Abstract
Background and Aim: Bone marrow mesenchymal stem cells (MSC) are receiving increasing attention for skin wound repair. However, the specific mechanisms underlying MSC-mediated improvement in wound healing have not been fully elucidated. This study aims at testing whether epidermal growth factor (EGF) can promote MSC-mediated wound healing and hair follicle regeneration. Methods: Excisional wounds in rats were transplanted with different collagen-chitosan scaffolds: control, MSC, and MSC + EGF. Regenerated tissues were harvested 1, 3, or 5 weeks following transplantation, stained with hematoxylin and eosin and evaluated microscopically. The formation of sebaceous glands was examined by Oil red staining and the regeneration of hair follicles by immunohistochemical staining and Western blot to test the expression of hair follicle-specific factors. Results: Gross observations showed that the wounds were much smaller and the hairs grew faster in the MSC + EGF group. Histological analysis demonstrated that there were more hair follicles, sebaceous glands, and newly formed blood vessels in the MSC + EGF group compared with that in the MSC group. In addition, oil red staining showed that MSCs + EGF induced sebaceous gland regeneration. Finally, immunohistochemistry and western blot revealed that MSCs + EGF increased the expression of hair follicle-specific factors. Conclusion: MSCs alone cannot achieve the regeneration of hair follicles and EGF can promote MSC-mediated wound healing and hair follicle regeneration.
Keywords: EGF, hair follicles, MSC, wound healing
Introduction
As skin is the largest organ of the body, skin loss caused by trauma, burning, and chronic diseases becomes one of the most serious clinical problems. Millions of patients suffering from skin loss require treatment with skin substitutes such as wound dressings, allografts, and autografts. However, traditional autografts are limited by their availability in terms of both time and donor sites [1,2]. Recent advances in regenerative medicine and tissue engineering have expanded our understanding of wound healing and led to the development of diverse methods for skin repair and regeneration. In full-thickness skin defects, the absence of dermis prevents the operation of autonomous repair mechanisms. In such cases, a proper dermal equivalent, which can function as a regenerating template for wound healing, is highly required.
In the dermis, there are hair follicles, sebaceous glands, sweat glands, and other accessory organs, in addition to blood vessels and nerve tissues. These skin accessory organs have many functions, such as body temperature regulation, tactile perception, and maintenance of the body fluid and electrolyte balance. Therefore, these accessory organs play important roles in maintaining normal skin functions, as well as during dermis regeneration and wound healing [3-5]. Regeneration of accessory organs such as hair follicles and sebaceous glands not only accelerates wound healing but also improves the functionality of the regenerated skin. Although artificial skin materials used for the treatment of full-thickness skin defects can repair the structure of the epidermis and the dermis during the course of several years, skin accessory organs such as hair follicles and sebaceous glands still cannot be regenerated [6,7].
Many studies have focused on wound healing and hair regeneration [8-11]. The use of mesenchymal stem cells (MSCs) is one of the most promising discoveries, which can contribute to wound closure acceleration, improvement of neovascularization, and reduction of scar formation. In addition, Rustad et al. suggested that MSC induced hair regeneration during wound healing [1]. While the specific mechanisms by which MSCs improve wound healing have not been fully elucidated, it has been proposed that MSC differentiation plays a significant role. A suitable environment is necessary for MSCs to differentiate into specific cells. However, in a wound following skin damage, the physiological environment of the skin is compromised, interfering with the differentiation pathways of transplanted MSCs. Consequently, hair and sebaceous gland regeneration is impeded. Epidermal growth factor (EGF) has been shown to induce differentiation of MSCs into dermal papilla cells, and to promote hair follicle regeneration during wound healing [12]. In this study, we generated three collagen-chitosan scaffolds coated with PBS, MSCs, or MSCs + EGF respectively, and tested whether the combination of MSCs and EGF could accelerate wound healing, improve neovascularization, and promote hair regeneration.
