Abstract
Irisin, a novel muscle-secreted peptide, has been proposed to play a potential role in improving myocardial remodeling that leads to impaired myocardial function and heart failure. It has been reported that controlling reactive oxygen species (ROS) exposure could increase cardiomyocyte survival and prevent pathological remodeling of the myocardium. Therefore, we aimed to determine the potential protective effects of Irisin pretreatment against ROS in cardiomyocytes and explored the potential mechanisms. H9c2 cells that were subjected to H2O2 in vitro were used to mimic myocardial remodeling. Then, the effects of Irisin on myocardial cell proliferation, apoptosis and cellular ROS levels were evaluated during this process by using MTT assay, flow cytometry analysis and 2’7’-Dichloro fluoresc in diacetate (DCFH-DA). In order to determine whether Irisin could regulate any microRNA (miRNA) during this process, six miRNAs that are known to be involved in apoptosis of cardiomyocytes were assessed by qRT-PCR. The protective effects of Irisin on cardiomyocytes mediated by miR-19b were evaluated by detecting cell proliferation and apoptosis. In addition, the potential target of miR-19b was predicted with bioinformatics tools and verified using dual-luciferase reporter assay. Finally, the protein levels of members of the phosphatidylinositol 3-kinase (PI3K)/Akt/signaling pathway were also examined by Western Blot. Our study showed that Irisin treatment improved H2O2-induced cell viability and attenuated the levels of intracellular ROS and the apoptosis of cardiomyocytes in a dose-dependent manner. We also demonstrated that Irisin promoted cell viability and inhibited cell apoptosis via upregulating miR-19b expression. In addition, PTEN was identified as a functional target gene of miR-19b that was responsible for its anti-apoptotic effects in cardiomyocytes. Further study demonstrated that Irisin-regulated miR-19b could reactivate the AKT/mTOR signaling pathway blocked by H2O2 in H9c2 cells. We demonstrated that Irisin strongly enhances cellular proliferation and preventsapoptosis of cardiomyocytes as well as attenuates the levels of intracellular ROS induced by H2O2. These effects might be mediated through the miR-19b/AKT/mTOR signaling pathway, which provide a new insight into the mechanism by which Irisin may have beneficial effect on myocardial remodeling.
Keywords: Cardiomyocytes, Irisin, microRNA-19b, apoptosis, AKT/mTOR signaling pathway
Introduction
Cardiovascular diseases (CVD) are the leading cause of death in the world. Despite great efforts have been made in treatment strategies in recent years, the morbidity and mortality of cardiovascular diseases remain high [1]. It has been recognized that myocardial remodeling constitutes the pathophysiological basis leading to the development of CVD. Myocardial remodeling is characterized by cardiac hypertrophy, myocardial fibrosis, oxidative stress and myocardial cell apoptosis [2,3]. Among them, oxidative stress and apoptosis are the most important pathologic features in the pathogenesis and progression of various cardiovascular diseases [4-7]. Therefore, treatment strategies that can reverse myocardial remodeling are effective in improving prognosis and lowering mortality.
Irisin is a recently identified myokine that mediates beneficial effects of exercise by promoting the browning of white adipose tissue [8]. A large body of evidence indicates that Irisin levels was decreased in the serum of patients with metabolic diseases, suggesting that Irisin could be therapeutic for metabolic disease and other disorders which are linked to cardiovascular diseases such as coronary artery disease, abdominal aortic aneurysm, heart failure, and diabetic heart diseases [9-11]. Two recent studies have focused on the potential relationship between Irisin and myocardial remodeling. One study found that Irisin improved endothelial function in type 2 diabetes through reducing oxidative stress that play fundamental roles in myocardial remodeling [12]. Another study revealed that Irisin inhibited high glucose-induced apoptosis, oxidative stress and increased antioxidant enzymes expression in HUVECs [13]. However, the underlying mechanisms involved are still unknown.
MicroRNAs (miRNAs) are a class of endogenous small noncoding RNAs with a length of 17-24 nucleotides, which repress translation of target mRNAs or induce degradation of target mRNAs by binding to the 3’UTR of target mRNAs [14]. Increasing evidence supports that miRNAs dysregulation play indispensable roles in multiple cardiovascular diseases including cardiac hypertrophy, myocardial infarction, myocardial fibrosis, cardiac arrhythmia and heart failure [14-18]. A recent study demonstrated that Irisin significantly reduces atherosclerosis in apolipoprotein E-deficient mice via promoting endothelial cell proliferation through microRNA-126-5p [13]. These studies suggest that Irisin could exert its biological function through regulation of miRNAs. However, it is still unknown whether Irisin exerts a protective effect on myocardial remodeling via miRNAs.
