Abstract
This study was to investigate the differentiation potential of iPSCs into olfactory receptor neurons and mitral/tufted cells in vitro. We extracted mouse embryonic fibroblast and prepared feeding layer, where mouse iPSCs were inoculated on; RT-PCR were used to identify iPSCs pluripotency genes Oct4, Nanog and Sox2. Then, we separated the olfactory epithelium and olfactory bulb from mice, which contained respectively olfactory receptor neurons and mitral/tufted cells, co-cultured iPSCs with olfactory epithelium cell and olfactory bulb, and identified differentiated cells with the olfactory receptor neurons markers (OMP, GAP43, NCAM), mitral/tufted cells markers (TBX21, Iba1) after 14 days co-culturing by immunofluorescence and RT-PCR. We successfully established a stable culture system of mouse iPSCs and RT-PCR showed that pluripotency genes (Oct4, Nanog, Sox2) were expressed in mouse iPSCs. Immunocytochemical analysis or RT-PCR results indicated that the differentiated iPSCs can express olfactory receptor neurons markers (OMP, GAP43, NCAM) and mitral/tufted cells markers (TBX21, Iba1) after being co-culture with olfactory epithelium or olfactory bulb. We conclude that Mouse iPSCs can be differentiated into olfactory receptor neuron-like cells and mitral/tufted-like cells in vitro.
Keywords: Induced pluripotent, stem cells, olfactory receptor neurons, mitral/tufted cells, cell differentiation, repair
Introduction
Tissue regenerative medicine refers to the use of biological and engineering methods to exchanging impaired cells or tissues with new functional cells or tissues. Sensorineural dysosmia may negatively impact quality of life, safety, and nutrition [1], which remains a clinical challenge. The first step in regenerative medicine for sensorineural dysosmia is the generation of olfactory receptor neurons (OSNs) and mitral/tufted cells. Olfactory receptor neurons have round or oval cell bodies and possess a bipolar morphology. The neurons extend a dendrite to the apical surface of the olfactory epithelium, where odor receptors are localized; and its axons form functional synaptic connections with the dendrites of mitral/tufted cells within the glomeruli of the olfactory bulb [2]. Although neurons has the ability to regenerate, neurogenesis following damage involves that a number of steps in the regenerative process be executed properly [3].
Induced pluripotent stem cells (iPSCs) were firstly generated by Takahashi and Yamanaka through using a combination of 4 reprogramming factors including Oct4 (Octamer binding transcription factor-4), Sox2 (Sex determining region Y)-box 2, Klf4 (Kruppel-like factor-4), and c-Myc in 2006 [4]. It can not only be derived from different types of somatic cells by introduction of several defined transcription factors [5], but also demonstrates both self-renewing and pluripotency like embryonic stem cells (ESCs) without immune rejection or ethical issues [6], which could be used as an alternative for ESCs in various clinical research. Previously, several studies have reported the differentiation to olfactory receptor neurons-like or mitral/tufted-like cells from neural stem cells and mesenchymal stem cells. Lee et al. [7] engrafted neural stem cells transnasally into the mouse anosmia model and found that the control group had better survival rate and better recovery of olfactory function in terms of the food-finding test and the expression of OMP (olfactory marker protein) than those of the control group. Bone marrow-derived cells have been suggested to be transplanted in the olfactory bulb through the tail vein injection and a few cells can differentiate into mitral/tufted cells in the olfactory bulb [8]. Franceschini V et al. reported that human adipose tissue-derived stem cells were intravenously injected to immune deficient mice and this was shown to provide conditions for regeneration of the olfactory neuroepithelium after permanent damage was induced by dichlobenil [9].
As shown in the above-mentioned studies, Cell-based therapy might be a promising option for olfactory impairment. iPSCs can be differentiated to different cell types and be applied in the study or treatment of numerous diseases and drug discovery [10]. However, there is less research in regard to the potential treatment of iPSCs for dysosmia. In the present study, we intended to evaluate the therapeutic potential of iPSCs in restoring olfactory. The regenerative potential of iPSCs was demonstrated through indirect co-culture with olfactory epithelium and olfactory bulb in vitro and investigated whether iPSCs could be differentiated into olfactory receptor neurons or mitral/tufted cells, which may provide an experimental basis for the treatment of sensorineural olfactory disorder.
