Abstract
Background: Generalized gingival fibromatosis is characterized by a progressive overgrowth of the gingiva, which can be caused by a variety of factors. According to these factors, it can be defined as hereditary gingival fibromatosis, non-familial gingival fibromatosis or drug-induced gingival fibromatosis. Non-familial gingival fibromatosis is a rare entity that needs to be documented. Case presentation: Here, we describe two cases of Chinese non-familial gingival fibromatosis. A six-year-old girl and a five-year-old boy presented with generalized gingival overgrowth. Clinical appearance, histological examination, and family history lead to the diagnosis of non-familial gingival fibromatosis. Real time PCR was performed to examine the expression of the α2-integrin gene, ITGA2, and identified decreased expression of ITGA2 in the gingiva of patients compared to both their parents and healthy volunteers. This is the first evidence that suggests a role for ITGA2 in non-familial gingival fibromatosis. Conclusion: Our findings provide evidence that altered cell-matrix interactions via collagen receptors, such as α2-integrin, may play a role in non-familial gingival fibromatosis. Thus, targeting collagen receptors might be an attractive target for the treatment of gingival fibromatosis.
Keywords: Gingival fibromatosis, α2-integrin, fibroblast phagocytosis
Introduction
Gingival fibromatosis (GF) is a rare, benign, fibrous enlargement of gingiva, frequently characterized by the expansion and accumulation of connective tissue and occasionally increased cell infiltration, which can result in esthetic and functional problems, including altered speech and mastication [1]. In some cases, GF is associated with other systemic alterations, such as Ramon Syndrome, Cross Syndrome and Zimmermann-Laband Syndrome [2]. Pharmacologic treatments for these syndromes, such as phenytoin, cyclosporine, or nifedipine, in addition to inheritance factors are thought to be main causes of gingival fibromatosis [3]. However, some cases of GF occur in the absence of a family history or drug therapy, which are diagnosed as non-familial GF. Non-familial GF patients exhibit similar a classical presentation and radiographic appearance of hereditary gingival fibromatosis (HGF) and drug-induced GF [4]. Additionally, all forms of GF are associated with increased extracellular matrix (ECM) production by fibroblasts [5]. The genes responsible for HGF were mapped to chromosomes 2p21-p22, 2p22.3-p23.3 and 5q13-q22 [6-8], which encodes for the son of sevenless one (SOS1) gene. Importantly, mutations in SOS1 have been linked to HGF in previous report [9].
At the molecular level, GF was previously thought to be the result of alterations in fibroblast proliferation or/and impairment in ECM metabolism [1]. Alterations in fibroblast proliferation are mainly controlled by growth factors, such as transforming growth factor beta-1 (TGF-β1). TGF-β1 is reported to be an autocrine stimulator of fibroblast proliferation by mediating G1/S transition in HGF fibroblasts [10,11]. In addition, connective tissue growth factor (CTGF/CCN2) is also involved in promoting and maintaining fibrosis, and increased expression of CTGF/CCN2 has also been found in fibrotic gingival tissue [12]. Moreover, GF is typically related to elevated collagen synthesis and reduced collagen degradation [13]. Type I collagen is the predominate collagen type synthesized in the gingiva of GF patients. The molecular chaperone for type I collagen, heat shock protein (HSP) 47, has been found to play an important role in the overproduction of type I collagen in HGF gingiva via post-translational regulation [11]. In addition to type I collagen, other ECM components, including fibronectin and glycosaminoglycan, are also elevated in HGF gingiva [14]. The degradation of ECM in gingival tissue occurs mainly through two pathways: degradation through matrix metalloproteinase (MMP) family members and through fibroblast phagocytosis [15,16]. Decreased expression levels and diminished activity of MMP-1 and MMP-2 have been reported in HGF gingiva [17]. Inhibition of MMP-1 and MMP-2 may contribute to poor connective tissue degradation and resulting in ECM accumulation in gingiva. Fibroblast phagocytosis is also pivotal for ECM degradation, which is regulated by integrins [18]. Abnormalities in α2β1 integrin lead to impaired fibroblast adherence to collagen, which could inhibit phagocytic function. This is a proposed etiologic mechanism of GF [19]. Decreased collagen phagocytosis and reduced α2β1 expression by fibroblasts is related to cyclosporine A (CsA)-induced gingival overgrowth [20]. In contrast, variable integrin α2β1 expression in gingival fibroblasts has been documented in patients with HGF [21,22]. In addition, it has been suggested that the loss of α2-integrin expression could induce the expression of TGFβ and CTGF/CCN2, ultimately resulting in GF [23]. These findings suggest that α2β1 integrin may contribute to fibrotic changes in non-familial gingival fibromatosis. Nevertheless, the association between integrin-α2 and non-familial GF has not been examined to date. In the present study, we aim to address the contribution of integrin-α2 to non-familial GF.