In the present study, we explored the protective effect of Irisin on myocardial proliferation and apoptosis in the cell model of oxidative stress. Moreover, the interactions between Irisin, miR-19b and AKT/mTOR signaling pathway were also studied in order to reveal the underlying mechanisms of Irisin in the protection of myocardial damage induced by oxidative stress. Our findings can help important implications for the future development and application of Irisin for myocardial remodeling treatment.
Materials and methods
Materials
Irisin (purity >99%) was purchased from Cayman Chemical (Michigan, USA) and was dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA) which was stored at -20°C. The final concentrations of icariin used in the culture were 1, 2 and 5 ng/ml.
Cell culture and H2O2 treatment
The embryonic rat cardiac myoblast H9c2 cell line was obtained from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Carlsbad, USA) supplemented with 10% fetal bovine serum (Beyotime Institute of Biotechnology, Haimen, China) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). H9c2 cells were treated with 200 μM H2O2 for 12 hours to establish the oxidative stress-induced injury model in cardiomyocytes, in accordance with a previously published method [19].
Cell viability assay
The effect of Irisin on cell viability was quantitatively assessed with the MTT assay. H9c2 cells were seeded in 96-well plates at a density of 1×104 cells/well. The cultures were treated for 24 h with different concentrations of Irisin (1, 2 and 5 ng/ml) before exposing the cultures to H2O2 for 1 h. Then, 20 μl of MTT solution (5 mg/ml) was added to each well for 4 h. After removal of MTT solution, dimethyl sulfoxide (DMSO) was added, and the absorbance at 490 nm was measured with Microplate Reader (Bio-Tek Instruments, Inc.). All experiments were done in triplicate.
Cell apoptosis detection by flow cytometry
After exposure to different experimental conditions, H9c2 cells (1×106) were collected and then washed twice with PBS. The cells were resuspended in 500 μl binding buffer and then mixed with Annexin V-FITC (1 µg/ml; Invitrogen, Carlsbad, CA) in the dark for 15 min at room temperature. Five minutes after propidium iodide [8] solution was added, the percentages of dead cells and cells undergoing apoptosis were evaluated by flow cytometry (Becton-Dickinson, San Jose, CA, USA).
Detecting the ROS generation
The generation of myocardial ROS was assessed using 2’,7’-DCF diacetate (DCF-DA; Sigma-Aldrich) as previously reported [20]. Briefly, after cell culture medium was discarded, cells were incubated with 20 μmol/L DCFH for 30 min at 37°C, then cells were washed twice with PBS. ROS formation was stimulated by H2O2 (final concentration: 200 μM) for 6 h at 37°C. Before and 6 h after H2O2 addition, the fluorescence levels of the samples were measured using a fluorescence microplate reader at 488 nm excitation and 525 nm emission wavelengths. Fold-increases in ROS level were determined by comparison with the control group.
Transfection
The miR-19b mimic, inhibitor, and their negative controls were all purchased from RiboBio (Guangzhou, China). Before transfection, H9c2 cells were grown in 25 cm2 cell culture flasks with 4 ml medium. The miRNA was transfected to H9c2 cardiomyocytes using lipofectamine 2000 (Invitrogen, Carlsbad, CA), which were incubated at 37°C for 36 h for further experiments.
RNA extraction and real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen,Carlsbad, CA) following the manufacturer’s instruction. MiR-19b was reverse transcribed using the PrimeScript RT reagent Kit (TaKaRa, Tokyo, Japan) and quantified by real-time PCR with the TaqMan MicroRNA assay kit (Applied Biosystems). qRT-PCR analyses for PTEN and the normalization control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were performed using SYBR Premix Ex Taq (TaKaRa) on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems). The relative expression of each gene was calculated and normalized using the 2-ΔΔCt method relative to RNU6B or GAPDH. All reactions were conducted in triplicate.