Materials and methods
Cell line and cell culture
Specific pathogen Free (SPF) CD-1/ICR mice were ordered from Vital River Experimental Animal Technology Co. Ltd (Beijing, China). Mouse Embryonic Fibro lasts (MEF) were extracted from E12.5 CD-1/ICR mice embryos. A mouse induced pluripotent stem (iPS) cell line (0203-001) obtained from the SiDanSai biological technology Co. Ltd (Shanghai, China) was maintained on 1×104 cells per cm2 mitomycin C-inactivated MEF as a feeder layer on 0.1% gelatin-coated tissue culture dishes in iPSC medium containing KNOCKOUT Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 15% fetal bovine serum (FBS; Gibco), 1% nonessential amino acids (Gibco), 1% L-GlutaMax 100×(Gibco), 0.1 mM β-mercaptoethanol (Gibco), 1 ul/ml leukemia inhibitory factor (LIF; Millipore, American). The primary olfactory epithelium [11] or olfactory bulb was obtained from CD-1/ICR mice at postnatal day 5 and was cultured in medium containing 97% DMEM/F12 (1:1) (Hyclone) supplemented with 2% N2 (Gibco), 1% B27 (Gibco) and 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco).
All animal experiments were approved by the Laboratory Animal Ethics Committee of the First Affiliated Hospital of Nanchang University.
Mouse iPSC differentiation in indirect co-culture model
For generation of embryoid bodies (EBs), iPS cells were trypsinized and re-suspended in EB medium (above-mentioned iPS medium without LIF). To induce EB formation, the hanging drop method was used and drops of 20 μl containing 2000 cells were pipetted onto the lids of 10 cm cell culture dishes, then the lids were placed back on the 10 cm dish containing 10 mL PBS to prevent drying out of hanging drops and incubated at 37°C for three days. 24 mm Transwell with 0.4 um pore polyester membrane insert (product #3450, Corning, American) which are packaged 6 inserts in a 6 well plate were used for co-culture. 3-day-oldEBs were first carefully transferred to cell slides coated with fibronectin, which were placed in 6-well plates and each well was inoculated 15 EBs, then the inserts were placed in same well of 6-well plate followed by primary olfactory epithelium or olfactory bulb seeding on the inserts in medium containing 97% DMEM/F12 (1:1) (Hyclone) supplemented with 2% N2 (Gibco), 1% B27 (Gibco). Medium was changed every day. The cells seeding on the upper chamber (the insert) stayed physically separated from the subnatant EBs, but may communicate via cytokine through the 0.4 µm pores.
Immunofluorescence
After 14-day co-culture, for staining of iPSCs, the cell slides in co-culture system were taken out and washed three times by PBS, and then they were fixed in 4% paraformaldehyde for 15 minutes and permeabilized for 20 minutes in 0.5% Triton X-100/phosphate-buffered saline (PBS). Next, blocking was performed by incubation in concentrated normal goat serum (Boster, Wuhan, China) for 30 minutes. Then the cells were incubated with primary antibodies overnight at 4°C. Primary antibodies were anti-OMP (1:100; Santa Cruz Biotechnology, sc-365818), anti-GAP43 (1:100; Abcam Biotechnology; ab-11136), anti-NCAM (1:100; Abcam Biotechnology; ab-9018), anti-T-bet/TBX21 (1:100; Abcam Biotechnology; ab-181400), anti-Iba1 (1:100; Abcam Biotechnology; ab178680). Then cells were incubated with secondary antibody for 2 hour at room temperature. Secondary antibodies were goat anti-rabbit (IgG-cy31:100; Boster, Wuhan, China; BA1032), goatanti-mouse IgG-cy3 (1:100; Boster, Wuhan, China; BA1031). Finally, cells were stained with 4’,6-diamidino-2-phenylindole (DAPI) for nuclear counterstaining and mounted with Antifade Mounting Medium (Southern Biotech; 0100-01). All fluorescent photographs were acquired using a using an Olympus BX53 fluorescence microscope. Secondary antibodies were from the goat.
RNA extraction and RT-PCR
To identify iPSCs pluripotency genes Oct4, Nanog and Sox2, semi-quantitative RT-PCR was used. Total RNA of iPSCs was extracted using TRIzol reagent (Life Technologies, Darmstadt, Germany), according to the manufacturer’s instructions. cDNA was synthesized using Ex Taq TM (TAKARA; DRR100A). The resulting cDNA was amplified by PCR using specific primers. Primer sequences are listed in Table 1. Thermal cycling was performed as follows: 94°C (4 min), 30 cycles of 94°C for 30 s, 56°C for 25 s and 72°C for 25 s, then 72°C (4 min) and 4°C (4 min). β-actin expression was used as a normalizing internal control. PCR products were resolved by electrophoresis on 1.5% agarose gels.
Table 1.