Case report
The study and the consent procedures were approved by the Ethical Committees of West China School of Stomatology, Sichuan University. Written informed consent was obtained from all participants before enrollment in the study. The case presentation portion was prepared under the recent standardized guidelines [24].
A six-year-old girl (Case 1) and a five-year-old boy (Case 2) reported to the outpatient department of Department of Periodontics, West China Hospital of Stomatology, Sichuan University presenting with enlargement of gingival tissues.
The female patient and her parents noticed the overgrowth of gingiva dating back four years, which progressively increased in size causing occlusion disorder and poor esthetic. The male patient also exhibited the overgrowth of gingiva, but the time of onset was unknown. Further history revealed that no other family members were affected with a similar condition. Thus, the family and postnatal history was noncontributory. Routine medical history and physical examination revealed no underlying systemic disease, nor was any history of epilepsy or mental disorder apparent. The dental history was not significant, and extraoral examination showed no fluctuancy, compressibility, translucency, nor associated bleeding.
Intraoral examination of the maxilla and mandible in Case 1 (Figure 1A) revealed a gross generalized growth of gingiva covering more than two thirds of the maxillary or mandibular teeth. The mass in anterior palatine region had covered the anterior palatine fold. The mass was found to be firm and nontender with a smooth surface and a pink to pinkish-white color. There was neither associated bleeding nor pain with growth. In Case 2 (Figure 1B), gross generalized growth of gingiva was apparent, and the enlarged gingiva involved both free and attached gingivae and nearly covered the occlusal surface of the molars. The mandibular teeth were hardly visible in mandibular rest position. The mass was firm and pink with mildly pigmentation. The patient experienced difficulty maintaining oral hygiene measures, which resulted in the accumulation of material Alba and dental plaque. There was no bleeding or pain noted.
Figure 1.

Clinical views of two patients with GF. Pre-operative frontal views and maxillary views of patients A (A and B) and B (C and D). Pre-operative facial views showing generalized gingival enlargement covering teeth surfaces.
The patients were referred for panoramic radiograph. The panoramic radiograph of both children (Figure 2A and 2B) showed no abnormal permanent teeth germ. On the basis of clinical and radiological findings, a provisional diagnosis of non-familial gingival fibromatosis was made. Patients were referred for incisional biopsy and routine investigations were done and incisional biopsy was performed.
Figure 2.
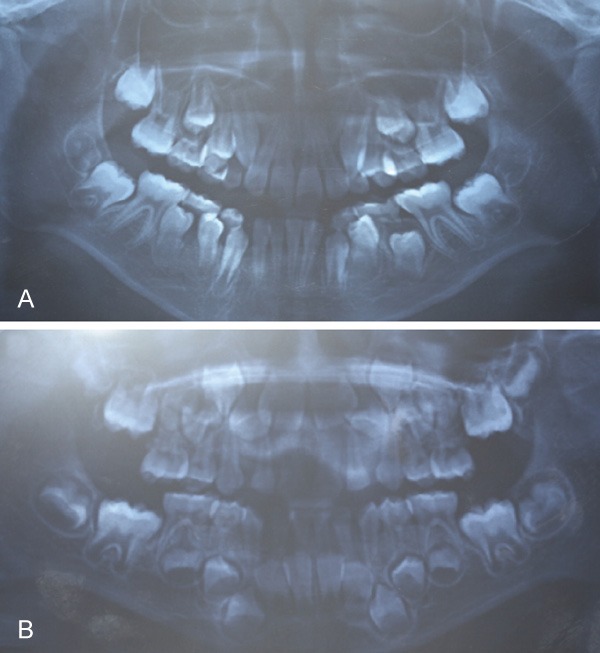
Pre-operative panoramic radiograph views of patients A (A) and B (B). The views show numerous displaced and unerupted teeth.