Western blot analysis
Total proteins were extracted from H9c2 cells using RIPA buffer (Beyotime, Jiangsu, China), and then the cell lysates were centrifuged at 3,000× g for 30 min. The concentrations of protein were determined using a Protein Assay Kit (Bio-Rad, Hercules, CA, USA). For western blot analysis, 50 μg proteins were fractionated on 12% gels by SDS-polyacrylamide gel electrophoresis and then transferred onto PVDF membranes (Millipore, Bedford, MA). After blocking in 10% nonfat dry milk at room temperature for 1 h, the membranes were incubated with the primary antibody overnight at 4°C. The β-actin, Bcl-2, cleaved caspase-3, cleaved PARP, PTEN, AKT, pAKT, mTOR, and p-mTOR antibodies (all used at 1:1000 dilution) were obtained from Cell Signaling Technology (CST, BSN, USA). On the following day, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Thermo, Waltham, MA, USA) for 1 h at room temperature. Signals were visualized by ECL chemiluminescence. Equal protein loading was assessed by the expression of β-actin. The bands were semi-quantified using Image J software.
Luciferase reporter assays
A whole fragment of 3’UTR PTEN mRNA and a mutant form were cloned into pGL-3-Luc. The HEK 293T cells were seeded in 12-well plates and co-transfected with pGL-3-PTEN wild-type or mutant portion and TK100 Renilla combined with miR-19b mimic, miR-19b inhibitor or NC control using Lipofectamine 2000 (Invitrogen). Cells were harvested 48 hours after transfection, and luciferase activity was measured using a Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI) according to the manufacturer’s protocol. All of the dual-luciferase reporter assays were done in triplicate within each experiment.
Statistical analysis
All of the data are representative of three independent experiments performed as triplicate determinations. Statistical analyses of the data were evaluated using one-way ANOVA. Values of P<0.05 were considered statistically differences.
Results
Irisin attenuates H2O2-induced apoptosis and ROS in H9c2 cardiomyocytes
To assess the potential protective effects of Irisin, H9c2 cells were treated with varying concentrations of Irisin, followed by 200 μM H2O2 treatment, and then examined the cell viability by MTT assay. As shown in Figure 1A, Irisin improved the cell viability induced by H2O2 in a dose-dependent manner and achieved the strongest effect at 5 ng/ml. Therefore, we used 5 ng/mL Irisin for the following analysis.
Figure 1.
Irisin alleviated H2O2 induced apoptosis and ROS productionin H9c2 cardiomyocytes. H9c2 cells were treated for 24 h with different concentrations of Irisin (1, 2 and 5 ng/ml) before exposing the cultures to H2O2 for 1 h. A. Cell viability was measured using the MTT assay. B. The generation of myocardial ROS was assessed using 2’,7’-DCF diacetate. C. Flow cytometry analysis for the apoptosis of H9c2 cardiomyocytes. D. Immunoblot analysis for Bcl-2, cleaved-Caspase 3 and cleaved-PARP. The data are expressed as the mean ± SD of three independent experiments. *P<0.05, **P<0.01 vs control, ##P<0.01 vs H2O2 group.
Next, we evaluate the effect of Irisin on intracellular ROS that is the main inducer of oxidative stress and found that the levels of intracellular ROS were decreased by Irisin compared with control group in a dose-dependent manner (Figure 1B). As oxidative stress may lead to cardiomyocyte apoptosis, which plays a key role in the progression of cardiovascular diseases [21], we investigated the effects of Irisin on H2O2-induced cardiomyocyte apoptosis. As shown in Figure 1C, Irisin reduced H2O2-induced the apoptosis of H9c2 cells in a dose-dependent manner.
To examine the mechanism underlying the induction of apoptosis in more detail, we examined the expression of Bcl-2, cleaved caspase-3 and cleaved PARP proteins. Western blot analysis showed that the level of the cleaved caspase-3 and cleaved PARP protein was markedly decreased in the group of Irisin + H2O2 group compared to the H2O2 group, whereas the level of anti-apoptotic proteins Bcl-2 was increased after Irisin pretreatment following H2O2 exposure. These data indicated that Irisin pretreatment prevented the oxidative stress-induced apoptosis in cardiomyocytes dose-dependently, possibly through the intrinsic apoptotic pathway.
MiR-19b was upregulated in Irisin-treated H9c2 cells
Previous study demonstrated that miRNAs play important roles in multiple cardiovascular diseases by analyzing the miRNA profiles [22,23]. A recent study showed that the therapeutic effect of Irisin might be partially achieved through modulating miRNAs expression in atherosclerosis [24]. Therefore, we explored whether Irisin could affect the expression of six miRNAs that is associated with cardiomyocytes apoptosis. Through qRT-PCR analysis using Irisin-treated H9c2 cells, we found Irisin significantly increased the expression of miR-19b, whereas the expressions of miR-1 [25-27], miR-133 [26], miR-181a [28], miR-378 [29] and miR-195 [30,31] that were associated with the apoptosis of cardiomyocytes had no changes (Figure 2A). Moreover, a previous study showed that miR-19b could attenuate apoptosis in H2O2-treated H9c2 cardiomyocytes [24]. Thus, we chose miR-19b for further study. To investigate whether miR-19b involved in the protective role of Irisin in oxidative stress cell model, we treated H9c2 cells with increasing doses of Irisin for 24 h, and examined the relative expression of miR-19b by qPCR. We found that H2O2 treatment downregulated the expression of miR-19b in H9c2 cells, while administration of Irisin attenuated the inhibitory effect in a dose dependent manner (Figure 2B). These results suggest that Irisin may exert its anti-apoptotic and antioxidant activity through regulation of miR-19b in H2O2-treated H9c2 cells.
