Abstract
A new biosorbent, composed of Amberlite XAD-4 loaded with Anoxybacillus kestanboliensis, was developed and surface morphologies were investigated by SEM and FT-IR. It was used for solid phase column preconcentrations of Co(II) and Hg(II) before their measurements by ICP-OES. LODs were calculated as 0.04 and 0.06 ng mL−1 for Co(II) and Hg(II) respectively. The maximum biosorption capacities were determined as 24.3 and 27.8 mg g−1 for Co(II) and Hg(II) respectively. Preconcentration factors were achieved for Co(II) and Hg(II) as 80. The method validation was performed by analyzing certified reference materials. The new process was successfully utilized for the preconcentration of these metals in various food samples. It should be highlighted that the sensitivity of ICP-OES was critically improved by applying developed method. Hence, ICP-OES could be an effective alternative for ICP-MS and/or GF-AAS.
Subject terms: Pollution remediation, Biocatalysis
Introduction
Some metal ions are known to be highly toxic. Therefore, a number of recent studies have focused on metal ion toxicity and carcinogenicity on human beings and other living organisms even at very low levels1,2. Among the various types of pollutants, toxic metal ions cannot be easily eliminated from nature3. Accumulation of toxic and deleterious metal ions in soft tissues of animals and humans even at low concentrations could be a major concern as they are not metabolized and can create significant damage to the body tissues4. Co and Hg are two typical metal ions in environmental samples which are also detected in plants and food samples. Toxicological influences such as vasodilation and cardiomyopathy are reported for Co exposure. Hg is also familiar as it is the most neurotoxic metal ion and can harm most of the human systems3.
The usage of the biological processes is an idea for the operation of environmental metal pollution. Biological processes for the bioremediation depend on the physical nature of binding sites and chemical conditions4. The utilization of biomass, including alive and dead microorganisms in the possibly retention and removal of metal ions from environmental samples, has acquired significant reliability over the past few years due to its efficiency, ecofriendliness and economic choice. The major properties of biosorbents are (a) having a variety of metal binding sites, (b) having small and identical sizes, (c) being less subject to interference from alkali-earth and alkali metals than ion-exchange resins (d) being generally cheaper than functionalized polymers (e) having the possibility to be obtained via green technologies5.
Concentrations of toxic metal ions are known as trace levels that are lower than the quantification limit of the instruments6. The determination, separation and analysis of low concentration of toxic metal ions in natural samples such as soil, food and water have become significant7. Therefore, it is necessary to develop analytical methods to preconcentrate and determine trace metal ions6.
Alternative methods are recommended for the preconcentration and separation of metals before their measurements. Solid phase extraction, coprecipitation, cloud point extraction, membrane filtration, electro deposition, liquid liquid extraction, photo-degradation, photocatalysis and, ion exchange are the methods for the preconcentration of metals in a variety of samples8–13. Solid phase extraction (SPE) has attained special interest related to features such as easy procedure, low operational time, high preconcentration factor, high retention, low cost, low pollution, flexibility and reusability of absorbents/biosorbents14–16.
The aim of the study is the development of an analytical method based on the use of thermophilic Anoxybacillus kestanboliensis immobilized biosorbent as a solid phase extractor for Co(II) and Hg(II). According to our literature survey, further researches are required for the use of immobilized thermophilic bacteria as a biosorbent material for the preconcentration of metal ions. Development of SPE sorbent based on immobilized thermophilic A. kestanboliensis on XAD-4 resin for the preconcentrations of Co(II) and Hg(II) is aimed.
Materials and Method
Instrumentation and chemicals
ICP-OES (PerkinElmer Optima™ 2100 DV, PerkinElmer,) was used for the measurements of Co(II) and Hg(II) concentrations at 228.616 and 194.168 nm, respectively. The ICP-OES operating conditions of were indicated in our previous paper (Delay time was set as 15 s extra to avoid the memory effect on Hg measurement and monitored routinly) pH was measured by Mettler Toledo MPC 227 0.1.0 cm × 10.0 cm filtration column was used for SPE experiments. Flow rate were controlled by using Watson-Marlow 323 peristaltic pump. FT-IR spectra was recorded on a Perkin-Elmer infrared spectrometer on KBr pellets. SEM images were obtained with a LEO 440 SEM, which was used for investigation of surface macrostructure.
1000 µg mL−1 of Co(II) and Hg(II) solutions were buyed from High Purity Standards, Charleston, SC, USA). Concentrated H2O2 (35%), HNO3 (65%), HCl (36.5–38.0%) and NH4OH were obtained from Sigma Aldrich, Germany. NCSZC 73014 tea leaves, NCS DC78301 river sediment and DORM2dogfish muscle were already available in the laboratory.
Cultured of Thermophilic Anoxybacillus kestanboliensis
In this experimental study, Anoxybacillus kestanboliensis was isolated from Afyonkarahisar Omer spring mud sample, Turkey. The morphological and biochemical tests were analyzed. 16 S rRNA analysis was also made in OINTEK, ITU, Istanbul, Turkey for the identification of bacteria. Thermophilic A. kestanboliensis was cultured in 1 L glass flasks containing 0.25 L Nutrient Broth (NB) at 120 rpm and 55 °C for one day on a rotary shaker.
Preparation of the Dried Dead Anoxybacillus kestanboliensis and loaded Biomass
The preparation of dried dead A. kestanboliensis and the loading of bacteria on XAD-4 was prepared according to Ozdemir, et al.17 and Ozdemir, et al.18 with some modification.