Primers for reverse transcription polymerase chain reaction to identify iPSCs pluripotency
| Genes | Sequence |
|---|---|
| Nanog | F: 5’-CTCCGCTCCATAACTTCGGG-3’ |
| R: 5’-GCCCAGATGTTGCGTAAGTC-3’ | |
| Oct4 | F: 5’-AGCCGACAACAATGAGAACC-3’ |
| R: 5’-TGATTGGCGATGTGAGTGAT-3’ | |
| Sox2 | F: 5’-GTGGTTACCTCTTCCTCCCAC-3’ |
| R: 5’-TCTCCCCTTCTCCAGTTCG-3’ |
To quantitate olfactory receptor neurons-specific markers (OMP, GAP43, NCAM) expression and mitral/tufted cells specific makers (TBX21, Iba1) expression in differentiated iPS cells, the synthesized cDNA of iPSCs after 14-day co-cultured was followed the same steps as mentioned above with b-actin expression as an internal control. Real-time quantitative PCR (qPCR) was performed using the 2×All-in-One™ qPCR Mix (GeneCopoeia, American). Specific primers for reverse transcription and PCR reaction are shown in Table 2. The conditions for qPCR were 50°C (2 minutes), 95°C (10 minutes), followed by 40 cycles of 95°C for 30 s and 60°C for 30 s.
Table 2.
Primers for reverse transcription polymerase chain reaction to identify the differentiated iPSCs after 14-day co-culture
| Genes | Sequence |
|---|---|
| NCAM | F: 5’-CGCAGAGTATGAAGTCTATGTGGTA-3-3’ |
| R: 5’-CAGGACGAAGATGACAATGAGGAT-3’ | |
| GAP43 | F: 5’-AGCCTAAACAAGCCGATGTGCCT-3’ |
| R: 5’-TCTTCTTTACCCTCATCCTGTCG-3’ | |
| OMP | F: 5’-TCTTCTTTACCCTCATCCTGTCG-3’ |
| R: 5’-AGCAGATGCGGCTCCGAGTAGA-3’ | |
| TBX21 | F: 5’-GTTCCCATTCCTGTCCTTCACCG-3’ |
| R: 5’-ACACTGCACCCACTTGCCGCTCT-3’ | |
| Iba1 | F: 5’-CCTCGATGATCCCAAATACAGCA-3’ |
| R: 5’-GCCACTGGACACCTCTCTAATTAA-3’ |
All experiments were done in triplicate. Data were normalized to the internal control and relative expression levels were evaluated using the 2-ΔΔCt method. Specific primers for reverse transcription and PCR reaction are shown in Tables 1 and 2.
Statistical analysis
The experiment was repeated at least three times. All the data are presented as mean ± SEM. Statistical significance for two comparisons was evaluated by t test. Differences with P < 0.05 were considered statistically significant.
Results
Characterization of MEF cells
The MEF were extracted from E12.5 CD-1/ICR mice embryos, after one-day culture, the cells grew with adherence, which showed a long spindle shaped, cytoplasmic full and strong in stereo dimension (Figure 1A). They grew rapidly and were passaged after 3 day culture; while MEF were inactivated by mitomycin C for 3 hours and became feeder cells. The feeder cells lose their proliferative capacity and were as a feeder layer for culturing IPS cells (Figure 1B).
Figure 1.
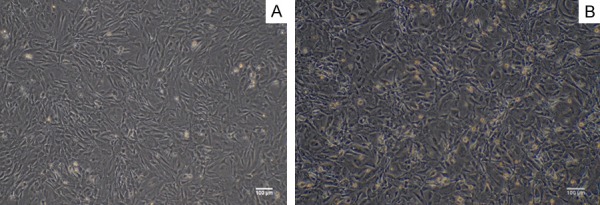
Characterization of MEF cells. A. The primary MEF cells showed a long spindle shaped, cytoplasmic full and strong in stereo dimension. Bar = 100 micrometer. B. Mitomycin C-inactivated MEF as a feeder layer cells: the cell became slender and some black particles uniformly distributed in the cells. Bar = 100 micrometer.
Identification of mouse iPS cells
Mouse iPS cells were cultured with feeder cells in the above mentioned iPSC medium. Figure 2A shows undifferentiated iPSCs colonies grew on feeder cells; After 3-4 days culture, we collected the mouse iPS cells and form EBs (Figure 2B). Initially, EBs were formed by hanging drop culture and largely composed of densely packed iPSCs, creating simple EBs. After cultured in suspension 2-3 days, the EBs were collected and were cultured on 6-well plates. Under fluorescence microscope, the undifferentiated EBs emitted green fluorescence with Oct4-GFP+ (Figure 2C).
Figure 2.