Figure 2.

Irisin upregulated the expression of miR-19b in H9c2 cardiomyocytes. A. The expression of six miRNAs (miR-1, miR-133, miR-181a, miR-378 and miR-19b) was measured by qRT-PCR in H9c2 cardiomyocytes treated with Irisin before exposing the cells to H2O2 for 1 h. B. The H9c2 cells were treated with different concentrations of Irisin (1, 2 and 5 ng/ml) for 24 h before exposing the cultures to H2O2 for 1 h. Then, the expression of miR-19b was evaluated by qRT-PCR. The data are expressed as the mean ± SD of three independent experiments. *P<0.05, **P<0.01 vs control, ##P<0.01 vs H2O2 group.
Irisin prevents cardiomyocytes from apoptosis induced by H2O2 through upregulating miR-19b expression
To examine whether the up-regulation of miR-19b contributes to the anti-apoptotic role of Irisin in H9c2 cells under H2O2 exposure, cells were transfected with miR-19b mimics and miR-19b inhibitor and then cell viability and apoptosis were evaluated. The miR-19b inhibitor or mimics were transfected into H9c2 cells, and the expression level of miR-19b was significantly decreased or increased after transfection (Figure 3A, 3C). Then, we observed Irisin treatment significantly decreased the apoptosis of H9c2 cells induced by H2O2, whereas knockdown of miR-19b by its inhibitor reversed the protective effect of Irisin on H2O2 induced H9c2 cells apoptosis (Figure 3B). In contrast, we observed that overexpression of miR-19b promoted Irisin-mediated H9c2 apoptosis (Figure 3D). Likewise, we investigated the role of miR-19b in the promoting effect of Irisin on the H9c2 cell viability. The MTT analysis showed that inhibition of miR-19b attenuated the promoting effect of Irisin on cell viability, while overexpression of miR-19b enhanced the promoting effect of Irisin (Figure 3E, 3F). Taken together, our results suggest that Irisin prevents cardiomyocytes from apoptosis induced by H2O2 through upregulating miR-19b expression.
Figure 3.
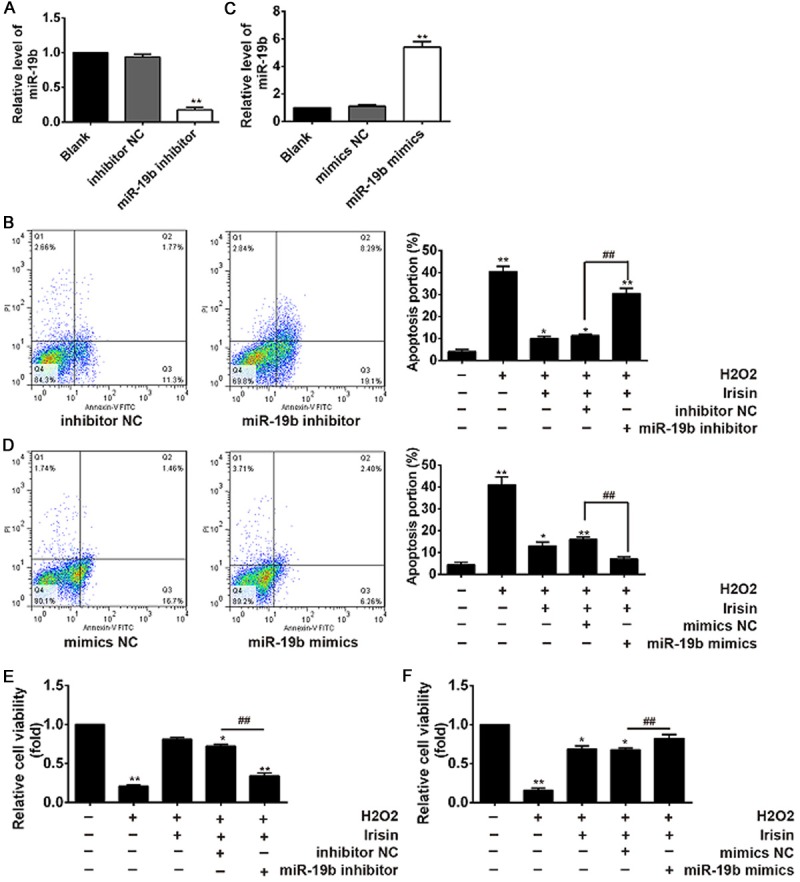
Irisin treatment promotes cell viability and inhibits cell apoptosis in H2O2-treated H9c2 cardiomyocytes through regulation of miR-19b. A, C. qRT-PCR analysis for miR-19b level in H9C2 cardiomyocytes transfected with miR-19b mimics, inhibitor, or respective negative controls. B, E. H9c2 cells were incubated in the presence of H2O2 or Irisin+ H2O2 or Irisin + H2O2 + miR-19b inhibitorand the cell viability and cell apoptosis of H9c2 cardiomyocytes were examined. D and F. H9c2 cells were incubated in the presence of H2O2 or Irisin+ H2O2 or Irisin + H2O2 + miR-19b mimics and the cell viability and cell apoptosis of H9c2 cardiomyocytes were examined. The data are expressed as the mean ± SD of three independent experiments. *P<0.05, **P<0.01 vs control, ##P<0.01 vs H2O2 + Irisin group.