General sorption studies
The model solution (50 mL), consisted of Cd(II), Ni(II), Co(II), Hg(II), As(III), Cu(II), Fe(II), Pb(II), Mn(II) and Cr(III) at 10.0 ng mL−1, was used for the SPE procedure. The solution pH was adjusted to 3.0 and 6.0, considering our previous experiences. They were passed through the SPE column at the flow rate of 1 mL min−1. Concentrations of metal ions were measured in eluate (5.0 mL of 1.0 mol L−1 HCl) by ICP-OES.
Sample preparation
Soil and tap water (after flushing 1.0 min) samples were received from Mardin, Turkey. The others were bought from local market. 100 mL portions of aqueous samples were directly applied to the method. Food samples were firstly digested in an analytical microwave oven (Berghof MWS3). 1.0 g portion of samples were weighed. 5.0 mL of HNO3:HCl (1:1, v/v) was added, and then the mixture was heated on a hot plate. It was dried and 6.0 mL of HNO3:HCl:H2O2 (1:1:0.2, v/v/v) was added before being transferred into a microwave vessel. It was heated to 170 °C by microwave irradiation and kept for 5.0 min. Then, the temperature was heated to 200 °C in 15 min and the mixture was kept there for 1.0 min. Next, the temperature was reduced to 100 °C and the mixture was kept there for another 20 min. After these procedures, the last volume was completed to 50.0 mL and the solution pH was adjusted to the requested value prior to the SPE process. The portions of tea leaves (NCSZC 73014), river sediments (NCS DC78301) and dogfish muscles (DORM2) (0.5 g) were digested, applying the same procedure reported for the environmental and food samples.
Results and Discussion
Surface functionalities of the A. kestanboliensis loaded XAD-4 resin with and without Co(II) and Hg(II) were examined through FT-IR spectral comparison. It was comparatively presented in Fig. 1. The peaks (from Fig. 1c) around 650, 1100, 1270, 1300 and 1400 cm−1 were attribuated to successfully loading of A. kestanboliensis to XAD-4. From Fig. 1b., the peaks on approximately 3750 cm−1, doublet on 2900 cm−1, 1700 cm−1, 1550 cm−1, 1450 cm−1 were attributed to O-H stretching of alcohol, N-H stretching from amine salt, C=O stretching, N-O stretching, C-H bending, respectively. By considering the hard and soft acids and bases theory, Hg and Co could be accepted as soft and borderline acids. Thus we can conclude that the targeted metal ions could interact with functional groups such as C6H5N, N3−, NO2−, SO3−2 and R−, C2H4, C2H6, RNC, CO, SCN−, R3P, R2S, RSH, RS−, S2O3−2 on bacteria loaded resin surface. Interactions could be due to complexation, ion exchange as well as physical adsorption17. No different peaks were observed after interaction of Co(II) and Hg(II). However, approximately 10 cm−1 shifting were observed and attributed to metal ions complexation with functional groups of bacterial surface. SEM analysis of thermophilic A. kestanboliensis immobilized XAD-4 was presented in Fig. 1f,g. As shown in Fig. 1f,g, the porous surface of the immobilized thermophilic A. kestanboliensis was clearly seen. It is well known that the porous surface is significant because of the high interaction between analytes and surface functional binding groups.
Figure 1.
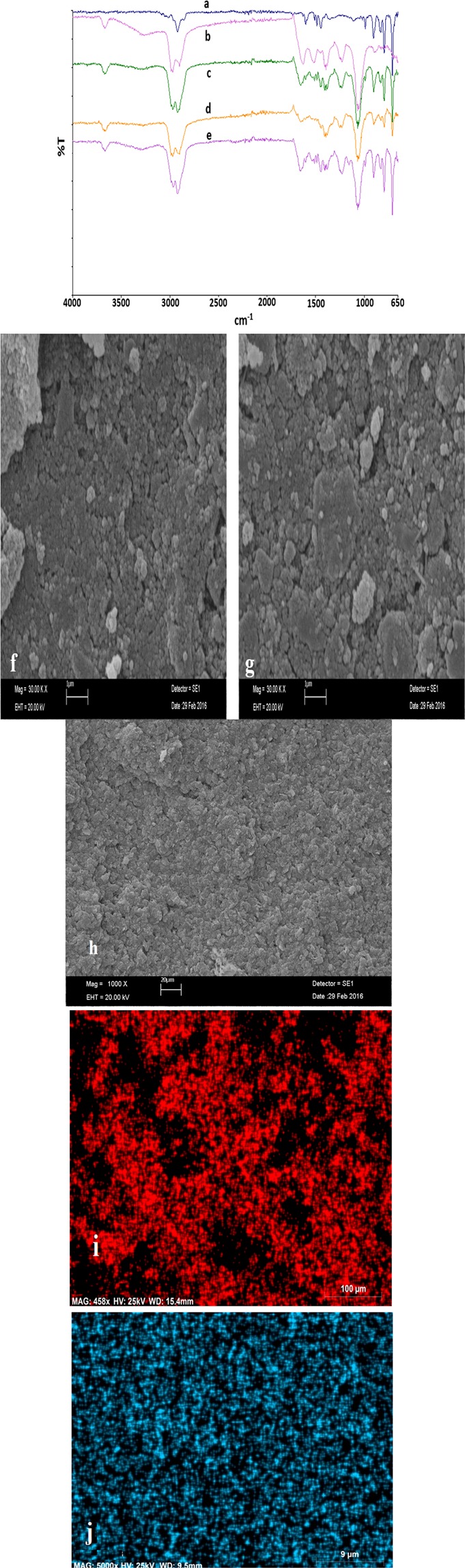
FT-IR spectral comparison of (a). Amberlite XAD-4, (b). A. kestanboliens, (c). A. kestanboliens on Amberlite XAD-4, (d). Co(II) loaded on A. kestanboliens-Amberlite XAD-4, (e). Hg(II) loaded on A. kestanboliens-Amberlite XAD-4, and SEM image of surface macrostructure of (f). A. kestanboliensis immobilized XAD-4 with Co(II), (g). A. kestanboliensis immobilized XAD-4 with Hg(II) (h). A. kestanboliensis immobilized XAD-4 without Co(II) and Hg(II), (i). EDX mapping of A. kestanboliensis immobilized XAD-4 with Co(II), (j). EDX mapping of A. kestanboliensis immobilized XAD-4 with Hg(II).
