61.16% in IHC 3+. The concordance of 54.67% was observed with a Kappa coefficient of 0.189 (P = 0.000), and a positive correlation was found between IHC and FISH (r = 0.427, P = 0.000). Moreover, the expression of ER was negatively correlated with HER-2 gene amplification and the expression of Her-2/neu protein (r = -0.419, P < 0.001; r = -0.144, P < 0.001; respectively), and the PR expression and ER/PR status was negatively correlated with HER-2 gene amplification (r = -0.226, P < 0.001; r = -0.258, P < 0.001), but not significantly with Her-2/neu expression. Interestingly, the tumor size and histological grade were positive correlation with HER-2/neu protein expression. The coincidently positive rate between FISH and IHC in breast cancer is very high, IHC score 0/+ is strongly consistent with HER-2 gene amplification, IHC score 2+~3+ is a preferred method to detect the HER-2 gene status, FISH still remains a standard method of evaluation of the HER-2 gene amplification.
Keywords: Breast neoplasms, human epidermal growth factor receptor 2 (HER-2), clinical pathological features, fluorescence in situ hybridization (FISH), immunohistochemistry (IHC)
Introduction
The human epidermal growth factor receptor-2 (HER-2) proto-oncogene, which plays a crucial role in the proliferation and differentiation of cells, is located on chromosome 17q11.2-q12, and encodes Her-2/neu protein. Amplification of this gene is associated with rapid progression of the disease, metastatic potential and resistance to tamoxifen [1]. Patients who with HER-2 gene amplification and Her-2/neu protein overexpression may indicate a worse situation, and are less responsive to hormonal therapies, whereas the use of anti-Her-2/neu monoclonal antibody, trastuzumab (Herceptin), especially with the combination with chemotherapy, is effective for those populations [2]. Therefore, to evaluate HER-2 status has become critical for treatment of breast cancer patients. Currently, the most commonly used method is immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), whereas the correlation between the results from these two methods, as well as the significance of the correlation in clinicopathology of breast cancer, has not been well established yet, on the basis of a new guideline in China.
Materials and methods
Specimens
A total of 589 paraffin-embedded samples from patients of invasive breast cancer were studied, of which 487 samples were obtained from Department of Pathology, the Affiliated Hospital of Southwest Medical University from November 2010 to July 2015, and 102 samples were provided by the Department of Pathology, People’s Hospital of Deyan, Sichuan, China, from October 2010 to March 2013. Patients came from southwest China including parts area of Sichuan, Yunnan, Guizhou and Chongqing province, and the samples had IHC markers (ER, PR and Ki67) and were confirmed by clinicopathological information including age, tumor size, lymph node metastasis, and histological grade. The tissues were sectioned (3 µm thickness) for both FISH and IHC. This experimental protocol was pre-approved by the Medical Ethics Committee of Southwest Medical University (No. 20130051).
Immunohistochemistry
Her-2/neu protein expression was detected by using EnVision technology. Briefly, the paraffin embedded tissue were sectioned with 3 µm thick and placed on poly-L-Lysine coated slides, every case was both with positive and negative control. The slides were dried overnight at 60°C. After deparaffinization and blocking of endogenous peroxidase, sections were transferred in retrieval solution (Tris-EDTA pH: 9.0) for 3 min, followed by washing with tap water. The sections were then incubated with the primary antibody (erbB-2, 1:100, clone 4B5, Roche, Germany) at 37°C for 30 min. Thereafter, sections were rinsed with Tris-buffered saline (TBS) three times, and then incubated with the secondary antibody (DAKO Rabbit/Mouse Chengdu China) at room temperature for 30 min, and washed with TBS. Finally, the DAB was used to illuminate the positive staining signals, and the sections were counterstained with hematoxylin. The positive staining signals were sorted into 4 grades, according to the standard procedures [3].