PTEN is a direct target of miR-19b
To explore the molecular mechanism by which miR-19b functions in the protective effects of Irisin on H9c2 cells against oxidative stress, the PicTar and TargetScan algorithms were used and PTEN attracted our attention for the highest score in both algorithms. Moreover, previous research proved that PTEN was a target of miR-19b in cardiomyocytes [32,33]. Thus, PTEN was selected for further investigation. The predicted bindingsites for miR-19b in the PTEN sequence are illustrated in Figure 4A. In addition, qRT-PCR and Western Blot analysis showed that miR-19b overexpression decreased the levels of PTEN mRNA and protein expression, whereas inhibition of miR-19b increased the expressions (Figure 4B-E).
Figure 4.

PTEN is a direct target of miR-19b. A. The predicted binding sites for miR-19b in the 3’UTR of PTEN and the mutations in the binding sites are shown. B and C. PTEN protein expression was measured in H9c2 cells transfected with miR-19b inhibitor and miR-19b mimic. β-actin was used as the internal control. D. The bands were semi-quantitatively analyzed by using Image J software, normalized to β-actin density. E. PTEN mRNA expression was measured in H9c2 cells transfected with miR-92a inhibitor and miR-92a mimic. U6 was used as the internal control. F. Relative luciferase activity in HEK-293 cells co-transfection with wild-type or mutant-type 3’UTR PTEN reporter plasmids and miR-19b mimics or inhibitor. wt, wild-type; Mut, mutant-type. The data are expressed as the mean ± SD of three independent experiments. **P<0.01 vs mimic NC group, ##P<0.01 vsinhibitor NC group.
To validate whether PTEN is the direct target of miR-19b, a luciferase assay was performed in HEK293 cells. The predicted PTEN binding site (PTEN-wt) and its mutant type (PTEN-mt) were amplified and directly fused to the downstream of the luciferase reporter gene in the pmirGLO-basic vector. Co-transfetion of miR-19b mimic and pmirGLO-PTEN-wt significantly decreased the luciferase activity, whereas co-transfection of miR-19b inhibitor and pmirGLO-PTEN-wt increased the luciferace activity (Figure 4F). Likewise, cells co-transfected with miR-19b and pmirGLO-PTEN-mut showed no obvious change in luciferase activity (Figure 4F). Our results demonstrate that miR-19b targeted PTEN and negatively regulated its expression in H9c2 cells, suggesting that miR-19b exert its anti-apoptotic and anti-oxidative stress role through the inhibition of PTEN.