A mixture of model solution including Cd(II), Ni(II), Co(II), Hg(II), As(III), Cu(II), Fe(II), Pb(II), Mn(II) and Cr(III) at 10.0 ng mL−1 concentration was utilized to SPE procedure. Considering the ICP-OES results, it was decided to optimize the method for Co(II) and Hg(II) as recovery percentages were clearly lower for other cations.
Effect of pH
pH solution influences the overall feature of the adsorbate and adsorbent18, and it also controls the electrostatic interaction between adsorbate and adsorbent19. The adsorbent chemical properties depend extremely on the solution pH18. The influence of pH on the recovery of Co(II) and Hg(II) of the immobilized thermophilic A. kestanboliensis on XAD-4 resin was investigated in the pH varieties of 2.0 to 9.0 (Fig. 2). The maximum pH for quantitative retentions of the tested metal ions by XAD-4 resin modified with thermophilic A. kestanboliensis was determined as 5.0 and 4.0 for Co(II) and Hg(II), respectively. It was determined that at lower pH, the existence of H+ complicates the cationic nature of Co(II) and Hg(II) metal ions which affects the biosorption rate as demonstrated in Fig. 2. At lower pH degrees, concentration of H+ is high and occupies the binding groups of cell surface and competes with analytes, as a result; the metal biosorption percentages decreased significantly20, however; at higher pH degrees, the metal ions displayed a trend to precipitate leading to lower biosorption21. All following experiments were tested at pH 5.0 and 4.0 for Co(II) and Hg(II), respectively.
Figure 2.
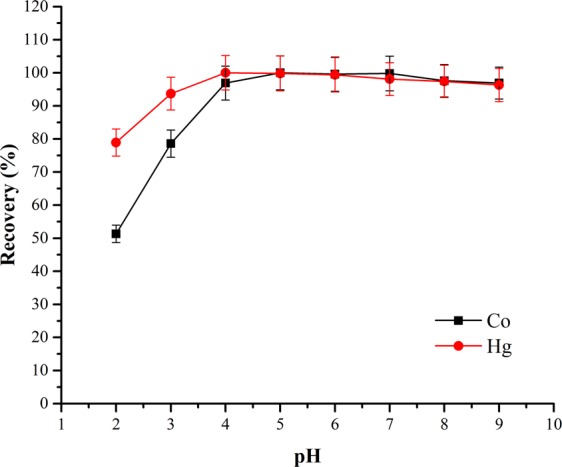
Effect of pH on the SPE preconcentrations of Co(II) and Hg(II).
The effect of flow rate
The time course is significant for the biotechnological approach and controlling the performance of the biosorption process5. As represented in Fig. S1, flow rate also acts as a significant parameter in metal biosorption. For this reason, the determination of maximum flow rate is important to obtain the optimum metal ion retention. When the flow rate rised from 3.0 mL min−1 to 6.0 mL/min for recovery of Co(II) and Hg(II), the biosorption percentages reduced from 97.6% to 81.5% and 98.1% to 82.7% for Co(II) and Hg(II), respectively. The reason of the reduction in recoveries could be due to the insufficient contact time between binding sites of biomass and metal ions22. The maximum flow rate was determined as 2.0 mL min−1 for both studied metal ions. From this point of view, the flow rate of 2.0 mL min−1 was used in following studies.
Effects of amounts of biosorbent and resin
The amount of biosorbents is also a critical parameter that affects the biosorption performance. Various concentrations of biosorbents can vary the quantity of the biosorbed metal ions on biosorbents as well as the recovery capacity23. The impact of the biosorbent concentration was examined in the variety of 100–300 mg. When the amount of biosorbent was increased from 50 mg to 200 mg, the recovery percentage was increased from 92% to 100%. This increase may be due to the availability of biosorbent metal binding sites as greater availability of specific biosorbents surfaces24,25. As seen in Fig. S2, the maximum biosorption yield was obtained at concentration of 200 mg of biosorbents. Appropriate quantity of biosorbents can bind the metal ions quantitatively from the metal solution samples. As the biosorbent concentration was less than 200 mg, the biosorbed metal ions reduced. On the other hand, in case of more than 200 mg of biosorbents, the biosorption percentage cannot change up to 300 mg. Similar findings reported by Sharma et al.25. Thus, following experiments were studied using 100 mg of biosorbents. In addition to this, the parameter of quantities of XAD-4 was experimented in the variety of 600–1000 mg. The results were given in Fig. S3. The retentions of Co(II) and Hg(II) rose up to 800 mg of XAD-4, however; that the amount was more than 800 mg of resin did not affect the metal ions retentions. So, 800 mg of XAD-4 was chosen as the best resin quantity for further processes.