For the female patient (Case 1), histological examination revealed moderately dense collagenous connective tissue with collagen bundles arranged in a haphazard manner. The overlying surface of the epithelium exhibited hyperkeratosis with elongation of rete ridges. The collagenous connective tissue was relatively avascular along with scant inflammatory cell infiltrates showing dense wavy bundles of collagen fibers containing numerous fibrocytes and fibroblasts (Figure 3A). The histological examination of the male patient (Case 2) showed moderate hyperplasia of a dense, hyperkeratotic epithelium with elongated rete ridges. The connective tissue exhibited an accumulation of excess collagen, and elastic and oxytalan fibers, but had relatively few fibroblasts and blood vessels (Figure 3B). The histopathologic features led to a final diagnosis of non-familial gingival fibromatosis for both patients.
Figure 3.

Histologic sections of GF patients A (A) and B (B) showing stratified squamous epithelium with long slender rete pegs, connective tissue and dense collagenous stroma. (H&E; original magnification ×50).
Upon consultation with the patients and the parents, the patients were treated by surgical removal of the lesion with quadrant gingivectomy. Gingiva samples were collected from the patients, their patients, and healthy volunteers. The transcriptional level of ITGA2 in these samples was quantified relative to ACTB. The total RNA of these samples was isolated, purified and reverse transcribed into cDNA, as described previously [25]. Primers for amplification of target gene (ITGA2) and endogenous control (ACTB) are given in Table S1. A four-point standard curve was generated for each gene to derive amplification efficiency (E). The curve for ITGA2 was linear (R2 = 1) with a slope of -3.32 (PCR efficiency = 200.1%) and the curve for ACTB was also linear (R2 = 1) with a slope of -3.30 (PCR efficiency = 200.9%). The plots were then used to derive relative expression levels for ITGA2 in all six samples. ITGA2 levels were significantly lower in both patients compared to their patients and healthy volunteers (Figure 4).
Figure 4.

Relative expression levels of ITGA2 for patients A (A) and B (B). ITGA2 expression was lower in patients compared to the corresponding parents and healthy volunteers. Presented data came from an average of three independent experiments and is the mean ± standard deviation. *P < 0.05, **P < 0.01.
Discussion
Here, we report two Chinese cases of non-familial gingival fibromatosis. The patients had no history of any systemic disease, syndromic association, or medication that could contribute to gingival overgrowth. The clinical and histopathological features led to the diagnosis of non-familial GF.
GF is characterized by generalized gingival overgrowth [1]. The enlargement of the gingiva may cover the teeth and extend into the attached gingiva, resulting in phonetic, esthetic, and functional problems [5]. Non-familial gingival fibromatosis is a rare form of gingival fibromatosis [4]. Both HGF and non-familial gingival fibromatosis are characterized by the excessive accumulation of ECM; thus, recent studies have focused on the molecular mechanism of collagen synthesis and degradation in this disease.
Integrins are a large family of heterodimeric membrane glycoproteins that are of paramount importance in cell-ECM and cell-cell interactions [26]. Recently, fibroblasts were reported to actively regulate collagen networks via these integrins [27]. Studies on HGF and drug-induced GF have implicated the involvement of α2-integrin in GF [20-22]. Flow cytometry analysis found CsA-treated fibroblasts had lower expression of α2-integrin than control fibroblasts [20]. Consistent with this, the gene encoding for α2-integrin (ITGA2) was down-regulated in CsA-treated fibroblasts compared with the controls [20]. In contrast, studies have shown inconsistent results regarding the role of α2-integrin in HGF [21,22]. Zhou et al. demonstrated that the expression of α2-integrin was significantly higher in HGF fibroblasts compared to control fibroblasts [22]. However, in a recent study, no difference in α2-integrin expression was observed between HGF fibroblasts and control fibroblasts by immunohistochemistry [21]. These findings suggest that reduced α2-integrin expression may be one etiological factor of gingival overgrowth induced by CsA. The exact role of α2-integrin in the pathogenesis of HGF should be determined in future studies. Although excess ECM accumulation seems to be a common feature of non-familial gingival fibromatosis, few studies have investigated the role of integrins in this condition.