FISH
FISH was performed using the HER-2/neu probes kit (Jingpujia Medical Technology Co, Ltd, Beijing, China). Firstly, 3 µm thick sections were mounted on poly-L-Lysine coated slides and baked overnight at 60°C. After deparaffinizing in xylene for 10 min two times and dehydrated in 100%, 85% and 70% ethanol for 2 min each and air-dry. Secondly, immersed the slides in distilled water in Pressure-cooker (121°C) for 3 min and for 4 min after disconnect the power supply, after washed in sodium saline citrate (2×SSC, pH 7.0) for 5 min twice at 37°C, the side were then digest for 18 min in protease solution (200 µg/ml) at 37°C, and then washed in 2×SSC for 5 min twice again. After that, 10 µl hybridization buffer containing the probe was applied to the target tissue on the slides. The sections were covered with a coverslip, and then the coverslip was sealed using rubber cement. The hybridization was conducted in a hybridization oven (ThermoBrite Statspin) under the following conditions: denature at 83°C for 8 min, incubation at 42°C in a humidified chamber for 16 hrs. Finally, slides were washed in 2×SSC for 10 min at 46°C, followed by 0.1% NP40 at 46°C for 5 min, and rinsed in 70% ethanol. After air-dry, 10 µl DAPI was applied and coverslip was gently placed. The hybridization signals were analyzed using a Nikon 80i fluorescence microscope (Nikon, Tokyo, Japan). The results were re-analyzed based on the methods of the latest ASCO-CAP HER2 Test Guideline Recommendations (Wolff AC et al., 2013), e.g. if HER-2 signal (red) were clustered obvious, it was determined that clustered amplification, otherwise, 30 invasive tumor nuclei ratio of HER-2 to CEP17 of ≥ 2.0 or < 2.0 but the average HER2 copy number ≥ 6.0 signals/cell was considered as HER2 amplification whereas ≤ 2.0 and with an average HER2 copy number < 4.0 signals/cell was reported as non-amplification, while when HER2/CEP17 ratio < 2.0 with an average HER2 copy number ≥ 4.0 and < 6.0 signals/cell was taken as an equivocal result that required additional 70 nuclei or repeat of the test.
Statistical evaluation
Statistical analysis was carried out using SPSS version 13.0. IHC and FISH results were done using “Kappa” as measure of concordance. Groups were compared using Wilcoxon test, and spearman rank correlation analysis and chi-square test. P < 0.05 was considered as statistically significant.
Results
In the present study, 589 specimens of invasive breast cancer paraffin sections of southwest China women were detected. The IHC slide was scanned for immunostaining evaluation scored IHC 0, 1+, 2+, 3+ (Figure 1A-D). Of the 589 samples detected by FISH, 65.87% (388/589) showed HER-2 negative (Figure 2A), 3.06% (18/589) were detected report equivocal (Figure 2B), and the rate of HER-2 gene amplification identified was 31.07% (183/589; Figure 2C, 2D). Concordance was detected in 93.67% with IHC 0/+, 32.26% in IHC 2+, 61.16% overall in IHC 3+. The concordance of 54.67% was observed with a Kappa coefficient of 0.189 (P = 0.000) and a positive correlation was found between IHC score and FISH results (r = 0.427 P = 0.000) (Table 1).
Figure 1.
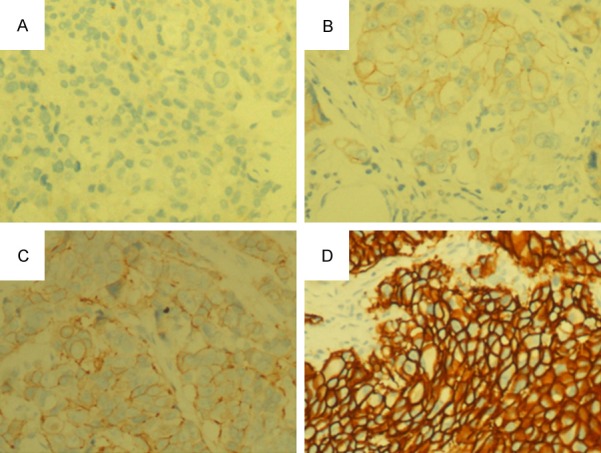
IHC scored of Her-2/neu protein. A. IHC 0: Negative membrane staining, ×200. B. IHC 1+: Weak membrane staining, ×200. C. IHC 2+ Moderate membrane staining, ×200. D. IHC 3+: Strong membrane staining, ×200.