Irisin promotes the activation of AKT/mTOR signal pathway in H9c2 cells under H2O2 exposure
It is reported that PTEN is a negative regulator of the AKT/mTOR signaling pathway [34]. Additionally, recent studies imply that activation of the AKT/mTOR pathway is essential for the cardiac physiology and protection against pathological remodeling and failure [35].Following on from the above findings, we sought to further explore whether the miR-19b/PTEN/AKT/mTOR axis was involved in the anti-apoptotic effects of Irisin in the cell culture model. Western blot was performed to detect the protein levels of Akt, phosphorylated-Akt (p-Akt), mTOR, phosphorylated-mTOR (p-mTOR) and PTEN in H9c2 cells under H2O2 exposure.We confirmed that p-Akt and p-mTOR were significantly decreased after H2O2 treatment. Interestingly, Irisin abolished the inhibitory effect of H2O2 on p-Akt and p-mTOR (Figure 5A, 5B). Moreover, after H2O2 treatment, the expression level of PTEN was markedly increased, whereas Irisin attenuated the enhancement of H2O2 on the expression of PTEN (Figure 5A, 5B). These data imply that Irisin may activate the AKT/mTOR signaling pathway through regulation of miR-19b/PTEN, finally inhibiting cell apoptosis and ROS induced by H2O2.
Figure 5.

Irisin attenuates H2O2 induced the inactivation of AKT/mTOR signaling pathway in H9c2 cells. H9c2 cells were treated for 24 h with 5 ng/ml Irisin before exposing the cultures to H2O2 for 1 h. A. AKT and mTOR phosphorylation were determined by Western blot. Total AKT and mTOR were used as a loading control. B. The bands were semi-quantitatively analyzed by using Image J software. The data are expressed as the mean ± SD of three independent experiments. **P<0.01 vs H2O2 group.
Discussion
In the present study, we demonstrated that Irisin could promote cell viability and inhibit cell apoptosis and ROS production in H9c2 cells under H2O2 exposure. Furthermore, we demonstrate that Irisin prevent H9c2 cells from apoptosis by regulating miR-19b/PTEN/AKT/mTOR axis. These results suggest that Irisin treatment could serve as a potential therapeutic strategy to protect against myocardial remodeling.
Several reports have demonstrated that Irisin, a newly discovered myokine, has great potential as therapeutic applications for metabolic disturbances. A study from Xiang et al. showed that circulating Irisin levels were positively associated with endothelium-dependent vasodilation [36] in newly diagnosed type 2 diabetic patients [37]. Recently, the relationship between Irisin and cardiovascular diseases has gained increasing attention [38-40]. For example, Lu J et al. found that Irisin protected against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice [13]. In our study, we demonstrated that Irisin pretreatment promoted cell proliferation, inhibited cell apoptosis and ROS production in H9c2 cellstreated with H2O2. In addition, a previous study reported that controlling ROS exposure could increase cardiomyocyte survival and prevent pathological remodeling of the myocardium [41]. These data suggest that Irisin may improve myocardial remodeling induced by oxidative stress, at least in part, by inhibiting apoptosis of cardiomyocytes.
Recently, miRNAs have been reported to be involved in the development of myocardial remodeling [15,16]. For example, Rui Li et al. identified miR-7a/b play an important role in myocardial infarction (MI)-induced cardiac remodeling by regulating the expression of Sp1 [42]. Liu, N., et al. found that miR-135a was markedly up-regulated under H2O2 treatment in rat cardiomyocytes and regulated H2O2-induced apoptosis via the modulation of Bcl-2 [43]. A recent research showed that Irisin exhibited unique activities that regulate miRNA expression in atherosclerosis [24]. Here, we found that Irisin treatment upregulated the expression of miR-19b in H9c2 cells under H2O2 treatment. miR-19b inhibition attenuated the anti-apoptotic effects of Irisin and the pro-proliferation effects of Irisin on cardiomyocytes. Moreover, miR-19b has been proved to be able to protect cardiomyocyte apoptosis and improve cell survival [33]. This indicates that the inhibitory effects of Irisin on H2O2 induced apoptosis may be through the upregulation of miR-19b.
PTEN is a dual protein/lipid phosphatase inside the cells, which has been shown to be repressed by miR-19b in several cancer cells [44-47]. Meanwhile, inactivation of PTEN has been reported to activate downstream signaling including AKT and/or ERK [48,49]. Moreover, a recent study performed by Xu, J., et al. demonstrated that miR-19b attenuated H2O2-induced apoptosis in rat H9c2 cardiomyocytes via targeting PTEN [32]. Therefore, it is not difficult to understand that miR-19b-mediated decrease of PTEN expression can activate downstream AKT signaling, and inhibit cellular apoptosis. As expected, PTEN was validated as a target gene of miR-19b and negatively regulated by miR-19b at the protein and mRNA level in H9c2 cardiomyocytes. Moreover, we found that p-Akt and p-mTOR were decreased after H2O2 treatment in H9c2 cells, whereas Irisin could abolish the inhibitory effects of H2O2 on p-Akt and p-mTOR. Taken together, these data indicated that Irisin inhibited apoptosis induced by H2O2 through activation of miR-19b/PTEN/AKT/mTOR axis.