Influence of eluent type, concentration and volume
An acceptable eluent can effectively elute the biosorbed metal ions from surface binding sites for a high preconcentration factor. In addition to this, it should not influence the accurate determination of the metal ions and regeneration of solid phase extraction column26. For this purpose, various concentrations (0.5 mol L−1 and 1 mol L−1) and volumes (3 and 5 mL) of HCI and HNO3 were evaluated as an eluent. The experimental results of eluent types, concentrations and volumes were demonstrated in Table S1. When using 3 mL of 0.5 mol L−1 and 1 mol L−1 of HCI and HNO3, the recoveries were found as 90 ± 0.6% and 93 ± 0.8% and 88 ± 0.4% and 91 ± 0.9% for Co(II), respectively and 91 ± 0.8% and 94 ± 0.5% and 89 ± 0.7% and 92 ± 0.3% for Hg(II), respectively. The most satisfactory eluent was determined as 5 mL 1 mol L−1 HCI for the quantitative elution of Co(II) and Hg(II).
Influence of matrix ions on retention of Co(II) and Hg(II)
The influences of some possible matrix ions such as K(I), Na(I), Mg(II), Ni(II), Zn(II), Ca(II), Cu(II), Fe(II), Cd(II) and Al(III), existing in natural samples on the uptakes of the Co(II) and Hg(II), were also tested by the addition, known as the amount of every matrix ion, into the sample solution containing the experimented metal ions. The tolerated quantities of every ion were less than 5% change on the absorbance. Not all the experimented matrix ions showed an interfering effect under experimental conditions (Table 1). These results demonstrated that the recommended SPE method for the examined metal ions should be applied to different samples.
Table 1.
Effect of possible interfering ions on the SPE preconcentrations of Co(II) and Hg(II).
| Recoverya (%) | |||
|---|---|---|---|
| Interferences | Interferic ion to metal ion ratio | Co(II) | Hg(II) |
| Na(I) | 8000 | 98 ± 0.4 | 99 ± 0.2 |
| K(I) | 8000 | 97 ± 0.6 | 99 ± 0.7 |
| Ca(II) | 200 | 98 ± 1.4 | 100.1 ± 0.9 |
| Mg(II) | 200 | 98 ± 0.8 | 99 ± 0.5 |
| Zn(II) | 20 | 97 ± 1.4 | 98 ± 0.8 |
| Fe(II) | 20 | 97 ± 1.0 | 98 ± 0.9 |
| Cd(II) | 10 | 98 ± 1.3 | 99 ± 1.1 |
| Cu(II) | 10 | 97 ± 1.4 | 98 ± 0.7 |
| Ni(II) | 10 | 96 ± 0.9 | 97 ± 1.4 |
| Al(III) | 10 | 97 ± 0.7 | 97 ± 1.3 |
aConcentrations of the heavy metal ions are 10 µg L−1.
Effect of sample volume and determination of enrichment factor
The parameter of the sample volume is very important to obtain a high preconcentration factor and credible analytical results and to determine the analytes at very low levels in natural samples27. As a result, the sample volume must be experimented in SPE studies. Various sample volumes were tested under the optimum experimental conditions for determination of the highest sample volume. The influence of the sample volume on the recoveries of the studied metal ions is represented in Fig. S4. The recovery yields of Co(II) and Hg(II) is higher than 95% up to sample volume of 400 mL. The preconcentration factor was found as 80 for both metal ions.
Reusage of the SPE column
In SPE studies, the regeneration of the SPE column is also a significant factor for the economical perspective17.To analyze the regeneration of SPE column, 50 mL 1 mg L−1 of Co(II) and Hg(II) solutions were passed through the thermophilic A. kestanboliensis loaded onto XAD-4 column at a flow rate of 2.0 mL min−1. After 30 reusage of the SPE column, the recovery percentages of Co(II) and Hg(II) were found as 98.3% and 98.7%, respectively. It could be concluded that the developed column could be used up to 35 times with ≥95% retentions (Fig. 3).
Figure 3.
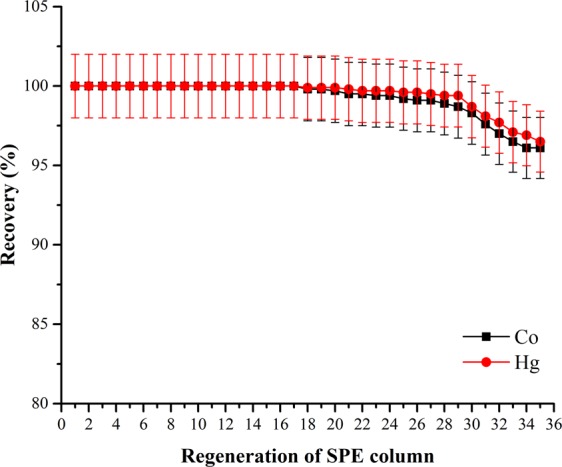
Effect of reusage of SPE column on the preconcentrations of Co(II) and Hg(II).