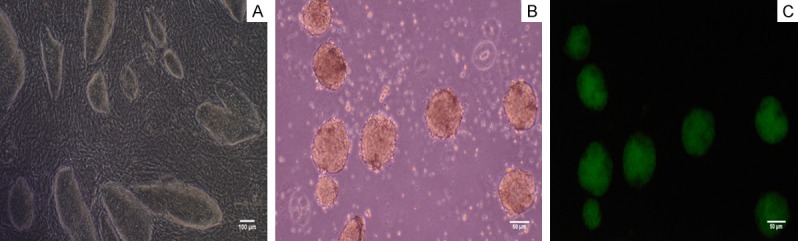
Characterization of mouse iPS cells. A. The iPS cells grew with adherence and formed colonies. Bar = 100 micrometer. B. Embryoid bodies (EBs) formed readily the hanging drop method. Bar = 50 micrometer. C. EBs emitted green fluorescence under the excitation of the blue light under the fluorescence microscope. Bar = 50 micrometer.
Semi-quantitative PCR showed the pluripotency genes (Nanog, Oct4, and Sox2) were expressed in the iPS cells but not in the MEF, which identify iPSC pluripotency (Figure 3).
Figure 3.
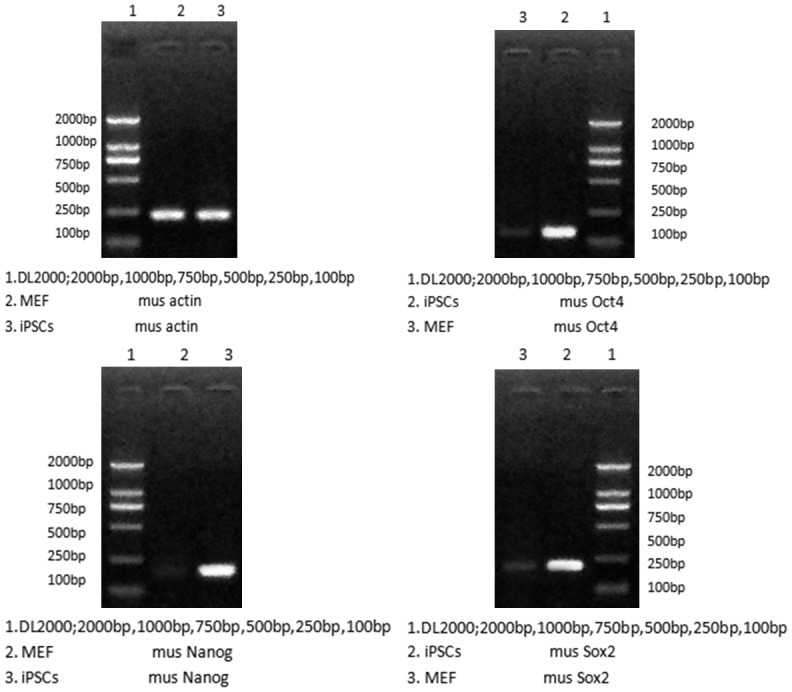
Upregulation of pluripotency genes (Nanog, Oct4, and Sox2) in the iPS cells compared with MEF by RT-PCR analysis. Actin was the internal control.
Differentiation to OSNs or M/T cells in vitro
In vitro differentiation of iPSCs was induced using a co-culture model established by Corning Transwell. In the co-culture model, the ORNs or M/T cells were seeded on hanging cell culture inserts to prevent direct contact with the EBs, which were seeded on the 6-well plates. At 14-day co-culture, we collected the EBs on the Transwell 6-well for analysis.