Materials and methods
MSC isolation
Rat MSCs were isolated by short-term adherence to plastic [11]. Briefly, following anesthesia, the rat tibia was punctured with a needle. Using a sterile tube, bone marrow (1-2 mL) was aspirated into a 10-mL syringe containing 5000 U of heparin. The bone marrow was centrifuged to remove fat and heparin. The precipitated cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% v/v fetal bovine serum (FBS), 100 mg/mL penicillin, and 100 U/mL streptomycin until a confluent cell layer was formed. The cells from the monolayer were detached with 0.25% trypsin and were subsequently routinely passaged.
Preparation of collagen-chitosan scaffolds
A porous collagen-chitosan scaffold was fabricated as previously described [13-16]. Briefly, collagen and chitosan were dissolved in 0.5 M acetic acid to form a 0.5% (w/v) solution, in a mass ratio of 9:1. The collagen-chitosan composite was injected into homemade molds, frozen at -20°C for 2 h, and lyophilized for 24 h to produce a porous collagen-chitosan scaffold.
EGF, MSC, and MSC + EGF scaffolds were prepared according to a multi-point injection method. MSC scaffolds were prepared by injecting 1.0 × 106-1.0 × 107 MSCs into the previously prepared collagen-chitosan scaffold. MSC + EGF scaffolds were prepared by injecting both 1.0 × 106-1.0 × 107 MSCs and ~40 μg EGF into the collagen-chitosan scaffold.
Excisional wound healing model
All animal studies conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-27, revised 1996) and were approved by the Institutional Animal Care and Use Committee of Zhejiang University. Twenty-seven male Sprague-Dawley (SD) rats (2 months old, 250 ± 12 g) were randomized into three treatment groups: Control, MSC, and MSC + EGF. Prior to the operation, the rats were anesthetized by intraperitoneal administration of 3% pentobarbital sodium solution (Sigma) at a dose of 1.0 ml/kg. An additional dose (0.5 ml/kg) was administered as required to maintain deep anesthesia. The back hair of the rat was removed with 8% Na2S aqueous solution (Figure 1A). Three standardized full-thickness excisional skin wounds (diameter = 2.0 cm) were created at the rat dorsum with a distance of no less than 2.0 cm from each other (Figure 1B). The excised skin was trimmed into thin split-thickness skin. The scaffolds were then extended on the wound bed and sutured to the adjacent skin (Figure 1C, 1D). The operative incisions were sterilized with 2.5% povidone iodine, the rats received a pressure dressing (petrolatum gauze and elastoplast) and were housed in single cages. The rats were inspected every day to ensure that the pressure dressings were intact. One week post-surgery, the wounds healed and the dressings were removed. The animals were killed in batches 1, 3, and 5 weeks post-surgery. Following imaging of the wound sites, tissue specimens were harvested and were sectioned along the uniform direction, and maintained in 10% buffered formalin, liquid nitrogen, and physiological saline solution for histological investigation and molecular biology analysis respectively.
Figure 1.

Surgical transplantation of scaffolds into full-thickness skin wounds in rats.
Macroscopic observation
Images of the wounds were acquired at different time points and analyzed using the image processing software package Photoshop (ver.11.0.1; Adobe, San Jose, CA, USA). The wound area was calculated from the images according to a method previously described [10,11].
Histology and oil red staining
Five-millimeter-thick sections were prepared from paraffin-embedded tissue samples, and were stained with hematoxylin and eosin (H&E) according to standard protocols [6,17,18]. Sebaceous glands budding from the follicle epithelial root are an accessory for hair follicle formation. Therefore, the regeneration of sebaceous glands indirectly indicates hair follicle regeneration. We investigated the regeneration of sebaceous glands during wound healing by oil red staining. Five-millimeter-thick sections were prepared from paraffin-embedded tissue samples, and were stained using oil red according to standard protocols.
Immunohistochemistry
The expression of factors related to hair follicle formation, such as alkaline phosphatase (ALP), was investigated by immunohistochemistry. ALP immunohistochemical staining was carried out to identify newly hair follicle formation in the transplanted scaffolds. Briefly, following deparaffinization and hydration, the paraffin sections were blocked in 5% goat serum for 30 min, exposed to rabbit anti-ALP primary antibody (1:100, Abcam, Cambridge, UK) at 4°C overnight, incubated with goat anti-rabbit secondary antibody (1:200, Dako, Ely, UK) at 37°C for 30 min, developed with 3,30-diaminobenzidine tetrahydrochloride solution and counterstained using haematoxylin. Successful staining was ensured if the outline of the lumen of blood vessels appeared brown.