Analytical figures of merit
Analytical figures of merit were summarized in Table S2 in view of and preconcentration factor LOD, RSD, LOQ, linear range. Linear calibration curves for Co(II) and Hg(II) were acquired in the ranges of 0.25–12.5 ng mL−1 for Co(II) and Hg(II) with correlation coefficients as 0.9985 and 0.9989, respectively. LODs were 0.04 and 0.06 ng mL−1 for Co(II) and Hg(II), respectively. RSDs were found lower than 6.8%. Considering the 400 mL of original and 5 mL of last sample volume, the preconcentration factor was found as 80.
A list of the same analytical parameters for different procedures for Co(II) and Hg(II) preconcentrations is presented in Table 228–37. It could be finalized that the developed procedure has more advantages due to its simplicity, being easy to handle and use of low cost bacterial biomass.
Table 2.
Comparison of analytical characteristics of the preconcentrations methods for Co(II) and Hg(II).
| Method | Instrument | LOD, ng mL−1 | PF1 | Linear range, ng mL−1 | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Co(II) | Hg(II) | Co(II) | Hg(II) | Co(II) | Hg(II) | |||
| SPE on Amberlite XAD-2 resin anchored with pyrocatechol | FAAS | 0.95 | — | 24 | — | — | — | 28 |
| SPE ON 2-hydroxyacetophenone-functionalized polyurethane foam | FAAS | 0.8 | — | 36 | — | 2.7–150 | — | 29 |
| SPE on Duolite XAD 761 modified with a new Schiff base | FAAS | 2.2 | — | 26 | — | 15–340 | — | 30 |
| SPE on chelating resin | FAAS | 0.44 | — | 150 | — | 5–900 | — | 31 |
| Cationic micellar precipitation | ICP-OES | 0.009 | — | 40 | — | 0.03–700 | — | 32 |
| Magnetic SPE on magnetic core-shell nanoparticles modified with thiourea-derived chelating agents | Direct mercury analyzer | — | 0.017 | — | 100 | — | — | 33 |
| SPE on 2-nitroso-1-naphthol-4-sulfonic acid modified natural clinoptilolite zeolite | Derivative spectrophotometry | — | 0.1 | — | 95 | — | — | 34 |
| Cloud point extraction | ICP-OES | — | 1.1 | — | 51.3 | — | 10–100 | 35 |
| Magnetic SPE on Fe3O4@SiO2@PT nanocomposite | Cold vapor AAS | — | 0.02 | — | 267 | — | 0.8–70 | 36 |
| Preconcentration on an ion-imprinted polymer coated maghemite nanoparticles | FAAS | — | 4.1 | — | 100 | — | 20–1000 | 37 |
| SPE on Amberlite XAD-4 resin loaded with Anoxybacillus kestanboliensis | ICP-OES | 0.04 | 0.06 | 80 | 80 | 0.25–12.5 | 0.25–12.5 | This method |
Tea leaves (NCSZC 73014), river sediment (NCS DC78301) and dogfish muscle (DORM2) were utilized to the developed procedure to validate the procedure. Results are presented in Table 3. As can be seen, good correlation was obtained between the determined and certified values.
Table 3.
Langmuir and Freundlich isotherms for Anoxybacillus kestanboliensis loaded Amberlite XAD-4 as sorbent for Co(II) and Hg(II).
| Metal ion |
Langmuir isotherm | Freundlichisotherm | ||||
|---|---|---|---|---|---|---|
| As (mg/g) | Kb (L/mg) | r2 | KF | n | r2 | |
| Co | 45.0 | 0.13 | 0.9954 | 6.0 | 1.86 | 0.9203 |
| Hg | 48.8 | 0.11 | 0.9975 | 5.6 | 1.72 | 0.9387 |
Biosorption capacity
The biosorption capacity of the biomass is a major parameter as it detects how much biosorbent is necessary quantitatively23. 50.0 mL of 100.0 mg L−1 of Co(II) and Hg(II) solution at pH 5.0 and 4.0, respectively on 100.0 mg A. kestanboliensis loaded Amberlite XAD-4 were applied to the batch method. They were shaked at 120 rpm (2 g, radius of motor is 100 mm) for 120 min at 25 °C. Then the sorbent was separated at 10000 rpm (11200 g, radius of motor is 100 mm) for 10 min by centrifugation. The amounts of the Co(II) and Hg(II) in upper solution were directly determined. The pellet was digested in HNO3 before determination. The quantity of biosorbed Co(II) and Hg(II) was calculated using the equation given in literature38. The highest capacity of the thermophilic A. kestanboliensis loaded onto XAD-4 column was found to be 24.3 and 27.8 mg g−1 for Co(II) and Hg(II), respectively. The results demonstrated that the biosorption capacity of Co(II) and Hg(II) probably differ in size, level of hydration and their binding constant with the biosorbent39. In addition, results were utilized to Langmuir and Freundlich isotherms to define the mechanism of biosorption. Results are shown in Table 3. It was possible to conclude that biosorptions of Co(II) and Hg(II) on A. kestanboliensis loaded onto XAD-4 were mono-layer considering the high correlation coefficient of Langmuir isotherm.
Real sample analysis
By considering the results from the validation experiments, it was decided to apply the developed method to real environmental and food samples for Co(II) and Hg(II) preconcentrations before their determinations by ICP-OES. In order to demonstrate the usability of the procedure, tap water, river water, mineral water, tuna fish, tomato, potato, macaroni, cabbage, red lentil, black and green teas, mint, baby rice powder and biscuit, coffee, bovine liver, gluten free biscuit, meet, beef and soil samples were analyzed (Table 4). Tap water, mint and baby biscuit samples were also spiked with known concentrations of analyte. They were recovered quantitatively. Hg(II) concentrations in tuna fish, potato and soil were determined after preconcentrations procedure, while it was lower than LOD for other samples. Concentrations of Co(II) in samples varied from <LOD to 16.9 ± 1.4 mg kg−1.