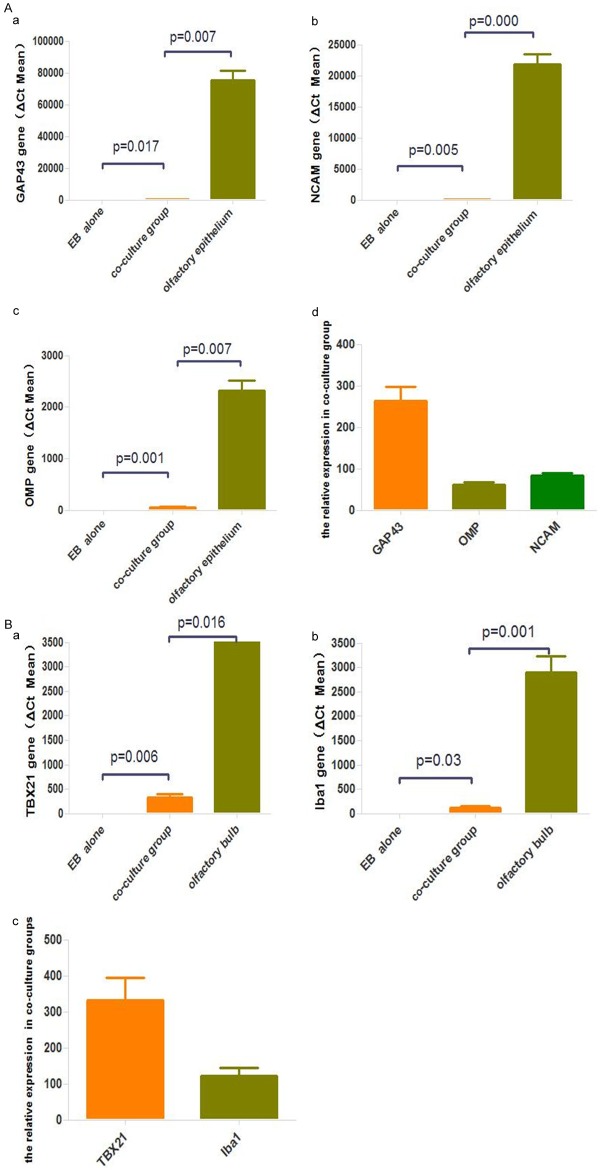
Immunofluorescence analysis of OSNs co-culture group showed that the expression and distribution of the ORNs proteins as OMP (Figure 4A), NCAM (Figure 4B) and GAP43 (Figure 4C) were more obvious than that in the control. As the same, M/T cells co-culture group also show M/T cells specific proteins TBX21 (Figure 5A), Iba1 (Figure 5B) expression, not in control group.
Figure 4.
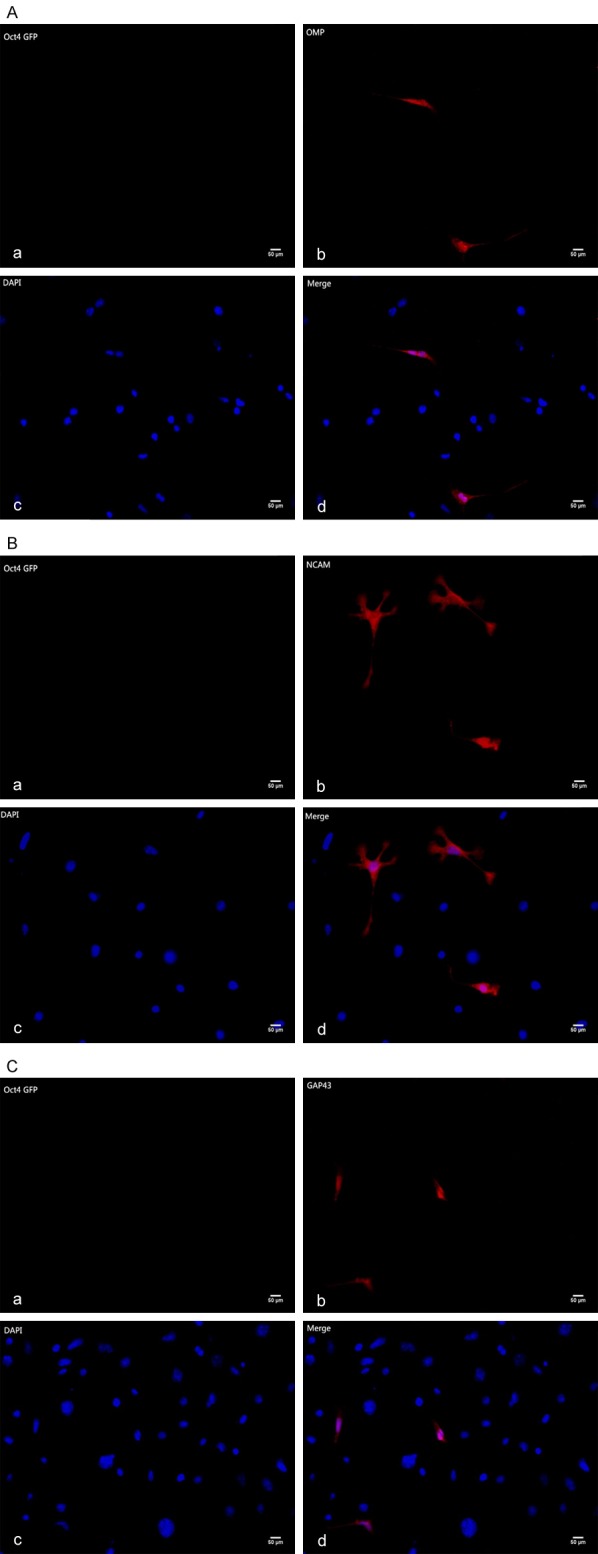
A. Immunofluorescence staining of olfactory receptor neuron specific markers (OMP, Olfactory Maker Protein). Aa. The iPS cells co-cultured with OSNs hardly expressed Oct4 GFP Gene. Ab. The differentiated iPSCs expressed OMP after 12-day co-culture Scale bars = 50 um. Ac. 4’,6-diamidino-2-phenylindole (DAPI) staining. Ad. Image D is merged by image B and image C. Scale bars = 50 um. B. Immunofluorescence staining of olfactory receptor neurons specific Markers (NCAM, Neural Cell Adhesion Molecule). Ba. The iPS cells co-cultured with OSNs hardly expressed Oct4 GFP Gene. Bb. The differentiated iPSCs expressed NCAM after 12-day co-culture. Bc. 4’,6-diamidino-2-phenylindole (DAPI) staining. Bd. Image D is merged by image B and image