Western blot analysis
Frozen regenerated tissue was homogenized and lysed by radio immunoprecipitation assay buffer (RIPA Lysis Buffer, Beyotime, China), containing protease and phosphatase inhibitors (1 mM phenylmethanesulfonyl fluoride (PMSF), Beyotime, China). The homogenates were centrifuged at 13,000×g for 5 min at 4°C and the protein concentration in the supernatant was determined by the bicinchoninic acid (BCA) assay (BCA Protein assay kit, Beyotime, China). The supernatant was mixed with 5× concentrated SDS sample buffer (Beyotime, China), boiled for 5 min to ensure protein denaturation, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were electrotransferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was incubated consecutively with blocking buffer for 30 min at room temperature, with the primary antibody overnight at 4°C and with a horseradish peroxidase-linked secondary antibody for 1 h at room temperature. Between the incubations in primary and secondary antibody, the membrane was washed three times, 10 min each, with Tris-buffered saline containing 0.1% Tween 20 (TBS-T). The membrane was treated with a standard chemical luminescence reagent (Beyotime, China). For signal detection, Kodak X-Omat AR films were exposed to the membrane, were scanned on a gel imaging and system, and analyzed by the Quantity One 4.4 software (Bio-Rad, Hercules, CA, USA).
Results
Gross observations of wound healing and hair growth
Images were taken to record and analyze wound size changes and healing (Figure 2). Repaired wounds could be readily distinguished from normal skin, owing to the naked appearance of the skin on the wound site, displaying little hair growth and attachment. At day 7, the appearance of the skin on the wound sites of the rat in the MSC + EGF group was more red and fresh, and with more scales, compared to that of the rat in the control and MSC groups (Figure 3A-C). These results suggest that MSC + EGF improved wound healing and promoted vascularization. At day 21, the wound area in the rat in the MSC + EGF group was much smaller than in the other two groups (Figure 3D-F). At day 35, we found that the skin in the wound area was nearly completely regenerated in the MSC + EGF group whereas there were still a few wounds unhealed in the control and MSC groups (Figure 3G-I).
Figure 2.

Images of regenerated skin following scaffold transplantation.
Figure 3.
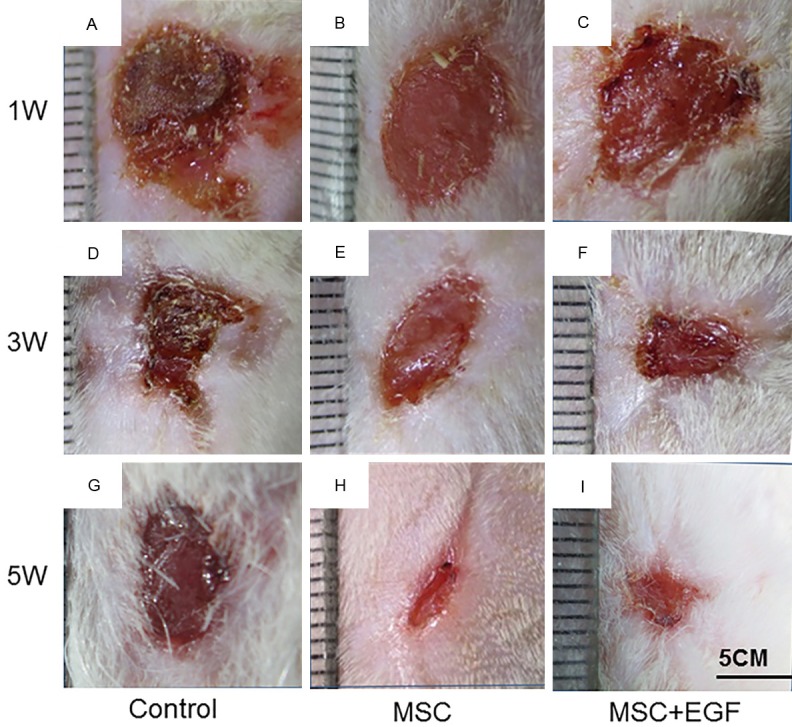
Images of wounds following scaffold transplantation. Wounds transplanted with empty scaffold (A, D, G), MSC scaffold (B, E, H) and MSC + EGF (C, F, I) were imaged 1 week (A-C) 3 weeks (D-F) and 5 weeks (G-I) following transplantation.