Table 4.
Application of developed method for the preconcentrations of Co(II) and Hg(II).
| Samples | Co mg kg−1 | Hg mg kg−1 |
|---|---|---|
| NCSZC 73014, certified | 220 ± 20a | 3.8 ± 0.8a |
| NCSZC 73014, determined | 215 ± 26a | 3.8 ± 0.6a |
| DORM2, certified | 182 ± 31a | 4.64 ± 0.26 |
| DORM2, determined | 179 ± 40a | 4.58 ± 0.29 |
| NCS DC78301, certified | 16.5 | 0.22 |
| NCS DC78301, determined | 16.3 ± 1.1 | ± |
| Tap water | <LOD | <LOD |
| Tap waterb | 0.010 ± 0.001 | 0.009 ± 0.001 |
| Tigris River water | <LOD | <LOD |
| Mineral water | <LOD | <LOD |
| Tuna fish | 0.20 ± 0.02 | 0.026 ± 0.003 |
| Tomato | 0.66 ± 0.03 | <LOD |
| Potato | 0.65 ± 0.03 | 0.016 ± 0.001 |
| Macaroni | 0.044 ± 0.003 | <LOD |
| Cabbage | 0.66 ± 0.01 | <LOD |
| Red lentil | 0.14 ± 0.01 | <LOD |
| Green tea | 0.17 ± 0.01 | <LOD |
| Black tea | 1.6 ± 0.2 | <LOD |
| Mint | 0.051 ± 0.004 | <LOD |
| Mintb | 0.060 ± 0.003 | 0.009 ± 0.001 |
| Baby biscuit | 0.044 ± 0.002 | <LOD |
| Baby biscuitb | 0.053 ± 0.003 | 0.010 ± 0.001 |
| Baby rice powder | 0.069 ± 0.005 | <LOD |
| Coffee | 0.058 ± 0.004 | <LOD |
| Gluten free biscuit | 0.060 ± 0.005 | <LOD |
| Bovine liver | 0.86 ± 0.04 | <LOD |
| Meat | 0.083 ± 0.006 | <LOD |
| Beef | 0.069 ± 0.004 | <LOD |
| Soil | 16.9 ± 1.4 | 0.65 ± 0.03 |
ang g−1.
bSpiked with known amounts of Co(II) and Hg(II) to give final concentrations as 0.010 mg kg−.
Direct determinations of Co(II) and Hg(II) at ultra-trace levels in environmental and food samples are not possible by ICP-OES. Thus, the use of GF-AAS and/or ICP-MS is an obligation for this purpose. We contribute to overcome this problem by developing Co(II) and Hg(II) specific SPE method. As a brief discussion of obtained results we can clearly highlight that the sensitivity of conventional ICP-OES was improved and the use of above mentioned instruments were eliminated. Results from certified reference samples sign the accuracy of the method. We can recommend the routine usability of the developed method.
Conclusion
In this research paper, A. kestanboliensis was successfully loaded onto XAD-4, and used as SPE column material for the preconcentration and determinations of Co(II) and Hg(II). FT-IR and SEM analyzes were evaluated for surface characterization. The developed SPE column composed of bacteria loaded resin was used for over 35 cycles of biosorption/desorption without any loss in its biosorption behaviour. The developed method has much better features in comparison with literature due to high tolerance to foreign ions, too. Preconcentration factors were achieved as 80 for both of targeted metal ions. What we have achieved with this study is to critically increase the sensitivity of the ICP through the Co(II) and Hg(II). Hence low cost laboratories could use this environmentally friend method for routine analysis. The proposed method possesses lower LOD with a high preconcentration factor that makes it suitable for the preconcentrations of tested metals prior to their determinations by ICP-OES in real food samples.
Supplementary information
A Novel Biosorbent for Preconcentrations of Co(II) and Hg(II) in Real Samples.
Acknowledgements
This academic work was linguistically supported by the Mersin TTO Academic Writing Center of Mersin University, Turkey.
Author contributions
E.K., S.Ö. and F.S. participated in the study design and coordination, conducted experimental studies and prepared the manuscript. All authors have read and approved the last article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ersin Kılınç, Email: kilinersin@gmail.com.
Sen Fatih, Email: fatihsen1980@gmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-019-57401-y.
