Abstract
Objective: MicroRNA-320 (MiR-320) had been reported to be down-regulated in several cancers. However, its clinical significance in gastric cancer remained unknown. In this study, we aimed to detect the expression of miR-320 and its clinical significance in gastric cancer. Methods: The relative expression levels of miR-320 in plasma of gastric cancer patients and healthy controls were detected using quantitative real-time polymerase chain reaction (qRT-PCR). A receiver operating characteristic (ROC) curve was established to estimate the diagnostic value of miR-320 in gastric cancer. Moreover, its prognostic value was assessed via Kaplan-Meier and Cox regression analyses. Results: Compared with healthy individuals, the plasma miR-320 expression in patients with gastric cancer was significantly decreased (P<0.001). The low plasma miR-320 expression was closely associated with TNM stage and lymph node metastasis (P<0.05). Furthermore, plasma miR-320 could be used to distinguish gastric cancer patients from healthy controls with an area under the curve (AUC) of 0.861. The sensitivity and specificity were 82.4% and 75.9%, respectively. Kaplan-Meier analysis revealed that patients with high expression of miR-320 had an obviously longer overall survival than those with low miR-320 expression (log rank test, P=0.003). MiR-320 could be an independent prognostic factor for patients with gastric cancer via univariate and multivariate analyses. Conclusions: Plasma miR-320 is down-regulated and correlated with the progression of gastric cancer. What’s more, miR-320 may be a potential bio-marker for the diagnosis and prognosis of gastric cancer patients.
Keywords: Gastric cancer, MiR-320, diagnosis, prognosis
Introduction
Gastric cancer becomes one of the most frequent malignant tumors in the world [1]. And it remains a lethal cancer, although the morbidity rate is decreased [1,2]. The surgical resection is a curative therapeutic method for gastric cancer patients with early stage. However, lack of typical symptoms in early stage of gastric cancer, most patients were at the advanced stages with diagnosis [3]. In the current, gastroscopic screening and some serum molecules (carcinoembryonic antigen) are the major methods for early detection of gastric cancer [4]. Nevertheless, there were big limitations, such as high cost, invasive nature and unsatisfied sensitivity as well as specificity [4-6]. A large number of studies had reported that the prognosis of gastric cancer was very poor and the 5-year overall survival was less than 25% [7,8]. Therefore, identifying novel non-invasive bio-markers for early diagnosis and prognosis of gastric cancer will provide an important clinical relevance.
MicroRNAs (MiRNAs), as small non-coding RNA molecules, had been reported to be aberrantly expressed in several human tumors, and correlated with carcinogenesis and tumor progression [9,10]. Recently, miRNAs had been proven to be highly stable in human blood, including plasma and serum, supporting their potency as blood-based bio-markers for the diagnosis, treatment and prognosis of cancers [11,12]. Among these miRNAs, miR-320 was identified as a tumor suppressor and reduced between miR-320 expression a variety of tumors [13,14]. It was shown to suppress cell proliferation and metastasis, suggesting the correlation between miR-320 expression and the progression of tumors [15]. Increasing evidences revealed that the expression of miR-320 could be served as potential diagnosis bio-marker and therapy target of tumors [16,17]. However, the diagnostic and prognostic value of miR-320 remains to be known in gastric cancer.
In this study, we detected the plasma expression level of miR-320 in gastric cancer patients and healthy controls. And the relationship between plasma miR-320 expression and clinical factors of gastric cancer patients was analyzed. Meanwhile, we also estimated the clinical significance of miR-320 in gastric cancer.
Materials and methods
Patients and samples
In this study, a total of 116 patients with confirmed histopathological diagnosis of gastric cancer were enrolled from Cangzhou Central Hospital. None of patients had received any chemotherapy, radiotherapy or other treatments before surgery. Additionally, 85 healthy donors were collected as controls and they had not detected with any tumors previously. This study was approved via the Ethical Committee of the hospital and written informed consents were provided by all participants in advance.
10 mL blood samples from each participator were collected into EDTA tubes. Then these samples were centrifuged for 10 min at 1500 rpm and the supernatant plasma was frozen at -80°C until use. The detailed clinicopathologic characteristics of patients were shown in Table 1. A 5-years’ follow-up was performed using a telephone or questionnaires, and patients who died from other disease or unexpected events were excluded from this study.
Table 1.