Figure 2.
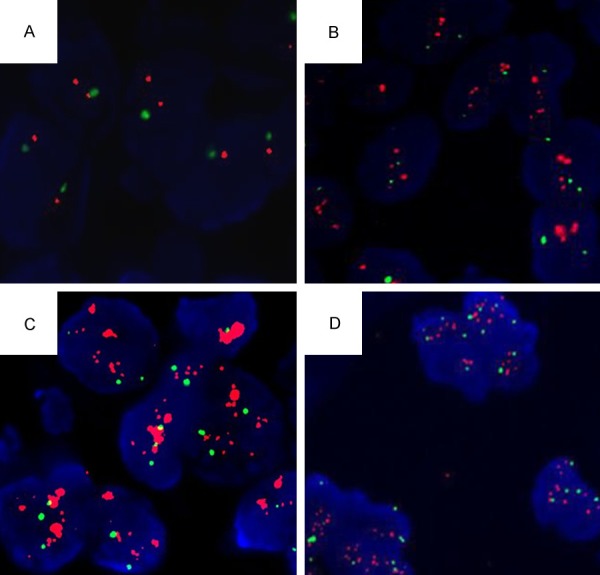
FISH results (CEP17 stained green and HER-2 gene stained red). A. Negative, a ration of HER-2 to CEP17 signals less than 2.0 and with an average HER2 copy number < 4.0 signals/cell. B. Equivocal: a ratio of HER-2 to CEP17 signals < 2.0 with an average HER2 copy number ≥ 4.0 and < 6.0 signals/cell. C. HER-2 gene amplification: red signal were clustered obvious. D. HER-2 gene amplification: a ratio of HER-2 to CEP17 signals < 2.0 but the average HER2 copy number ≥ 6.0 signals/cell.
Table 1.
Correlation between IHC and FISH in HER-2 status
| IHC Score | FISH Status | Total | Concordance | ||
|---|---|---|---|---|---|
|
| |||||
| Negative | Equivocal | Positive | |||
| -/+ | 148 | 1 | 9 | 158 | 93.67% (148/158) |
| ++ | 198 | 12 | 100 | 310 | 32.26% (100/310) |
| +++ | 42 | 5 | 74 | 121 | 61.16% (74/121) |
| Total | 388 | 18 | 183 | 589 | |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization. Kappa = 0.189, P = 0.000; r = 0.427, P = 0.000.
The relationship between FISH and IHC, and the relevance to cliniopathological characteristics, was also investigated in this study. There were significant differences between the expression of ER, PR, ER/PR status and FISH results (x2 = 108.171, P = 0.000; x2 = 30.914, P < 0.001; x2 = 39.845, P < 0.001), Expression of ER, PR also significant difference to HER-2/neu protein expression (x2 = 19.430, P < 0.001; x2 = 8.609, P < 0.05). The expression of ER was negatively correlated with HER-2 gene amplification and the expression of Her-2/neu protein (r = -0.419, P = 0.000; r = -0.144, P = 0.000). The PR expression and ER/PR status was negatively correlated with HER-2 gene amplification (r = -0.226, P = 0.000; r = -0.258, P = 0.000), but not significantly with Her-2/neu protein expression (P > 0.05), otherwise the tumor size and histological grade were positive correlation with HER-2/neu protein expression, but not with HER-2 amplification (Table 2).
Table 2.