Furthermore, since hair growth indicates hair follicle regeneration, we monitored hair regrowth in the regenerated wound area at day 35 and we found more hair growth in the animals in the MSC + EGF group compared to the those in the control and MSC groups (Figure 3G-I).
Histology
Histological analysis was performed to evaluate tissue regeneration, as shown in Figure 4. On day 7, some messy and not-yet-mature follicles began to appear in the MSC + EGF group (Figure 4A-C). On day 21, we clearly observed hair follicles wrapped by sebaceous glands in the MSC + EGF group, but no hair follicle or sebaceous gland was found in the control and MSC groups (Figure 4D-F). On day 35, mature hair follicles and sebaceous glands could be clearly identified in the MSC + EGF group, whereas still no hair follicle or sebaceous gland was observed in the control and MSC groups (Figure 4G-I).
Figure 4.

H&E staining of wound sections. Wounds were transplanted with empty scaffold (A, D, G), MSC scaffold (B, E, H) and MSC + EGF (C, F, I).
Newly formed blood vessels are necessary for skin regeneration. At day 7, newly formed blood vessels could be visualized in all three groups (Figure 5). Compared with that in the control group, blood vessel density in both the MSC and MSC + EGF groups were much higher (P<0.01).
Figure 5.

H&E staining of wound sections. Arrows indicate blood vessels.
Oil red staining
Oil red staining was performed to further investigate the ability of MSCs + EGF to induce sebaceous gland formation. As shown in Figure 6, there was no oil red-positive area in the control and MSC groups during the entire observation time. However, oil red-positive areas could be clearly identified in the MSC + EGF group already on day 7 (Figure 6C). With increasing time, these oil red-positive structures were gradually transformed into the sebaceous glands (Figure 6F, 6I). These results further indicate that MSCs + EGF can promote the formation of sebaceous glands from the follicle epithelial root, which can be accessory to hair follicle regeneration.
Figure 6.

Oil red staining of wound sections. Wounds were transplanted with empty scaffold (A, D, G), MSC scaffold (B, E, H) and MSC + EGF (C, F, I). Sebaceous gland positive aeras were stained red.
Immunohistochemistry
In normal skin, ALP is a hair follicle-specific factor in dermal papilla cells, which is continuously expressed throughout the development of the hair follicle. ALP can be used as an important indicator of hair follicle regeneration. Therefore, in order to further identify hair follicle regeneration during wound healing, we tested ALP expression by immunohistochemistry in the regenerated skin from the three experimental groups. ALP positive cells were stained yellow-brown. As show in Figure 7, few ALP-positive areas could be found in the control and MSC groups, while a large number of ALP-positive areas could be observed in the MSC + EGF group. These results indicated that MSC + EGF promoted hair follicle regeneration.
Figure 7.

ALP immunohistochemistry of wound sections. Wounds were transplanted with empty scaffold (A, D, G), MSC scaffold (B, E, H) and MSC + EGF (C, F, I). ALP positive areas were stained yellow or brown.
Western blot analysis
The immunohistochemistry results were confirmed by Western blot analysis. As shown in Figure 8, compared to the control and MSC groups, the expression of ALP, α-SMA, and versican increased in the MSC + EGF group (P<0.01).
Figure 8.

Western blot analysis of ALP, α-SMAs and versican expression. *denotes statistical significance (P<0.01).
Discussion
In this study, we utilized biomimetic hydrogel scaffolds to effectively deliver MSC or MSC + EGF into cutaneous wounds. Our results demonstrate that delivery of MSCs + EGF accelerated wound healing. Moreover, we found that MSCs + EGF could promote hair growth, angiogenesis, and sebaceous gland recovery.