Relationship between miR-320 expression and clinical characteristics of gastric cancer patients
| Clinical Features | Cases (n=116) | MiR-320 expression | x2 | P | |
|---|---|---|---|---|---|
|
| |||||
| High (n=50) | Low (n=66) | ||||
| Age (years) | 0.933 | 0.334 | |||
| ≤61 | 66 | 31 | 35 | ||
| >61 | 50 | 19 | 31 | ||
| Gender | 0.755 | 0.385 | |||
| Male | 77 | 31 | 46 | ||
| Female | 39 | 19 | 20 | ||
| Tumor size (cm) | 1.386 | 0.239 | |||
| ≤4 | 60 | 29 | 31 | ||
| >4 | 56 | 21 | 35 | ||
| Histological grade | 3.325 | 0.068 | |||
| Well/Moderate | 63 | 32 | 31 | ||
| Poor | 53 | 18 | 35 | ||
| TNM stage | 4.691 | 0.030 | |||
| I-II | 68 | 35 | 33 | ||
| III-IV | 48 | 15 | 33 | ||
| Lymph node metastasis | 6.153 | 0.013 | |||
| Negative | 66 | 35 | 31 | ||
| Positive | 50 | 15 | 35 | ||
RNA extraction and qRT-PCR analysis
Total RNA from plasma samples was severally isolated using Trizol Reagent (TaKaRa. Japan) according to the instructions of manufacturer. TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, US) was used to conduct reverse transcription. Then the RT-PCR reaction was performed by TaqMan Universal Master Mix (Applied Biosystems, US). Using small nuclear RNA U6 as the internal control, the sequences of primers for miR-320 and U6 were as follows: miR-320, forward-5’-AAAAGCTGGGTTGAGAGGGCGA-3’, and reverse-5’-GCGAGCACA- GAATTAATACGAC-3’; U6, forward-5’-CGCTTCGGCAGCACATATACTA-3’, and reverse-5’-CGCTTCACGAATTTGCGTGTCA-3’. All samples were detected in triplicate and the relative mRNA quantification of miR-320 expression was calculated using the 2-∆∆Ct method.
Statistical analysis
All statistical analyses were performed with the SPSS 18.0 software and the data were expressed as mean ± standard deviation (SD). Student’s t test was used to evaluate the differences between two groups. The relationship between miR-320 expression and clinical characteristics was analyzed by Chi-square test. Receiver operating characteristic (ROC) was applied to estimate the diagnostic value of miR-320 in gastric cancer. The overall survival of patients with different miR-320 expression was compared via Kaplan-Meier analysis with log rank test. Univariate and multivariate analysis with Cox regression was used to assess the prognostic value of miR-320 in gastric cancer. P<0.05 was considered as statistically significant.
Results
Demographic characteristics of the study subjects
The clinical characteristics of gastric cancer patients were shown in Table 1. There were 39 females and 77 males in gastric cancer group, with a mean age of 61 years (age rang, 42-87 years). The tumor size of 60 patients was less than 4 cm, and the others were more than 4 cm in size. According to the TNM staging system, 68 patients at stage I-II, and 48 patients of stage III-IV These patients were classified based on histological grade as follows: well/moderate, n=63 and poor, n=53. Lymph node metastasis of gastric cancer patients occurred in 50 of 116 patients. Another group of 85 healthy people were consisted of 38 female and 47 male with a mean age of 58 years (age range, 41-82 years).
The expression of miR-320 was down-regulated in gastric cancer patients
The relative mRNA expression levels of miR-320 in plasma of gastric cancer patients and healthy controls were detected by qRT-PCR. The results indicated that miR-320 expression was significantly lower in gastric cancer patients than that in healthy individuals (P<0.001, Figure 1).
Figure 1.
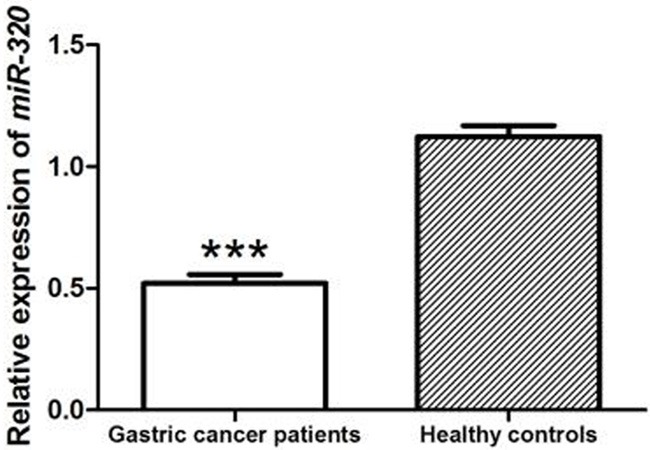
The relative mRNA expression of miR-320 in plasma of gastric cancer patients and healthy individuals. Compared with healthy controls, the plasma miR-320 expression was significantly down-regulated in gastric cancer patients (P<0.001).