Association of HER-2/neu protein expression, HER-2 gene status and clinical-pathological characteristics
| FISH results | P value (Ranksum test) | P value (Correlation test) | IHC Score | P value (Ranksum test) | P value (Correlation test) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Negative | Equivocal | Positive | -/+ | ++ | +++ | |||||
| Age | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||||||
| ≤ 45 | 200 | 6 | 81 | 82 | 151 | 54 | ||||
| > 45 | 188 | 12 | 102 | 76 | 159 | 67 | ||||
| ER | P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | ||||||
| - | 84 | 3 | 108 | 39 | 110 | 46 | ||||
| + | 81 | 7 | 51 | 27 | 78 | 34 | ||||
| ++ | 107 | 3 | 15 | 54 | 47 | 24 | ||||
| +++ | 116 | 5 | 9 | 38 | 75 | 17 | ||||
| PR | P = 0.000 | P = 0.000 | P = 0.035 | P > 0.05 | ||||||
| - | 133 | 5 | 104 | 56 | 141 | 45 | ||||
| + | 112 | 7 | 49 | 64 | 69 | 35 | ||||
| ++ | 86 | 3 | 18 | 29 | 52 | 26 | ||||
| +++ | 57 | 3 | 12 | 9 | 48 | 15 | ||||
| ER/PR | P = 0.000 | P = 0.000 | P > 0.05 | P > 0.05 | ||||||
| -/- | 71 | 4 | 74 | 36 | 82 | 31 | ||||
| +/- | 56 | 5 | 32 | 20 | 66 | 7 | ||||
| -/+ | 29 | 6 | 11 | 16 | 24 | 6 | ||||
| +/+ | 232 | 3 | 66 | 86 | 138 | 77 | ||||
| Ki67 | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||||||
| ≤ 50 | 259 | 11 | 118 | 107 | 199 | 82 | ||||
| > 50 | 129 | 7 | 65 | 51 | 111 | 39 | ||||
| Tumor size | P > 0.05 | P > 0.05 | P > 0.05 | P = 0.000 | ||||||
| < 2 | 101 | 4 | 50 | 52 | 70 | 33 | ||||
| ≥ 2, < 5 | 182 | 9 | 85 | 91 | 158 | 27 | ||||
| ≥ 5 | 105 | 5 | 48 | 15 | 82 | 61 | ||||
| lymph node | P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | ||||||
| 0 | 145 | 3 | 57 | 53 | 107 | 45 | ||||
| ≥ 1, < 4 | 114 | 10 | 58 | 42 | 106 | 34 | ||||
| ≥ 4 | 129 | 5 | 68 | 63 | 97 | 42 | ||||
| Histological grade | P > 0.05 | P > 0.05 | P = 0.022 | P = 0.003 | ||||||
| I | 109 | 6 | 42 | 51 | 87 | 19 | ||||
| II | 151 | 9 | 87 | 65 | 127 | 55 | ||||
| III | 128 | 3 | 54 | 42 | 96 | 47 | ||||
ER, estrogen receptor; PR, progesterone receptor.
Discussion
As the accuracy and importance of HER2 testing and its status evaluation, The ASCO/CAP (American Society of Clinical Oncology and the College of American Pathologists) have continuous updated the recommendations in HER-2 testing not only technical guidelines but also interpretation guidelines. We use the recently updated guidelines, the new ASCO/CAP 2013 criteria, in our experiment. The new guideline in order to avoid false negative results contrast to the former one, and the first time combined the HER-2/CEP17 ratio and the HER-2 gene copy number to define the negative and equivocal status. We analyzed the concordance between FISH and IHC for HER-2 status, and re-evaluated its clinicopathologic significance to got results as following using the new guidelines make sure inferred more reliable evaluation results.