In the present study, we addressed whether α2-integrin contributed to non-familial gingival fibromatosis pathogenesis and found decreased expression of ITGA2 in the gingiva of patients with GF compared to their parents and healthy volunteers. This finding suggests that overgrowth of gingival tissue in non-familial GF may be related to poor ECM degradation through fibroblast phagocytosis. However, whether the integrin-α2 decreased at the translational level was not investigated due to the inability to obtain large samples. In addition, this study presents only two Chinese cases of non-familial GF. Additional case-control studies are warranted in other populations in order to further determine the role of integrin-α2 in non-familial GF.
Conclusions
Our report documented two cases of non-familial gingival fibromatosis and revealed that inhibition of collagen phagocytosis by reducing α2-integrin expression in gingival fibroblasts may be one etiological factor of non-familial gingival fibromatosis. Cell-matrix interactions via collagen receptors seem to be crucial for maintenance of normal gingiva architecture and function. Thus, targeting collagen receptors, such as ITGA2, might be a new form of treatment for progressive fibrotic diseases.
Acknowledgements
This work was supported by the National key clinical specialist construction programs of China (grant number: 2010). We thank the patients, their parents and the healthy volunteers. We also thank the entire team of section of periodontology at West China Hospital of Stomatology, Sichuan University and Faqiang Zhang and Zengliang Xia at West China Hospital of Sichuan University.
Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Coletta RD, Graner E. Hereditary gingival fibromatosis: a systematic review. J Periodontol. 2006;77:753–764. doi: 10.1902/jop.2006.050379. [DOI] [PubMed] [Google Scholar]
- 2.Poulopoulos A, Kittas D, Sarigelou A. Current concepts on gingival fibromatosis-related syndromes. J Investig Clin Dent. 2011;2:156–161. doi: 10.1111/j.2041-1626.2011.00054.x. [DOI] [PubMed] [Google Scholar]
- 3.Chaurasia A. Hereditary gingival fibromatosis. Natl J Maxillofac Surg. 2014;5:42–46. doi: 10.4103/0975-5950.140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W, Chen X. Gingival fibromatosis. J Tongji Med Univ. 1996;16:55–57. doi: 10.1007/BF02889047. [DOI] [PubMed] [Google Scholar]
- 5.Gagliano N, Moscheni C, Dellavia C, Masiero S, Torri C, Grizzi F, Stabellini G, Gioia M. Morphological and molecular analysis of idiopathic gingival fibromatosis: a case report. J Clin Periodontol. 2005;32:1116–1121. doi: 10.1111/j.1600-051X.2005.00811.x. [DOI] [PubMed] [Google Scholar]
- 6.Hart TC, Pallos D, Bowden DW, Bolyard J, Pettenati MJ, Cortelli JR. Genetic linkage of hereditary gingival fibromatosis to chromosome 2p21. Am J Hum Genet. 1998;62:876–883. doi: 10.1086/301797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X, Shi L, Cheng Y, Peng Q, Huang S, Liu J, Huang M, Peng B, Bian Z. A novel locus for autosomal dominant hereditary gingival fibromatosis, GINGF3, maps to chromosome 2p22.3-p23.3. Clin Genet. 2005;68:239–244. doi: 10.1111/j.1399-0004.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- 8.Xiao S, Wang X, Qu B, Yang M, Liu G, Bu L, Wang Y, Zhu L, Lei H, Hu L, Zhang X, Liu J, Zhao G, Kong X. Refinement of the locus for autosomal dominant hereditary gingival fibromatosis (GINGF) to a 3.8-cM region on 2p21. Genomics. 2000;68:247–252. doi: 10.1006/geno.2000.6285. [DOI] [PubMed] [Google Scholar]
- 9.Hart TC, Zhang Y, Gorry MC, Hart PS, Cooper M, Marazita ML, Marks JM, Cortelli JR, Pallos D. A mutation in the SOS1 gene causes hereditary gingival fibromatosis type 1. Am J Hum Genet. 2002;70:943–954. doi: 10.1086/339689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Andrade CR, Cotrin P, Graner E, Almeida OP, Sauk JJ, Coletta RD. Transforming growth factor-beta1 autocrine stimulation regulates fibroblast proliferation in hereditary gingival fibromatosis. J Periodontol. 2001;72:1726–1733. doi: 10.1902/jop.2001.72.12.1726. [DOI] [PubMed] [Google Scholar]