Relationship between miR-320 expression and clinical features of patients with gastric cancer
According to the mean expression level of miR-320, 116 patients were divided into high miR-320 expression group and low miR-320 expression group to explore whether miR-320 was linked with the development of gastric cancer. Then we analyzed the association between miR-320 expression and clinical characteristics of gastric cancer patients. As shown in Table 1, the low plasma miR-320 expression was closely correlated with advanced TNM stage (P=0.030) and lymph node metastasis (P=0.013). However, no obvious association was found between miR-320 expression and age, gender, tumor size or histological grade (all, P>0.05, Table 1).
The diagnostic value of miR-320 in gastric cancer patients
To explore the diagnosis significance of miR-320 in patients with gastric cancer, ROC curve was established. The outcome demonstrated that miR-320 could be a valuable bio-marker to discriminate gastric cancer patients from healthy controls with an area under the curve (AUC) of 0.861 (95% CI=0.811-0.910, P<0.001) combing with the sensitivity of 82.4% and the specificity of 75.9% (Figure 2). Meanwhile, the ideal cutoff value of miR-320 expression was 0.765.
Figure 2.
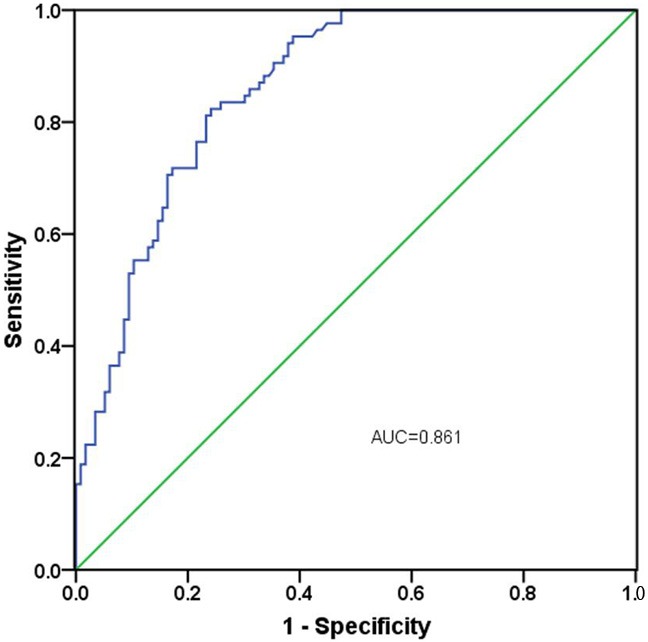
The ROC curve analysis for the diagnosis of gastric cancer using miR-320 expression. It indicated that the AUC was 0.861 with a sensitivity of 82.4% and a specificity of 75.9%
The prognostic value of miR-320 in gastric cancer patients
To investigate the prognostic value of miR-320 in gastric cancer patients, a 5 years’ follow-up was carried out. Based on the data from follow-up, Kaplan-Meier analysis with log rank test revealed that the gastric cancer patients with low miR-320 expression had a shorter overall survival than those with high expression of miR-320 (log rank test, P=0.003, Figure 3). Besides, the result of Cox regression analysis showed that miR-320 expression (HR=2.046, 95% CI=1.023-4.093, P=0.043) as well as TNM stage (HR=1.933, 95% CI=1.013-3.689, P=0.046) were both correlated with the prognosis of gastric cancer patients and they may be independent prognostic factors for gastric cancer (Table 2).
Figure 3.