C. Scale bars = 50 um. C. Immunofluorescence staining of olfactory receptor neurons specific Markers (GAP43, Growth Associated Protein-43). Ca. The iPS cells co-cultured with OSNs hardly expressed Oct4 GFP Gene. Cb. The differentiated iPSCs expressed GAP43 after 12-day co-culture. Cc. 4’,6-diamidino-2-phenylindole (DAPI) staining. Cd. Image D is merged by image B and image C. Scale bars = 50 um.
Figure 5.
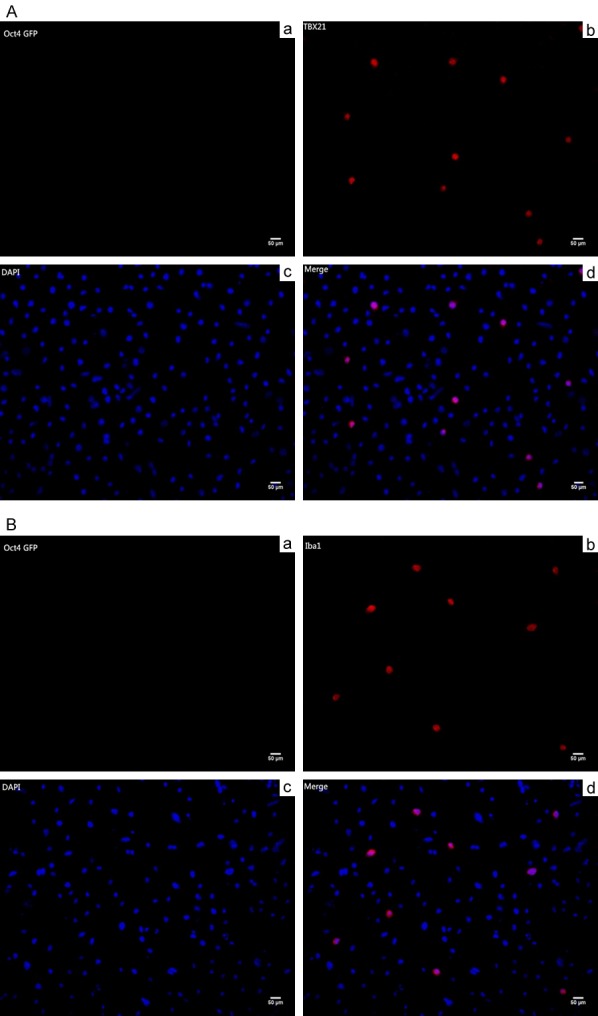
A. Immunofluorescence staining of mitral/tufted neurons specific markers (TBX21, T-box Transcription Factor-21). Aa. The iPS cells co-cultured with OSNs hardly expressed Oct4 GFP Gene. Ab. The differentiated iPSCs expressed TBX21 after 12-day co-culture. Ac. 4’,6-diamidino-2-phenylindole (DAPI) staining. Ad. Image D is merged by image B and image C. Scale bars = 50 um. B. Immunofluorescence staining of mitral/tufted neurons specific markers (Iba1, Ionized Calcium Binding Adaptor Molecule 1). Ba. The iPS cells co-cultured with OSNs hardly expressed Oct4 GFP Gene. Bb. The differentiated iPSCs expressed Iba1 after 12-day co-culture. Bc. 4’,6-diamidino-2-phenylindole (DAPI) staining. Bd. Image D is merged by image B and image C. Scale bars = 50 um.