Her-2 gene amplification and its receptor protein over expression are known to be associated with poor prognosis. It is significant that correct evaluation of HER-2 status of breast cancer patients to their treatment and prognosis [4]. Generally, IHC assay is used traditionally and relatively cheap and convenient to operate. When IHC 2+, indicating there is nondeterminacy in the Her-2/neu protein expression, at this point, FISH, considering as a gold standard, is a technology of evaluation HER-2 gene amplification at the levels of DNA [1,5]. Thus, both IHC and FISH are widely used to detect HER-2 status, the concordance between the two methods is extensively investigated, we used the most recent guidelines re-evaluated its concordance there to study further. Recent studies reported that strong correlation between IHC 0/1+ and FISH [1,5,6]. In our present study, the concordance between FISH and IHC 0/1+ was 93.67%, which is consistency with the researches, and increased compared with our previous reported 87.5% [7], Otherwise, 388 cases were detected negative in 589 samples which compared 88 cases negative in the 159 patients studied in the previous study, the ratio has increased than old criteria, not exactly the same as Stoss et al. [8] reported. Because the analyzed results do not came from same batch of data and also related to the small cases in previous research. Then we reported the concordance between FISH and IHC 2+ was 32.26%. It is consistency with Sudha SM et al. reported about 16-29% [9,10], and lower than 50.50% we calculated before [7]. It seems the most recent ASCO/CAP guidelines, which combine the HER2/CEP17 ratio and the mean HER2 gene copy number to define the equivocal status, can effectively reduce the redetection [8]. But showed IHC 2+ should be routinely performed FISH test because its lower concordance rate, in order to accurately make a clinical treatment plan for the patients. Otherwise, several reporters have published their researches to show high concordance rate about between FISH and IHC 3+ [1,3,6,11,12], by contrast, our study illustrated that numeric only 61.16%, just similar as the previous reported 58.8% [7]. The reasons might be attribute to false positive results of IHC, such as specimens fixation, method of antigen retrieval, differentt antibodies and subjective interpretations, as well as impact of chromosome 17. Overall, our study demonstrated consistency between two technologies by consistency test (Kappa coefficient of 0.189) and a positive correlation was found between FISH and IHC assay, which is consistent with Kovacs’ conclusion, but the consistency was not as the above, it’s not difficult to see the difference is mainly in IHC 3+ which group still have a great percentage of HER-2 gene without amplification, but patients who with IHC 3+ can directly use targeted drug therapy in currently therapy guideline in the world. Therefore, IHC 3+ may another detected HER-2 gene status by FISH and HER-2 gene amplification patients taking targeted therapy have better prognosis just as the research of Mass RD et al. [13]. A lot of researches were also supported the FISH test results to be the basic standards to determine whether taking the targeted drug [14]. In summary, we recommend that the breast cancer patients should test the HER-2 gene by FISH whatever the IHC assay results, and thus to improve the diagnosis, guide treatment and determine the prognosis.
In the published literatures, there is an inverse association between HER-2 gene amplification and ER expression [15-17], in comparison, our research also indicated the similar results. Furthermore, ER also showed a converse relationship with HER-2/neu protein expression, such as Mirtavoos-Mahyari et al. [18] reported that ER-negative breast cancer tumors were significantly more likely to be HER-2 positive (3+ by immunohistochemistry or positive by fluorescent in situ hybridization) than were ER-positive tumors. In addition, Huang HJ et al. [5] reported that there is an inverse association between PR and HER-2/neu in patients older than 45-year-old, and Han Zhang et al. [10] reported a significant difference of HER-2 gene amplification between ER+PR+ and ER-PR-. In our present results, PR expression and ER/PR status was negatively correlated with HER-2 gene amplification. Meanwhile, ER/PR status, such as ER-PR- or ER+PR-, seemed to have worse biologic characteristics and poor prognosis [19]. The reason could be the complex signaling pathways between ER, PR, HER-2/neu in breast cancer cells. Evidence certified that the expression of ER, PR, HER-2 in breast cancer may classify the patients into subtypes, which closely related to clinic [20,21].