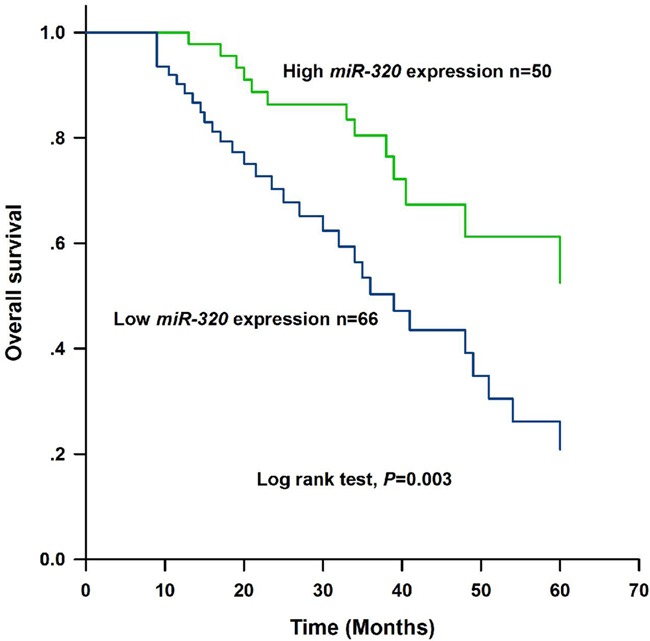
Kaplan-Meier analysis for estimating the overall survival of patients with gastric cancer. Patients with low expression of miR-320 had shorter overall survival than those with high miR-320 expression (log rank test, P=0.003).
Table 2.
Univariate and multivariate analyses with Cox regression for the overall survival in gastric cancer patients
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95.0% CI | P | HR | 95.0% CI | P | |
| MiR-320 (Low vs High) | 2.562 | 1.333-4.924 | 0.005 | 2.046 | 1.023-4.093 | 0.043 |
| Age (year) (>61 vs ≤61) | 1.020 | 0.548-1.899 | 0.951 | - | - | - |
| Gender (Male vs Female) | 1.108 | 0.598-2.053 | 0.744 | - | - | - |
| Tumor size (cm) (>4 vs ≤4) | 0.796 | 0.437-1.450 | 0.455 | - | - | - |
| Histological grade (Poor vs Well/Moderate) | 2.097 | 1.114-3.947 | 0.022 | - | - | - |
| TNM stage (III-IV vs I-II) | 2.470 | 1.337-4.564 | 0.004 | 1.933 | 1.013-3.689 | 0.046 |
| Lymph node metastasis (Positive vs Negative) | 2.139 | 1.109-4.126 | 0.023 | - | - | - |
Note: - indicated no data.
Discussion
It was reported that the expression of miR-320 was decreased in a variety of cancers, revealing that miR-320 may be involved in the carcinogenesis and progression of tumor [13,14]. Meanwhile, previous studies had demonstrated that altered expression of circulating miRNAs in serum and plasma could be potential bio-markers for tumors as well as other diseases [18,19]. Currently, the clinical significance of miR-320 in gastric cancer still remains unclear.
In the present study, the expression levels of miR-320 in plasma of gastric cancer patients and healthy controls were detected. The results revealed that plasma miR-320 expression was significantly down-regulated in gastric cancer patients compared to that in healthy individuals which was similar to the previous studies [20,21]. This result suggested that miR-320 could function as a tumor suppressor in gastric cancer. Then we analyzed the correlation between miR-320 expression and clinical features of patients to explore whether miR-320 was involved in the progression of gastric cancer. The outcome showed that the low plasma miR-320 expression was strongly associated with TNM stage and lymph node metastasis, which were clinical factors representing progression and metastasis of tumor. These findings were consistent with what had been found in cervical cancer [22]. Taken together, miR-320 may participate in tumorigenesis and the progression of gastric cancer.
Furthermore, we investigated the clinical significance of miR-320 in gastric cancer. ROC curve analysis demonstrated that the low plasma miR-320 expression was significantly associated with the early diagnosis of gastric cancer. It was indicated to be useful to discriminate gastric cancer from healthy controls with high values of AUC, sensitivity and specificity. Consistent with our result, accumulated evidence had confirmed that miR-320 could be used as a potential bio-marker for the diagnosis of diseases, including tumors [23-25]. For example, Neuro et al. showed that miR-320 was significantly associated with a glioblastoma multiforme diagnosis [23]. Liu et al. have reported that the expression of miR-320 was down-regulated in retinoblastoma patients samples and it may be considered as a valuable diagnostic biomarker in RB [24]. Moreover, in the study of Jiang et al., they proved that the expression levels of miR-320 may be useful for the diagnosis of erectile dysfunction in patients with diabetes [25].