- 11.Martelli-Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD. Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol. 2003;74:296–306. doi: 10.1902/jop.2003.74.3.296. [DOI] [PubMed] [Google Scholar]
- 12.Kantarci A, Black SA, Xydas CE, Murawel P, Uchida Y, Yucekal-Tuncer B, Atilla G, Emingil G, Uzel MI, Lee A, Firatli E, Sheff M, Hasturk H, Van Dyke TE, Trackman PC. Epithelial and connective tissue cell CTGF/CCN2 expression in gingival fibrosis. J Pathol. 2006;210:59–66. doi: 10.1002/path.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghili H, Goldani Moghadam M. Hereditary gingival fibromatosis: a review and a report of a rare case. Case Rep Dent. 2013;2013:930972. doi: 10.1155/2013/930972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tipton DA, Howell KJ, Dabbous MK. Increased proliferation, collagen, and fibronectin production by hereditary gingival fibromatosis fibroblasts. J Periodontol. 1997;68:524–530. doi: 10.1902/jop.1997.68.6.524. [DOI] [PubMed] [Google Scholar]
- 15.van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Phagocytosis of fibronectin and collagens type I, III, and V by human gingival and periodontal ligament fibroblasts in vitro. J Periodontol. 2001;72:1340–1347. doi: 10.1902/jop.2001.72.10.1340. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez Rios M, Sorsa T, Obregon F, Tervahartiala T, Valenzuela MA, Pozo P, Dutzan N, Lesaffre E, Molas M, Gamonal J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: initial evidence for MMP-13/MMP-9 activation cascade. J Clin Periodontol. 2009;36:1011–1017. doi: 10.1111/j.1600-051X.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 17.Coletta RD, Almeida OP, Reynolds MA, Sauk JJ. Alteration in expression of MMP-1 and MMP-2 but not TIMP-1 and TIMP-2 in hereditary gingival fibromatosis is mediated by TGF-beta 1 autocrine stimulation. J Periodontal Res. 1999;34:457–463. doi: 10.1111/j.1600-0765.1999.tb02281.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Sodek J, McCulloch CA. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J Cell Physiol. 1996;168:695–704. doi: 10.1002/(SICI)1097-4652(199609)168:3<695::AID-JCP22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Gurkan A, Emingil G, Afacan B, Berdeli A, Atilla G. Alpha 2 integrin gene (ITGA2) polymorphism in renal transplant recipients with and without drug induced gingival overgrowth. Arch Oral Biol. 2014;59:283–288. doi: 10.1016/j.archoralbio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka M, Seto H, Wada C, Kido J, Nagata T. Decreased expression of alpha2 integrin in fibroblasts isolated from cyclosporin A- induced gingival overgrowth in rats. J Periodontal Res. 2003;38:533–537. doi: 10.1034/j.1600-0765.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- 21.Vieira-Junior JR, de Oliveira-Santos C, Della-Coletta R, Cristianismo-Costa D, Paranaiba LM, Martelli-Junior H. Immunoexpression of alpha2-integrin and Hsp47 in hereditary gingival fibromatosis and gingival fibromatosis-associated dental abnormalities. Med Oral Patol Oral Cir Bucal. 2013;18:e45–48. doi: 10.4317/medoral.17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Meng LY, Ye XQ, Von den Hoff JW, Bian Z. Increased expression of integrin alpha2 and abnormal response to TGF-beta1 in hereditary gingival fibromatosis. Oral Dis. 2009;15:414–421. doi: 10.1111/j.1601-0825.2009.01567.x. [DOI] [PubMed] [Google Scholar]
- 23.Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rison RA. A guide to writing case reports for the journal of medical case reports and BioMed central research notes. J Med Case Rep. 2013;7:239. doi: 10.1186/1752-1947-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Niu Y, Zhou X, Xu X, Yang Y, Zhang Y, Zheng L. Cell cycle control, DNA damage repair, and apoptosis-related pathways control pre-ameloblasts differentiation during tooth development. BMC Genomics. 2015;16:592. doi: 10.1186/s12864-015-1783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujimura T, Moriwaki S, Imokawa G, Takema Y. Crucial role of fibroblast integrins alpha2 and beta1 in maintaining the structural and mechanical properties of the skin. J Dermatol Sci. 2007;45:45–53. doi: 10.1016/j.jdermsci.2006.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