Hair follicles are necessary accessory organs of the skin. Hairs have physiological functions, such as providing thermoregulation and protection against extrinsic insults, and they act as contact sensors through the elongation, connection with surrounding muscle and nerve tissues for piloerection, through the enduring hair cycles. To achieve fully functional hair follicle regeneration, many methods have been developed to reconstruct the variable region of the hair follicle.
In previous studies, cells derived from accessory organs were often used as seed cells for organ regeneration owing to their ability to maintain cell homology and differentiate into various different cell types. Asakawa et al. isolated epithelial and dermal cells from embryonic skin tissue and found that their transplantation to the wound could facilitate hair follicle regeneration [19]. Higgins et al. showed that dermal hair follicle cells could induce human hair-follicle growth [2]. In addition, Huang et al. found that transplantation of cultured sweat gland cells into the wound in biomimetic hydrogel scaffolds was a promising method for sweat gland regeneration [20]. These studies suggest that regeneration of the accessory organs could be achieved by transplantation of accessory organ-derived cells. However, it is not straightforward to isolate these seed cells because skin appendages are very small, and the separation and purification processes are complex and need to be performed under a microscope [21]. Moreover, cells isolated from an accessory organ often have low viability during in vitro culture, and lose the ability to differentiate into specific cells that are needed for organ regeneration. Therefore, transplantation of accessory organ-derived cells has not been widely applied in the clinic.
Currently, the application of stem cells in skin recovery is receiving increasing attention. Compared with the accessory organ-derived cells, stem cells have many advantages. They have the potential to differentiate into a variety of specific cell types and are much easily isolated. Therefore, stem cells have been widely used for the regeneration and repair of various tissues and organs during regeneration and repair. Recent studies have shown that a variety of pluripotent stem cells, including embryonic stem cells, epidermal stem cells, mesenchymal stem cells, adipose-derived stem cells, and MSCs, could be used in the regeneration of skin accessory organs [22-26]. Among these, MSCs have received the most attention for hair follicle regeneration. As seed cells, MSCs can differentiate into multiple cell types and participate in cutaneous wound healing. Transplantation of MSCs into full-thickness burn wounds induces epidermal thickening, increases the number of dermal nerve fibers, accelerates wound healing, and greatly improves healing quality [27]. Moreover, MSCs participate in wound reepithelialization and the regeneration of accessory organs like hair follicles and sweat glands [10,27]. However, there are studies reporting that although transplantation of MSCs can promote skin recovery, they have little effect on hair follicle regeneration [28]. Our results are consistent with those of these studies. We found that MSCs could accelerate wound healing and blood vessel formation, but had little effect on hair follicle or sebaceous glands regeneration.
Hair follicle regeneration requires a variety of different cell types and a suitable external environment that is essential for MSC differentiation [29]. When the skin is wounded, the physiological environment under the skin is often compromised. This might be the reason why MSC transplantation fails to regenerate hair follicle or sebaceous glands. Recent studies suggest that MSCs must migrate to wound sites before they can be involved in the repair process, and the ability of MSCs to secrete biologically active substances, such as cytokines and growth factors, play key roles in wound recovery [30,31]. Thus, to regenerate hair follicles during skin wound recovery, it is very important to both promote MSC migration and induce MSC differentiation into dermal papilla cells.
A wide variety of growth factors and cytokines are involved in the regulation of all phases of wound healing. Yamazaki et al. and Jindo et al. found that pHGF has a stimulatory effect on hair growth in vivo and in vitro [32,33]. Zhang et al. showed that Activin B could promote MSC-mediated cutaneous wound healing by regulating cell migration via the JNK-ERK signaling pathway [27]. EGF is a multi-functional cell growth factor, affecting physiological processes, such as epidermization, cell proliferation, and migration [12,34,35]. Therefore, EGF may play an important role in MSC-mediated skin repair. In this study, we examined the impact of combined administration of MSCs and EGF on wound healing in a rat model. We found that EGF did indeed promote MSC-mediated wound healing and hair follicle regeneration in vivo. These results indicated that combined administration of MSCs and EGF might be a promising therapeutic strategy for wound management.
Acknowledgements
This study was supported by the Plan Project of Zhejiang Provincial Department of Health, China (No. 2016RCA027).
Disclosure of conflict of interest
None.