RT-PCR showed expression levels of OMP, NCAM, GAP43 in the co-culture on days 14 (Figure 6A) compared with the control; besides, GAP43 expression was significantly higher than OMP, NCAM, which showed more iPSCs may differentiated to non-mature OSNs other than mature OSNs. TBX21, Iba1 (Figure 6B) were also expressed in olfactory bulb co-culture group, not in the control group.
Figure 6.
A. Real-time PCR analysis of OMP, GAP43, and NCAM in the iPS cells in experimental group and control group. Experiments were performed in triplicate. *Significant at P < 0.05. B. Real-time PCR analysis of TBX21, Iba1 in the iPS cells in experimental group and control group. Experiments were performed in triplicate. *Significant at P < 0.05.
Discussion
It is generally known that the olfactory nerve can spontaneously regenerate, which involves various factors. In some cases, damage can result in irreversible anosmia. Despite advances in our understanding of olfaction, effective treatments for common causes of olfactory loss, including head trauma, viral infection, and chronic rhinosinusitis, aging [12], neurodegenerative diseases [13] or environmental factors remain elusive at this time. As a result, we attempted to evaluate the effects of iPSCs on irreversible anosmia.
In the present study, we report cell differentiation in vitro that mouse iPSCs possess the potency to differentiate into olfactory receptor neurons and mitral/tufted neurons in the indirect co-culture system. These differentiated cells express olfactory receptor neuron markers (OMP, GAP43, NCAM) and mitral/tufted neuron markers (TBX21, Iba1) after 14-day co-culture.
Gene expression experiments in mouse iPSCs revealed that the expression of OSNs markers, including OMP, GAP43, NCAM were markedly increased in olfactory epithelium co-culture compared with iPSCs grown alone. The expression of olfactory maker protein (OMP), which expressed in fully differentiated and matured OSNs [14] indicated mouse iPSCs differentiated into matured OSNs. Growth associated protein-43 (GAP-43) is an important marker genes used to confirm differentiation of iPSCs into immature OSNs. Our Real time PCR analysis showed that the expression of GAP43 was higher than that of OMP, which showed that most of mouse iPSCs differentiated into immature OSNs in the olfactory epithelium.
T-bet/Tbx21 expression is confined to olfactory bulb and the thymus. In the olfactory bulb, T-bet/TBX21 express highly in the mitral/tufted cell layer as well as in a few scattered cells of the glomerular layer [15]; Ionized calcium binding adaptor molecule 1 (Iba1) is considered to be a marker of microglia/macrophages [16]; Iba1 and TBX21 stain the cytoplasm of microglia/macrophages and the nuclei of mitral/tufted cells respectively. The expression of TBX21 and Iba1 in the olfactory bulb co-culture indicated that the differentiation of iPSCs toward M/T occurred.
Indirect co-culture systems are cultures in which two or more types of cells are cultured in the same environment with physical separation. In this type, cells interact only through soluble factor signaling [17] so the differentiation mechanism may involves that olfactory epithelium and olfactory bulb in the upper chambers of Transwell plates secrete some cytokines or growth factors which promoted the differentiation of mouse iPSCs into OSN-like cells and M/T neuron-like cells.
However, there have been several studies assessing the paracrine effect of transplanted stem cells on restoration of olfactory disorder rather than its differentiation to olfactory receptor neurons. Kwon et al. [18] reported that bone marrow stromal cells (BMSCs) transplantation accelerated regeneration of chemically damaged olfactory mucosa through the paracrine effect of BMSCs on NGF (nerve growth factor). Jo et al. [19] demonstrated that BMSCs transplantation affect restoration of OE and olfaction, most likely via regulation of the neurotrophic factor expression.
However, our studies demonstrated that only a small number of iPSCs expressed OSNs markers and M/T neuron markers during culture. This suggests that the efficiency of iPSC differentiation is low. As Shu et al. mentioned [20] melatonin could promote neural differentiation of induced pluripotent stem cells. So neurotrophic factor or NGF could promote olfactory nerve regeneration, we presumed that we can improve the efficiency of induction by adding some cytokines which promote neural differentiation of iPSCs.
To our knowledge, this is the first time that mouse iPSCs differentiating to OSN-like cells and M/T neuron-like cell have been demonstrated and iPSCs transplantation has a possibility of a potential future treatment for olfactory disorder. Although further studies need to elucidate whether iPSCs can promote anosmia functionally after transplantation following olfactory damages. In future, we are going to do experiment in vivo.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81160126).
Disclosure of conflict of interest
None.