References
- 1.Khan M, et al. Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta. 2016;146:130–137. doi: 10.1016/j.talanta.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 2.Ramandi NF, Shemirani F. Selective ionic liquid ferrofluid based dispersive-solid phase extraction for simultaneous preconcentration/separation of lead and cadmium in milk and biological samples. Talanta. 2015;13:404–411. doi: 10.1016/j.talanta.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Xing A, Zhao K. Simultaneous determination of nickel, cobalt and mercury ions inwater samples by solid phase extraction using multiwalled carbon nanotubes as adsorbent after chelating with sodium diethyl dithiocarbamate prior to high performance liquid chromatography. J. Chromatogr A. 2014;1360:76–81. doi: 10.1016/j.chroma.2014.07.084. [DOI] [PubMed] [Google Scholar]
- 4.Ozdemir S, Kilinc E, Poli A, Nicolaus B, Guven K. Cd, Cu, Ni, Mn and Zn resistance and bioaccumulation by thermophilic bacteria, Geobacillus toebii sub sp. decanicus and Geobacillus thermoleovorans sub sp. stromboliensis. World J. Microbiol Biotechnol. 2012;28:155–163. doi: 10.1007/s11274-011-0804-5. [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir S, Kilinc E. Geobacillus thermoleovorans immobilized on Amberlite XAD-4 resin as a sorbent for solid phase extraction of uranium(VI) prior to its spectrophotometric determination. Microchim Acta. 2012;178:389–397. doi: 10.1007/s00604-012-0841-2. [DOI] [Google Scholar]
- 6.Stafiej A, Pyrzynska K. Solid phase extraction of metal ions using carbon nanotubes. Microchem J. 2008;89:29–33. doi: 10.1016/j.microc.2007.11.001. [DOI] [Google Scholar]
- 7.Zhu Xiang B, Cui Y, Chang X, Wang H. Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) Ions in wastewater using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta. 2016;146:358–363. doi: 10.1016/j.talanta.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 8.Sharma G, et al. Fabrication and characterization of sodium dodecyl sulphate@ironsilicophosphate nanocomposite: Ion exchange properties and selectivity for binary metal ions. Mat Chem and Physics. 2017;193:129–139. doi: 10.1016/j.matchemphys.2017.02.010. [DOI] [Google Scholar]
- 9.Kumar A, et al. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem Eng J. 2018;339:393–410. doi: 10.1016/j.cej.2018.01.105. [DOI] [Google Scholar]
- 10.Sharma G, et al. Applications of nanocomposite hydrogels for biomedical engineering and environmental protection. Environ Chem Letters. 2018;16:113–146. doi: 10.1007/s10311-017-0671-x. [DOI] [Google Scholar]
- 11.Sharma G, Naushad M, Pathania D, Kumar A. A multifunctional nanocomposite pectin thorium(IV) tungstomolybdate for heavy metal separation and photoremediation of malachite green. Desalin Water Treat. 2015;41:19443–19455. [Google Scholar]
- 12.Kvil YS, et al. Photocatalytic conversion of CO2 into methanol over Cu-C/TiO2 nanoparticles under UV light and natural sunlight. J. Photochem Photobiol A Chem. 2017;347:244–253. doi: 10.1016/j.jphotochem.2017.07.046. [DOI] [Google Scholar]
- 13.Sharma G, et al. Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J. Clean Prod. 2018;172:2919–2930. doi: 10.1016/j.jclepro.2017.11.122. [DOI] [Google Scholar]
- 14.Li Z, Li J, Wang Y, Wei Y. Synthesis and application of surface-imprinted activated carbon sorbent for solid-phase extraction and determination of copper(II) Spectrochim Acta A. 2014;117:422–427. doi: 10.1016/j.saa.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Liu F. Synthesis and application of ion-imprinted interpenetrating polymer network gel for selective solid phase extraction of Cd2+ Chem Eng J. 2014;242:117–126. doi: 10.1016/j.cej.2013.12.067. [DOI] [Google Scholar]
- 16.Nekouei S, Nekouei F, Tyagi I, Agarwal S, Gupta KV. Mixed cloud point/solid phase extraction of lead(II) and cadmium(II) in water samples using modified-ZnO nanopowders. Process Saf Environ. 2016;99:175–185. doi: 10.1016/j.psep.2015.11.005. [DOI] [Google Scholar]
- 17.Ozdemir S, Kilinc E, Erdogan S. Bacillus sp. immobilized on Amberlite XAD-4 resin as a biosorbent for solid phase extraction of thorium prior to UV–vis spectrometry determination. Microchim Acta. 2010;171:275–281. doi: 10.1007/s00604-010-0423-0. [DOI] [Google Scholar]
- 18.Sharmaa G, et al. Fabrication and characterization of Gum arabic-cl-poly(acrylamide) nanohydrogel for effective adsorption of crystal violet dye. Carbohydr Polym. 2018;202:444–453. doi: 10.1016/j.carbpol.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Sharmaa G, et al. Fabrication and characterization of chitosan-crosslinked-poly(alginic acid) nanohydrogel for adsorptive removal of Cr(VI) metal ion from aqueous medium. Int J. Biol Macromol. 2017;95:484–493. doi: 10.1016/j.ijbiomac.2016.11.072. [DOI] [PubMed] [Google Scholar]
- 20.Ghazaghi M, Mousavi HZ, Rashidi AM, Shirkhanloo H, Rahighi R. Graphene-silica hybrid inefficient preconcentration of heavy metal ions via novel single-step method of moderate centrifugation-assisted dispersive micro solid phase extraction. Talanta. 2016;150:476–484. doi: 10.1016/j.talanta.2015.12.074. [DOI] [PubMed] [Google Scholar]