It is an established observation that HER-2/neu protein positive status is associated with growing tumor size [22,23] and tumor grade [24]. It similarly showed a positive correlation with HER-2/neu protein expression in our present study. And other clinicopathologic features like Ki67, age and lymph node did not show significant correlation with HER-2 gene amplification and HER-2/neu protein expression, as Ning SF et al. reported [6]. But Mujtaba et al. [23] once reported HER-2/neu over-expression increased with increasing lymph node metastasis, this might be due to different date statistic.
In conclusion, Breast cancer patients, considering clinical therapy, should be detected ER, PR and HER-2/neu expression routine by IHC, but as the basis of targeted therapy, FISH strongly recommends a standard method to evaluation HER-2 gene amplification. The new 2013 guidelines improved consistency between IHC and FISH test, and calculating HER-2/CEP17 ratio combined with the HER-2 gene copy number show a more accuracy when evaluating negative and suspicious status.
Acknowledgements
This research was supported, in part by grants from the Foundation of the Health and Planning Commission of Sichuan Province of China (120364), The Foundation of the Affiliated Hospital of Southwest Medical University of China (15087, 2015-PT-003), and the Applied Basic Research Programs of Science and Technology Commission Foundation of Sichuan Province of China (14JC01613-LH44). The authors also thank all patients of histopathology section from Department of Pathology, the Affiliated Hospital of Southwest Medical University and Department of Pathology, People’s Hospital of Deyang, Sichuan, China to provide paraffin tissue and clinical data.
Disclosure of conflict of interest
None.
References
- 1.Panjwani P, Epari S, Karpale A, Shirsat H, Rajsekharan P, Basak R, Shet T, Chinoy R, Chacko R, Gursale S, Baraskar N, Gupta S, Hawaldar R, Desai S. Assessment of HER-2/neu status in breast cancer using fluorescence in situ hybridization and immunohistochemistry: experience of a tertiary cancer referral centre in India. Indian J Med Res. 2010;132:287–294. [PubMed] [Google Scholar]
- 2.Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: a review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010;70:215–239. doi: 10.2165/11203700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Singhai R, Patil V, Patil A. Immunhistochemical (IHC) HER-2/neu and fluorescent-in-situ hybridization (FISH) gene amplification of breast cancer in Indian women. Asian Pac J Cancer Prev. 2011;12:179–183. [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 5.Kokate P, Sawaimoon S, Bhatia S, Mandava S. Evaluation of genetic status of HER-2/neu and aneusomy 17 by fluorescence in situ hybridization and comparison with immunohistochemistry assay from Indian breast cancer patients. Genet Test Mol Biomarkers. 2012;16:239–245. doi: 10.1089/gtmb.2011.0125. [DOI] [PubMed] [Google Scholar]
- 6.Ning SF, Li JL, Luo CP, Wei CH, Lu YK, Liu HZ, Wei WE, Zhang LT. Human epidermal growth factor receptor 2 expression in breast cancer: correlation with clinical pathological features. Int J Clin Exp Pathol. 2014;7:8740–8747. [PMC free article] [PubMed] [Google Scholar]
- 7.Li SN, Tang H, Guo QX, Ruan SB, Zhang Y, Luo X, Sun XW, Tang MX. HER-2 gene amplification and HER-2/neu protein expresssion in invasive breast cancer and its relationship to clinicopathological characteristics. J Clin Exp Pathol. 2014;30:251–255. [Google Scholar]
- 8.Stoss OC, Scheel A, Negalmeier I, Schildhaus HU, Henkel T, Viale G, Jasani B, Untch M, Ruschoff J. Impact of updated HER2 testing guidelines in breast cancer-re-evaluation of HERA trial fluorescence in situ hybridization data. Mod Pathol. 2015;28:1528–1534. doi: 10.1038/modpathol.2015.112. [DOI] [PubMed] [Google Scholar]