References
- 1.Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, Longaker MT, Gurtner GC. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci U S A. 2013;110:19679–19688. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcin CL, Ansell DM. The battle of the bulge: Re-evaluating hair follicle stem cells in wound repair. Exp Dermatol. 2016;26:101–104. doi: 10.1111/exd.13184. [DOI] [PubMed] [Google Scholar]
- 4.Garcin CL, Ansell DM, Headon DJ, Paus R, Hardman MJ. Hair follicle bulge stem cells appear dispensable for the acute phase of wound re-epithelialization. Stem Cells. 2016;34:1377–1385. doi: 10.1002/stem.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Wang X, Liu J, Cai T, Guo L, Wang S, Wang J, Cao Y, Ge J, Jiang Y, Tredget EE, Cao M, Wu Y. Hair follicle and sebaceous gland de novo regeneration with cultured epidermal stem cells and skin-derived precursors. Stem Cells Transl Med. 2016;5:1695–1706. doi: 10.5966/sctm.2015-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo R, Xu S, Ma L, Huang A, Gao C. Enhanced angiogenesis of gene-activated dermal equivalent for treatment of full thickness incisional wounds in a porcine model. Biomaterials. 2010;31:7308–7320. doi: 10.1016/j.biomaterials.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Guo R, Xu S, Ma L, Huang A, Gao C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials. 2011;32:1019–1031. doi: 10.1016/j.biomaterials.2010.08.087. [DOI] [PubMed] [Google Scholar]
- 8.Mao Z, Shi H, Guo R, Ma L, Gao C, Han C, Shen J. Enhanced angiogenesis of porous collagen scaffolds by incorporation of TMC/DNA complexes encoding vascular endothelial growth factor. Acta Biomater. 2009;5:2983–2994. doi: 10.1016/j.actbio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Ma L, Liang J, Zhang B, Teng J, Gao C. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials. 2013;34:2038–2048. doi: 10.1016/j.biomaterials.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G, Li Q, Chen X, Ji J, Zhang Y, OuYang HW. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials. 2013;34:4404–4417. doi: 10.1016/j.biomaterials.2013.02.048. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Li B, Li Y, Jiang Y, Ouyang H, Gao C. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials. 2010;31:5953–5965. doi: 10.1016/j.biomaterials.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Doma E, Rupp C, Baccarini M. EGFR-rasraf signaling in epidermal stem cells: roles in hair follicle development, regeneration, tissue remodeling and epidermal cancers. Int J Mol Sci. 2013;14:19361–19384. doi: 10.3390/ijms141019361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong Y, Gong Y, Gao C, Shen J. Collagencoated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J Biomed Mater Res A. 2008;85:628–637. doi: 10.1002/jbm.a.31603. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, You C, Hu X, Zheng Y, Li Q, Feng Z, Sun H, Gao C, Han C. The roles of knitted meshreinforced collagen-chitosan hybrid scaffold in the one-step repair of full-thickness skin defects in rats. Acta Biomater. 2013;9:7822–7832. doi: 10.1016/j.actbio.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, Han C, Mao Z, Ma L, Gao C. Enhanced angiogenesis in porous collagen-chitosan scaffolds loaded with angiogenin. Tissue Eng Part A. 2008;14:1775–1785. doi: 10.1089/ten.tea.2007.0007. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Wu P, Hu X, You C, Guo R, Shi H, Guo S, Zhou H, Yu C, Zhang Y, Han C. Polyurethane membrane/knitted mesh-reinforced collagen-chitosan bilayer dermal substitute for the repair of full-thickness skin defects via a two-step procedure. J Mech Behav Biomed Mater. 2016;56:120–133. doi: 10.1016/j.jmbbm.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Haifei S, Xingang W, Shoucheng W, Zhengwei M, Chuangang Y, Chunmao H. The effect of collagen-chitosan porous scaffold thickness on dermal regeneration in a one-stage grafting procedure. J Mech Behav Biomed Mater. 2014;29:114–125. doi: 10.1016/j.jmbbm.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Guo R, Teng J, Xu S, Ma L, Huang A, Gao C. Comparison studies of the in vivo treatment of full-thickness excisional wounds and burns by an artificial bilayer dermal equivalent and J-1 acellular dermal matrix. Wound Repair Regen. 2014;22:390–398. doi: 10.1111/wrr.12171. [DOI] [PubMed] [Google Scholar]