References
- 1.Gregorio LL, Caparroz F, Nunes LM, Neves LR, Macoto EK. Olfaction disorders: retrospective study. Braz J Otorhinolaryngol. 2014;80:11–17. doi: 10.5935/1808-8694.20140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci. 2011;34:467–499. doi: 10.1146/annurev-neuro-112210-112917. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook EH, Iwema CL, Peluso CE, Schwob JE. The regeneration of P2 olfactory sensory neurons is selectively impaired following methyl bromide lesion. Chem Senses. 2014;39:601–616. doi: 10.1093/chemse/bju033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Merling RK, Sweeney CL, Choi U, De Ravin SS, Myers TG, Otaizo-Carrasquero F, Pan J, Linton G, Chen L, Koontz S, Theobald NL, Malech HL. Transgene-free iPSCs generated from small volume peripheral blood nonmobilized CD34+ cells. Blood. 2013;121:e98–107. doi: 10.1182/blood-2012-03-420273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cefalo MG, Carai A, Miele E, Po A, Ferretti E, Mastronuzzi A, Germano IM. Human iPSC for therapeutic approaches to the nervous system: present and future applications. Stem Cells Int. 2016;2016:4869071. doi: 10.1155/2016/4869071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Jeon SW, Seo BS, Mo JH, Jeon EH, Choi AR, Kim JW. Transplantation of neural stem cells in anosmic mice. Clin Exp Otorhinolaryngol. 2010;3:84–90. doi: 10.3342/ceo.2010.3.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noda Y, Nishizaki K, Yoshinobu J, Orita Y, Tsujigiwa H, Yamada M. The engraftment and differentiation of transplanted bone marrowderived cells in the olfactory bulb after methimazole administration. Acta Otolaryngol. 2013;133:951–956. doi: 10.3109/00016489.2013.803153. [DOI] [PubMed] [Google Scholar]
- 9.Franceschini V, Bettini S, Pifferi S, Menini A, Siciliano G, Ognio E, Brini AT, Di Oto E, Revoltella RP. Transplanted human adipose tissue-derived stem cells engraft and induce regeneration in mice olfactory neuroepithelium in response to dichlobenil subministration. Chem Senses. 2014;39:617–629. doi: 10.1093/chemse/bju035. [DOI] [PubMed] [Google Scholar]
- 10.Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2. doi: 10.3389/fcell.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Q. Culture of mouse olfactory sensory neurons. Curr Protoc Neurosci. 2012 doi: 10.1002/0471142301.ns0324s58. Chapter 3: Unit3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schubert CR, Fischer ME, Pinto AA, Klein BE, Klein R, Tweed TS, Cruickshanks KJ. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:710–715. doi: 10.1093/gerona/glw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barresi M, Ciurleo R, Giacoppo S, Foti Cuzzola V, Celi D, Bramanti P, Marino S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J Neurol Sci. 2012;323:16–24. doi: 10.1016/j.jns.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre JC, Titlow WB, McClintock TS. Axon growth and guidance genes identify nascent, immature, and mature olfactory sensory neurons. J Neurosci Res. 2010;88:3243–3256. doi: 10.1002/jnr.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faedo A, Ficara F, Ghiani M, Aiuti A, Rubenstein JL, Bulfone A. Developmental expression of the T-box transcription factor T-bet/Tbx21 during mouse embryogenesis. Mech Dev. 2002;116:157–160. doi: 10.1016/s0925-4773(02)00114-4. [DOI] [PubMed] [Google Scholar]
- 16.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 17.Paschos NK, Brown WE, Eswaramoorthy R, Hu JC, Athanasiou KA. Advances in tissue engineering through stem cell-based co-culture. J Tissue Eng Regen Med. 2015;9:488–503. doi: 10.1002/term.1870. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JW, Jo HG, Park SM, Ku CH, Park DJ. Engraftment and regenerative effects of bone marrow stromal cell transplantation on damaged rat olfactory mucosa. Eur Arch Otorhinolaryngol. 2016;273:2585–2590. doi: 10.1007/s00405-016-3957-x. [DOI] [PubMed] [Google Scholar]
- 19.Jo H, Jung M, Seo DJ, Park DJ. The effect of rat bone marrow derived mesenchymal stem cells transplantation for restoration of olfactory disorder. Biochem Biophys Res Commun. 2015;467:395–399. doi: 10.1016/j.bbrc.2015.09.142. [DOI] [PubMed] [Google Scholar]
- 20.Shu T, Wu T, Pang M, Liu C, Wang X, Wang J, Liu B, Rong L. Effects and mechanisms of melatonin on neural differentiation of induced pluripotent stem cells. Biochem Biophys Res Commun. 2016;474:566–571. doi: 10.1016/j.bbrc.2016.04.108. [DOI] [PubMed] [Google Scholar]