- 9.Murthy SS, Sandhya DG, Ahmed F, Goud KI, Dayal M, Suseela K, Rajappa SJ. Assessment of HER2/neu status by fluorescence in situ hybridization in immunohistochemistry-equivocal cases of invasive ductal carcinoma and aberrant signal patterns: a study at a tertiary cancer center. Indian J Pathol Microbiol. 2011;54:532–538. doi: 10.4103/0377-4929.85087. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Ren G, Wang X, Zhao J, Yao H, Bai Y, Bo W. HER-2 gene amplification by fluorescence in situ hybridization (FISH) compared with immunohistochemistry (IHC) in breast cancer: a study of 528 equivocal cases. Breast Cancer Res Treat. 2012;134:743–749. doi: 10.1007/s10549-012-2101-x. [DOI] [PubMed] [Google Scholar]
- 11.Parti R, Apple SK, He J, Gornbein JA, Chang HR. Histopathologic characteristics predicting HER-2/neu amplification in breast cancer. Breast J. 2005;11:433–439. doi: 10.1111/j.1075-122X.2005.00125.x. [DOI] [PubMed] [Google Scholar]
- 12.Moatter T, Aban M, Iqbal W, Azam I, Pervaiz A, Siddiqui F, Murad F, Pervez S. Status of HER2 amplification, polysomy17 and histopathological features of 425 Pakistani breast cancer patients. Asian Pac J Cancer Prev. 2011;12:3069–3073. [PubMed] [Google Scholar]
- 13.Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 14.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybirdization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y, Sheng L, Du X, Qiu G, Chen B, Wang X. Clinicopathological variables predicting HER-2 gene status in immunohistochemistry-equivocal (2+) invasive breast cancer. J Thorac Dis. 2014;6:896–904. doi: 10.3978/j.issn.2072-1439.2014.07.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 17.Purdie CA, Baker L, Ashfied A, Chatterjee S, Jordan LB, Quinlan P, Adamson DJ, Dewar JA, Thompson AM. Increased mortality in HER-2 positive, oestrofen receptor positive invasive breast cancer: a population-based study. Br J Cancer. 2010;103:475–481. doi: 10.1038/sj.bjc.6605799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirtavoos-Mahyari H, Khosravi A, Esfahani-Monfared Z. Human epidermal growth factor receptor 2 and estrogen receptor status in respect to tumor characteristics in non-metastatic breast cancer. Tanaffos. 2014;13:26–34. [PMC free article] [PubMed] [Google Scholar]
- 19.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, Bellon JR, Wong JS, Smith BL, Harris JR. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J. Clin. Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 21.Wiechmann L, Sampson M, Stempel M, Jacks LM, Patil SM, King T, Morrow M. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol. 2009;16:2705–2710. doi: 10.1245/s10434-009-0606-2. [DOI] [PubMed] [Google Scholar]
- 22.Azizun-Nisa , Bhurgri Y, Raza F, Kayani N. Comparison of ER, PR and HER-2/neu (C-crbB-2) reactivity pattern with histologic grade, tumor size and lymph node status in breast cancer. Asian Pac J Cancer Prev. 2008;9:553–556. [PubMed] [Google Scholar]
- 23.Mujtaba S, Haroon S, Faridi N, Lodhi FR. Correlation of human epidermal growth factor receptor 2 (HER-2/neu) receptor status with hormone receptors oestrogen receptor, progesterone receptor status and other prognostic markers in breast cancer: an experience at tertiary care hospital in Karachi. J Pak Med Assoc. 2013;63:854–858. [PubMed] [Google Scholar]
- 24.Hang HJ, Neven P, Drijkoningen M, Paridaens R, Wildiers H, Van Limbergen E, Berteloot P, Amant F, Christiaens MR, Vergote I. Assciation between HER2/neu and the progesterone receptor in oestrogen-dependent breast cancer is age-related. Breast Cancer Res Treat. 2005;91:81–87. doi: 10.1007/s10549-004-8235-8. [DOI] [PubMed] [Google Scholar]