- 19.Asakawa K, Toyoshima KE, Ishibashi N, Tobe H, Iwadate A, Kanayama T, Hasegawa T, Nakao K, Toki H, Noguchi S, Ogawa M, Sato A, Tsuji T. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep. 2012;2:424. doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Xu Y, Wu C, Sha D, Fu X. In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials. 2010;31:5520–5525. doi: 10.1016/j.biomaterials.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 21.Aoi N, Inoue K, Kato H, Suga H, Higashino T, Eto H, Doi K, Araki J, Iida T, Katsuta T, Yoshimura K. Clinically applicable transplantation procedure of dermal papilla cells for hair follicle regeneration. J Tissue Eng Regen Med. 2012;6:85–95. doi: 10.1002/term.400. [DOI] [PubMed] [Google Scholar]
- 22.Kamstrup M, Faurschou A, Gniadecki R, Wulf HC. Epidermal stem cells - role in normal, wounded and pathological psoriatic and cancer skin. Curr Stem Cell Res Ther. 2008;3:146–150. doi: 10.2174/157488808784223087. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Fu X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012;348:371–377. doi: 10.1007/s00441-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 24.Peng LH, Mao ZY, Qi XT, Chen X, Li N, Tabata Y, Gao JQ. Transplantation of bone-marrowderived mesenchymal and epidermal stem cells contribute to wound healing with different regenerative features. Cell Tissue Res. 2013;352:573–583. doi: 10.1007/s00441-013-1609-7. [DOI] [PubMed] [Google Scholar]
- 25.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326:725–736. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M, Sun L, Wang X, Chen S, Kong Y, Liu N, Chen Y, Jia Q, Zhang L, Zhang L. Activin B promotes BMSC-mediated cutaneous wound healing by regulating cell migration via the JNK-ERK signaling pathway. Cell Transplant. 2014;23:1061–1073. doi: 10.3727/096368913X666999. [DOI] [PubMed] [Google Scholar]
- 28.Purkrabkova T, Smetana K Jr, Dvorankova B, Holikova Z, Bock C, Lensch M, Andre S, Pytlik R, Liu FT, Klima J, Smetana K, Motlik J, Gabius HJ. New aspects of galectin functionality in nuclei of cultured bone marrow stromal and epidermal cells: biotinylated galectins as tool to detect specific binding sites. Biol Cell. 2003;95:535–545. doi: 10.1016/j.biolcel.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 30.Fu X, Qu Z, Sheng Z. Potentiality of mesenchymal stem cells in regeneration of sweat glands. J Surg Res. 2006;136:204–208. doi: 10.1016/j.jss.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki M, Tsuboi R, Lee YR, Ishidoh K, Mitsui S, Ogawa H. Hair cycle-dependent expression of hepatocyte growth factor (HGF) activator, other proteinases, and proteinase inhibitors correlates with the expression of HGF in rat hair follicles. J Investig Dermatol Symp Proc. 1999;4:312–315. doi: 10.1038/sj.jidsp.5640236. [DOI] [PubMed] [Google Scholar]
- 33.Jindo T, Tsuboi R, Takamori K, Ogawa H. Local injection of hepatocyte growth factor/scatter factor (HGF/SF) alters cyclic growth of murine hair follicles. J Invest Dermatol. 1998;110:338–342. doi: 10.1046/j.1523-1747.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 34.Myers SR, Leigh IM, Navsaria H. Epidermal repair results from activation of follicular and epidermal progenitor keratinocytes mediated by a growth factor cascade. Wound Repair Regen. 2007;15:693–701. doi: 10.1111/j.1524-475X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 35.Nanba D, Toki F, Barrandon Y, Higashiyama S. Recent advances in the epidermal growth factor receptor/ligand system biology on skin homeostasis and keratinocyte stem cell regulation. J Dermatol Sci. 2013;72:81–86. doi: 10.1016/j.jdermsci.2013.05.009. [DOI] [PubMed] [Google Scholar]