Previous study had reported that the expression of miR-320 was related to the probability of recurrence-free survival in colon cancer [26]. However, the prognostic value of miR-320 in human tumors is still poorly known. Next, the result of Kaplan-Meier analysis indicated that the overall survival of patients with high expression of miR-320 was markedly longer than those with low miR-320 expression, which reveled miR-320 was associated with the prognosis of gastric cancer. Then univariate and multivariate analysis showed that miR-320 was an independent prognostic factor of gastric cancer. Besides, our outcome demonstrated TNM stage was also an independent factor.
MiR-320 had been proven to inhibit stem cell-like properties of prostate cancer cells through modulating Wnt/beta-catenin signaling pathway [27]. Previous study demonstrated that miR-320 suppress cell proliferation of osteosarcoma via regulating the expression of fatty acid synthase [28]. To our knowledge, Helicobacter pylori is one of the strongest risk for gastric cancer. A study of Noto et al. mentioned that H. pylori could suppress the expression of miR-320, indicating that miR-320 was a tumor suppressor in gastric cancer [29]. However, little is known about the exact mechanisms by which miR-320 regulates gastric cancer, which is still required more researches.
In summary, miR-320 is decreased in gastric cancer and correlated with the progression of this tumor. It may be a potential and non-invasive bio-marker for the diagnosis and prognosis of gastric cancer. However, due to the limitation of sample size and its source, further studies may be urgently needed.
Acknowledgements
Key project in medical science research of Hebei health and family planning commission (20150340).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20:13767–13774. doi: 10.3748/wjg.v20.i38.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JS, Wang YF, Zhang XQ, Lv JM, Li Y, Liu XX, Xu TP. H19 serves as a diagnostic biomarker and up-regulation of H19 expression contributes to poor prognosis in patients with gastric cancer. Neoplasma. 2016;63:223–230. doi: 10.4149/207_150821N454. [DOI] [PubMed] [Google Scholar]
- 4.Wu C, Luo Z, Tang D, Liu L, Yao D, Zhu L, Wang Z. Identification of carboxyl terminal peptide of Fibrinogen as a potential serum biomarker for gastric cancer. Tumour Biol. 2016;37:6963–6970. doi: 10.1007/s13277-015-4394-y. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Kwong A, Sihoe A, Chu KM. Plasma miR-940 may serve as a novel biomarker for gastric cancer. Tumour Biol. 2016;37:3589–3597. doi: 10.1007/s13277-015-4019-5. [DOI] [PubMed] [Google Scholar]
- 6.Fu Z, Qian F, Yang X, Jiang H, Chen Y, Liu S. Circulating miR-222 in plasma and its potential diagnostic and prognostic value in gastric cancer. Med Oncol. 2014;31:164. doi: 10.1007/s12032-014-0164-8. [DOI] [PubMed] [Google Scholar]
- 7.Hou Y, Deng J, Zhang L, Xie X, Guo X, Sun C, Zhang R, Liang H. Lower expression of PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1) association with poor prognosis of gastric cancer. Int J Clin Exp Med. 2015;8:20481–20489. [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Song Y, Chen X, Zhao J, Gao P, Huang X, Xu H, Wang Z. Novel long non-coding RNA RP11-119F7.4 as a potential biomarker for the development and progression of gastric cancer. Oncol Lett. 2015;10:115–120. doi: 10.3892/ol.2015.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JL, Kim M, Song KS, Kim SY, Kim YS. Cell-free miR-27a, a potential diagnostic and prognostic biomarker for gastric cancer. Genomics Inform. 2015;13:70–75. doi: 10.5808/GI.2015.13.3.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Li G, Wang Z, Yao Y, Chen R, Pu X, Wang J. Circulating microRNA-21 is a potential diagnostic biomarker in gastric cancer. Dis Markers. 2015;2015:435656. doi: 10.1155/2015/435656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma Y, Chen Y, Pan F, Wang K, Ni J, Jin W, He X, Su H, Cui D. Circulating miR-16-5p and miR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics. 2015;5:733–745. doi: 10.7150/thno.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou X, Zhang M, Qiao H. Diagnostic significance of miR-106a in gastric cancer. Int J Clin Exp Pathol. 2015;8:13096–13101. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC, Hong TM. miR-320 regulates tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing neuropilin 1. Angiogenesis. 2014;17:247–260. doi: 10.1007/s10456-013-9394-1. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Shen H, Liu L, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137:557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 15.Sun JY, Xiao WZ, Wang F, Wang YQ, Zhu YH, Wu YF, Miao ZL, Lin YC. MicroRNA-320 inhibits cell proliferation in glioma by targeting E2F1. Mol Med Rep. 2015;12:2355–2359. doi: 10.3892/mmr.2015.3657. [DOI] [PubMed] [Google Scholar]