- 21.Hoque MI, Chowdhury DA, Holze R, Chowdhury AN, Azam MS. Modification of Amberlite XAD-4 resin with 1,8-diaminonaphthalene for solid phase extraction of copper, cadmium and lead, and its application to determination of these metals in dairy cow’s milk. J. Environ Chem Eng. 2015;3:831–842. doi: 10.1016/j.jece.2015.03.020. [DOI] [Google Scholar]
- 22.Yahaya YA, Don MM. Pycnoporus sanguineus as Potential Biosorbent for Heavy Metal Removal from Aqueous Solution: A Review. J. Phys Sci. 2014;25:1–32. [Google Scholar]
- 23.Ozdemir S, Kilinc E, Poli A, Nicolaus B. Biosorption of heavy metals (Cd2+, Cu2+, Co2+ and Mn2+) by thermophilic bacteria, Geobacillus thermantarcticus and Anoxybacillus amylolyticus: equilibrium and kinetic studies. Bioremediat J. 2013;17:86–96. doi: 10.1080/10889868.2012.751961. [DOI] [Google Scholar]
- 24.Sharma G, et al. Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int J. Biol Macromol. 2018;114:295–305. doi: 10.1016/j.ijbiomac.2018.03.093. [DOI] [PubMed] [Google Scholar]
- 25.Sharma G, et al. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly(alginic acid-cl-acrylamide) nanohydrogel. Process Saf Environ Prot. 2017;109:301–310. doi: 10.1016/j.psep.2017.04.011. [DOI] [Google Scholar]
- 26.Ghaedi M, et al. Flame atomic absorption spectrometric determination of trace amounts of heavy metal ions after solid phase extraction using modified sodium dodecyl sulfate coated on alumina. J. Hazard Mater. 2008;155:121–127. doi: 10.1016/j.jhazmat.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J. Preparation, characterization and application of thiosemicarbazide grafted multiwalled carbon nanotubes for solid-phase extraction of Cd(II), Cu(II) and Pb(II) in environmental samples. J. Environm Sci. 2013;25:2331–2337. doi: 10.1016/S1001-0742(12)60329-5. [DOI] [PubMed] [Google Scholar]
- 28.Lemos VA, et al. Synthesis of amberlite XAD-2-PC resin for preconcentration and determination of trace elements in food samples by flame atomic absorption spectrometry. Microchem J. 2006;84:14–21. doi: 10.1016/j.microc.2006.03.006. [DOI] [Google Scholar]
- 29.Lemos VA, Santos LN, Bezerra MA. Determination of cobalt and manganese in food seasonings by flame atomic absorption spectrometry after preconcentration with 2-hydroxyacetophenone-functionalized polyurethane foam. J. Food Compos Anal. 2010;23:277–281. doi: 10.1016/j.jfca.2009.11.004. [DOI] [Google Scholar]
- 30.Marahel F, et al. Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem Toxicol. 2011;49:208–214. doi: 10.1016/j.fct.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Turan Ş, Tokalıoğlu Ş, Şahan A, Soykan C. Synthesis, characterization and application of a chelating resin for solid phase extraction of some trace metal ions from water, sediment and tea samples. React Funct Polym. 2012;72:722–728. doi: 10.1016/j.reactfunctpolym.2012.07.002. [DOI] [Google Scholar]
- 32.Beiraghi A, Babaee S, Roshdi M. Simultaneous preconcentration of cadmium, cobalt and nickel in water samples by cationic micellar precipitation and their determination by inductively coupled plasma-optical emission spectrometry. Microchem J. 2012;100:66–71. doi: 10.1016/j.microc.2011.09.003. [DOI] [Google Scholar]
- 33.Yang C, Sheng L, Kaiju W, Yangzhong L, Zhangjun H. Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim Acta. 2015;182:1337–1344. doi: 10.1007/s00604-015-1452-5. [DOI] [Google Scholar]
- 34.Mohammadi SZ, Roohparvar R, Taher MA. Simultaneous Separation Preconcentration and Determination of Trace Amounts of Mercury and Cadmium in Fruits, Vegetables and Biological Samples. J. Anal Chem. 2016;71:42–49. doi: 10.1134/S106193481601007X. [DOI] [Google Scholar]
- 35.Shoaee H, Roshdi M, Khanlarzadeh N, Beiraghi A. Simultaneous preconcentration of copper and mercury in water samples by cloud point extraction and their determination by inductively coupled plasma atomic emission spectrometry. Spectrochim Acta A. 2012;98:70–75. doi: 10.1016/j.saa.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 36.Abolhasani J, et al. Determination of Hg(II) ions in sea food samples after extraction and preconcentration by novel Fe3O4@SiO2@polythiophene magnetic nanocomposite. Environ Monit Assess. 2015;187:1–11. doi: 10.1007/s10661-014-4167-x. [DOI] [PubMed] [Google Scholar]
- 37.Tayyebeh M, Borhan Z, Mazaher A, Abbas A. Selective extraction and sensitive determination of mercury(II) ions by flame atomic absorption spectrometry after preconcentration on an ion-imprinted polymer-coated maghemite nanoparticles. J. Iran Chem Soc. 2015;12:1235–1243. doi: 10.1007/s13738-015-0587-y. [DOI] [Google Scholar]
- 38.Ozdemir S, Kilinc E, Poli A, Nicolaus B, Guven K. Biosorption of Cd, Cu, Ni, Mn and Zn from aqueous solutions by thermophilic bacteria, Geobacillus toebii subsp. decanicus and Geobacillus thermoleovorans subsp. stromboliensis: Equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2009;152:195–206. doi: 10.1016/j.cej.2009.04.041. [DOI] [Google Scholar]
- 39.Nie R, Chang X, He Q, Hu Z, Li Z. Preparation of p-tert[(dimethylamino)methyl]-calix[4]arene functionalized amino propylpolysiloxane resin for selective solid-phase extraction and preconcentration of metal ions. J. Hazar Mater. 2009;169:203–209. doi: 10.1016/j.jhazmat.2009.03.084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Novel Biosorbent for Preconcentrations of Co(II) and Hg(II) in Real Samples.