In conclusion, we reported that Irisin specificallyprevented cardiomyocyte apoptosis under oxidative stress through miR-19b/AKT/mTOR pathway. This finding indicates that Irisin treatment could serve as a potential therapeutic strategy to protect myocardial remodeling development.
Acknowledgements
This study was supported by Science Foundation of The Sichuan Medical Association Shihuida Project (2016SHD008).
Disclosure of conflict of interest
None.
References
- 1.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2016 update: a report from the American heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz K, Carrier L, Guicheney P, Komajda M. Molecular basis of familial cardiomyopathies. Circulation. 1995;91:532–540. doi: 10.1161/01.cir.91.2.532. [DOI] [PubMed] [Google Scholar]
- 3.Teiger E, Than VD, Richard L, Wisnewsky C, Tea BS, Gaboury L, Tremblay J, Schwartz K, Hamet P. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996;97:2891–2897. doi: 10.1172/JCI118747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–196. [PMC free article] [PubMed] [Google Scholar]
- 5.Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan L, Moore PK, Ji Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29:203–213. doi: 10.7555/JBR.28.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho S, Cho M, Kim J, Kaeberlein M, Lee SJ, Suh Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1alpha in a FOXO3-dependent mechanism. Oncotarget. 2015;6:43–55. doi: 10.18632/oncotarget.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H, Luo G, Liao W, Bin J, Huang X, Liao Y. Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget. 2015;6:18829–18844. doi: 10.18632/oncotarget.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, Kim JG, Lee IK, Park KG. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100:96–101. doi: 10.1016/j.diabres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, Tavintharan S, Sum CF, Lim SC. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:365–369. doi: 10.1016/j.jdiacomp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, Imrich R, Kyselovicova O, Belan V, Jelok I, Wolfrum C, Klimes I, Krssak M, Zemkova E, Gasperikova D, Ukropec J, Ukropcova B. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592:1091–1107. doi: 10.1113/jphysiol.2013.264655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu D, Wang H, Zhang J, Zhang X, Xin C, Zhang F, Lee Y, Zhang L, Lian K, Yan W, Ma X, Liu Y, Tao L. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J Mol Cell Cardiol. 2015;87:138–147. doi: 10.1016/j.yjmcc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 2015;243:438–448. doi: 10.1016/j.atherosclerosis.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda S, Pu WT. Expression and function of microRNAs in heart disease. Curr Drug Targets. 2010;11:913–925. doi: 10.2174/138945010791591304. [DOI] [PubMed] [Google Scholar]
- 16.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 18.Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Just S, Borries A, Rudloff J, Leidinger P, Meese E, Katus HA, Rottbauer W. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- 19.Chang G, Zhang D, Yu H, Zhang P, Wang Y, Zheng A, Qin S. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med. 2013;32:1011–1020. doi: 10.3892/ijmm.2013.1475. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Vater C, Jacobi A, Liebers C, Zou X, Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrowderived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol. 2014;171:2440–2456. doi: 10.1111/bph.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Brown ME, Zhang H, Martinez M, Zhao Z, Bhutani S, Yin S, Trac D, Xi JJ, Davis ME. High-throughput screening identifies microRNAs that target Nox2 and improve function following acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2017 doi: 10.1152/ajpheart.00685.2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Song H, Zhang Y, Wu F, Mu Q, Jiang M, Wang F, Zhang W, Li L, Shao L, Li S, Yang L, Zhang M, Wu Q, Tang D. Irisin inhibits atherosclerosis by promoting endothelial proliferation through microRNA126-5p. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JZ, Tang XN, Li TT, Liu LJ, Yu SY, Zhou GY, Shao QR, Sun HP, Wu C, Yang Y. Paeoniflorin inhibits doxorubicin-induced cardiomyocyte apoptosis by downregulating microRNA-1 expression. Exp Ther Med. 2016;11:2407–2412. doi: 10.3892/etm.2016.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Peng J, Wang C, Deng H, Li Y. Calcitonin gene-related peptide suppresses isoprenaline-induced cardiomyocyte apoptosis through regulation of microRNA-1 and microRNA-133a expression. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:964–971. doi: 10.3969/j.issn.1672-7347.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhai C, Tang G, Peng L, Hu H, Qian G, Wang S, Yao J, Zhang X, Fang Y, Yang S, Zhang X. Inhibition of microRNA-1 attenuates hypoxia/re-oxygenation-induced apoptosis of cardiomyocytes by directly targeting Bcl-2 but not GADD45Beta. Am J Transl Res. 2015;7:1952–1962. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, Guo J, Mei Y, Liu WL. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell Longev. 2014;2014:960362. doi: 10.1155/2014/960362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang N, Zhong J, Han S, Li Y, Yin Y, Li J. MicroRNA-378 alleviates cerebral ischemic injury by negatively regulating apoptosis executioner caspase-3. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17091427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Gao CK, Liu H, Cui CJ, Liang ZG, Yao H, Tian Y. Roles of MicroRNA-195 in cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury. J Genet. 2016;95:99–108. doi: 10.1007/s12041-016-0616-3. [DOI] [PubMed] [Google Scholar]
- 31.Hang P, Sun C, Guo J, Zhao J, Du Z. BDNFmediates down-regulation of MicroRNA-195 inhibits ischemic cardiac apoptosis in rats. Int J Biol Sci. 2016;12:979–989. doi: 10.7150/ijbs.15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong C, Wang K, Liu Y, Lv D, Zheng B, Zhou Q, Sun Q, Chen P, Ding S, Xu Y, Huang H. miR-19b controls cardiac fibroblast proliferation and migration. J Cell Mol Med. 2016;20:1191–1197. doi: 10.1111/jcmm.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Tang Y, Bei Y, Ding S, Che L, Yao J, Wang H, Lv D, Xiao J. miR-19b attenuates H2O2-induced apoptosis in rat H9C2 cardiomyocytes via targeting PTEN. Oncotarget. 2016;7:10870–10878. doi: 10.18632/oncotarget.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 35.Ghigo A, Li M. Phosphoinositide 3-kinase: friend and foe in cardiovascular disease. Front Pharmacol. 2015;6:169. doi: 10.3389/fphar.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang L, Xiang G, Yue L, Zhang J, Zhao L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014;235:328–333. doi: 10.1016/j.atherosclerosis.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9–10. doi: 10.1038/nature11364. discussion E10-11. [DOI] [PubMed] [Google Scholar]
- 39.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lecker SH, Zavin A, Cao P, Arena R, Allsup K, Daniels KM, Joseph J, Schulze PC, Forman DE. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818. doi: 10.1161/CIRCHEARTFAILURE.112.969543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohjoismaki JL, Goffart S. The role of mitochondria in cardiac development and protection. Free Radic Biol Med. 2017;106:345–354. doi: 10.1016/j.freeradbiomed.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 42.Li R, Geng HH, Xiao J, Qin XT, Wang F, Xing JH, Xia YF, Mao Y, Liang JW, Ji XP. miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci Rep. 2016;6:29082. doi: 10.1038/srep29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu N, Shi YF, Diao HY, Li YX, Cui Y, Song XJ, Tian X, Li TY, Liu B. MicroRNA-135a regulates apoptosis induced by hydrogen peroxide in rat cardiomyoblast cells. Int J Biol Sci. 2017;13:13–21. doi: 10.7150/ijbs.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, Xie RY, Wang SC, Jin W, Gao F, Shi JW, Zhao WT, Jia JS, Shen HF, Ke JR, Liu B, Zhao YQ, Huang WH, Yao KT, Li DJ, Xiao D. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Invest. 2015;95:1056–1070. doi: 10.1038/labinvest.2015.76. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R, Wu J, Geng S, Zhong C. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res. 2014;28:1553–1560. doi: 10.1002/ptr.5167. [DOI] [PubMed] [Google Scholar]
- 46.Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS One. 2013;8:e75885. doi: 10.1371/journal.pone.0075885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia Z, Wang K, Zhang A, Wang G, Kang C, Han L, Pu P. miR-19a and miR-19b overexpression in gliomas. Pathol Oncol Res. 2013;19:847–853. doi: 10.1007/s12253-013-9653-x. [DOI] [PubMed] [Google Scholar]
- 48.Luo M, Tan X, Mu L, Luo Y, Li R, Deng X, Chen N, Ren M, Li Y, Wang L, Wu J, Wan Q. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci Rep. 2017;7:43427. doi: 10.1038/srep43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao Y, Zheng W, Li N, Su Z, Zhao L, Zhou H, Jia L. MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci Rep. 2017;7:41942. doi: 10.1038/srep41942. [DOI] [PMC free article] [PubMed] [Google Scholar]