- 16.Jurkovicova D, Lukackova R, Magyerkova M, Kulcsar L, Krivjanska M, Krivjansky V, Chovanec M. microRNA expression profiling as supportive diagnostic and therapy prediction tool in chronic myeloid leukemia. Neoplasma. 2015;62:949–958. doi: 10.4149/neo_2015_115. [DOI] [PubMed] [Google Scholar]
- 17.Wan LY, Deng J, Xiang XJ, Zhang L, Yu F, Chen J, Sun Z, Feng M, Xiong JP. miR-320 enhances the sensitivity of human colon cancer cells to chemoradiotherapy in vitro by targeting FOXM1. Biochem Biophys Res Commun. 2015;457:125–132. doi: 10.1016/j.bbrc.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Kotlabova K, Doucha J, Hromadnikova I. Placental-specific microRNA in maternal circulation--identification of appropriate pregnancyassociated microRNAs with diagnostic potential. J Reprod Immunol. 2011;89:185–191. doi: 10.1016/j.jri.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Su ZX, Zhao J, Rong ZH, Wu YG, Geng WM, Qin CK. Diagnostic and prognostic value of circulating miR-18a in the plasma of patients with gastric cancer. Tumour Biol. 2014;35:12119–12125. doi: 10.1007/s13277-014-2516-6. [DOI] [PubMed] [Google Scholar]
- 20.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong L, Li Y, Han C, Wang X, She L, Zhang H. miRNA microarray reveals specific expression in the peripheral blood of glioblastoma patients. Int J Oncol. 2014;45:746–756. doi: 10.3892/ijo.2014.2459. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Zou P, Wang T, Xiang J, Cheng J, Chen D, Zhou J. Down-regulation of miR-320 associated with cancer progression and cell apoptosis via targeting Mcl-1 in cervical cancer. Tumour Biol. 2016;37:8931–8940. doi: 10.1007/s13277-015-4771-6. [DOI] [PubMed] [Google Scholar]
- 23.Manterola L, Guruceaga E, Gallego Perez-Larraya J, Gonzalez-Huarriz M, Jauregui P, Tejada S, Diez-Valle R, Segura V, Sampron N, Barrena C, Ruiz I, Agirre A, Ayuso A, Rodriguez J, Gonzalez A, Xipell E, Matheu A, Lopez de Munain A, Tunon T, Zazpe I, Garcia-Foncillas J, Paris S, Delattre JY, Alonso MM. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro Oncol. 2014;16:520–527. doi: 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu SS, Wang YS, Sun YF, Miao LX, Wang J, Li YS, Liu HY, Liu QL. Plasma microRNA-320, microRNA-let-7e and microRNA-21 as novel potential biomarkers for the detection of retinoblastoma. Biomed Rep. 2014;2:424–428. doi: 10.3892/br.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang XI, Luo Y, Zhao S, Chen Q, Jiang C, Dai Y, Chen Y, Cao Z. Clinical significance and expression of microRNA in diabetic patients with erectile dysfunction. Exp Ther Med. 2015;10:213–218. doi: 10.3892/etm.2015.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68:6416–6424. doi: 10.1158/0008-5472.CAN-07-6110. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ, Lee KH, Yeh SD, Hong TM, Chen YL. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/beta-catenin signaling pathway. Carcinogenesis. 2013;34:530–538. doi: 10.1093/carcin/bgs371. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, Chen ZQ, Shi XT. MicroRNA-320 inhibits osteosarcoma cells proliferation by directly targeting fatty acid synthase. Tumour Biol. 2014;35:4177–4183. doi: 10.1007/s13277-013-1546-9. [DOI] [PubMed] [Google Scholar]
- 29.Noto JM, Piazuelo MB, Chaturvedi R, Bartel CA, Thatcher EJ, Delgado A, Romero-Gallo J, Wilson KT, Correa P, Patton JG, Peek RM Jr. Strain-specific suppression of microRNA-320 by carcinogenic Helicobacter pylori promotes expression of the antiapoptotic protein Mcl-1. Am J Physiol Gastrointest Liver Physiol. 2013;305:G786–796. doi: 10.1152/ajpgi.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


