Abstract
Type IV collagen (Col IV) is an important protein in the basement membrane (BM), which can regulate the attachment of epithelium and the lamina propria. To test the hypothesis that Col IV may play a significant role in the progression of oral leukoplakia, we have observed the morphology and distribution of Col IV in 40 patients with oral leukoplakia (epithelial hyperplasia and dysplasia). The results showed that the expression of Col IV in the BM was continuous in normal oral mucosa, while it was non-continuous or fragmented in epithelial dysplasia by immunofluorescent staining. Consequently, to analyze the correlated factors of the Col IV destruction, the expressions of gelatinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinases -1 (TIMP-1) were detected in oral leukoplakia tissues by immunochemical staining. Then statistical analyses were performed to determine their correlations with Col IV levels. The statistics showed that the decreasing of Col IV was closely related to the increasing of MMP-9 in both epithelial hyperplasia and dysplasia (P < 0.05), while MMP-2 expression was closely related to the increasing of Col IV expression in epithelial dysplasia (P < 0.05). Therefore, monitoring changes in the expression of Col IV, gelatinase and TIMP-1 is a useful technique for assessing prognosis of oral leukoplakia.
Keywords: Oral leukoplakia, Col IV, gelatinase, TIMP-1, prognosis
Introduction
Oral leukoplakia (OLK) is a predominantly white patch or plaque and represents a potentially precancerous lesion in oral mucosa, which cannot be characterized by any other definable lesion or known disease (WHO) [1-4]. Based on the clinical and pathological criteria, it is classified into epithelial hyperplasia and dysplasia [3]. The presence of dysplasia is a predicting malignant transformation factor for oral squamous cell carcinoma (OSCC) with the fragment of type IV collagen (Col IV) in basement membranes (BM) [5]. Most attention has been paid to the development of OSCC. However, there is still no consensus on the most appropriate diagnosis and management of oral leukoplakia [6]. The aims of this study include the evaluation of the potential factors associated with the genesis and development of oral leukoplakia.
Matrix metalloproteinases (MMPs) and their inhibitors are known to be associated with invasive lesions and the genesis of carcinomas through the extracellular matrix remodeling. However, little is known about their role in precancerous lesions, such as oral leukoplakia. During the progression of epithelial dysplasia into carcinoma, the destruction of basement membrane is a critical event [7,8]. MMPs are thought to constitute an important mechanism for the destruction of Col IV in the BM [9]. Gelatinase, one member of MMPs family, is divided into gelatinase A (the 72 kDa, MMP-2) and gelatinase B (the 92 kDa, MMP-9). They can degrade extracellular matrix and type IV collagen in BM [10,11]. The tissue inhibitors of metalloproteinases-1 (the 28.5 kDa nonglycosylated protein TIMP-1), provides a stable level of anti-MMP activity in tissues [7,9]. It could forms a 1:1 complex with all activated MMPs, including gelatinase [12].
Therefore, research on the gelatinase that mediate in the development of oral leukoplakia and the distribution or morphology of Col IV in basement BM is very necessary. In this study, the expression of gelatinase and TIMP-1, and the changes in the morphology of Col IV were investigated. Then their relationship during the progression of oral leukoplakia was analyzed to determine whether these results can be used to assess the prognosis of of oral leukoplakia disease.
Material and methods
Patients
We collected 40 tissues samples from oral leukoplakia (20 for epithelial hyperplasia and 20 for epithelial dysplasia) patients diagnosed and treated at the Department of Oral Medicine, Peking University School of Stomatology (Beijing, China). 20 tissue samples of normal tissues were obtained from oral mucosa adjacent to the mucous cyst or trauma. Clinical and pathological diagnoses were made according to modified WHO diagnostic criteria for oral leukoplakia [1-3,13]. All participants were informed with consent. The study was approved by the Peking University Biomedical Institutional Review Board (IRB00001052-12007).
Histological and immunochemical analysis
Samples were fixed with 4% formalin and embedded. They were sectioned at 4 μm thickness and stained with H&E staining for diagnosis. Sections adjacent to the H&E staining were deparaffinized with xylene and then rehydrated in graded ethanol. 3% hydrogen peroxide was used to block the endogenous peroxidase activity for 20 min. After sections were treated with heated antigen retrieval solution, 5% bovine serum albumin (BSA) was applied to inhibit non-specific antigens for 15 min. The tissue section was then incubated with primary antibodies overnight in a humidified chamber at 4°C respectively. For immunofluorescent studies, sections were incubated overnight with primary antibody mouse anti-human Col IV (1:200, Abcam, Cambridge, MA, USA), followed by Rhodamine-conjugated secondary antibodies (Bioworld Technology). Then samples were observed under a confocal laser scanning microscope (LSM 5, Carl Zeiss, Oberkochen, Germany). For immunohistochemical analysis, sections were incubated overnight with primary antibody rabbit anti-human MMP-2 (1:100), MMP-9 (1:100), TIMP-1 (1:200, Bioworld Technology, St. Louis Park, MN, USA). Immunohistochemical staining was performed by the universal two-step method. Subsequently, they were treated with the corresponding secondary antibody (PV6000, Zhongshan Goldenbridge Biotechnology, Beijing, China) for 30 min at 37°C. Then the antibody reaction was visualized using diaminobenzidine (DAB) chromogen (Zhongshan Goldenbridge Biotechnology). All slides were counterstained with hematoxylin. Sections were incubated with immunoglobulins of the same species at the same final concentrations as negative controls, and oral squamous cell carcinoma tissues were used as positive controls.
Evaluation of immunochemical results
All collected samples were reviewed by two independent investigators who were blinded to the clinical manifestation of the patients. Compared to normal tissue, the basement membrane alterations were evaluated as follows: Grade 1: N, normal (well-defined borderline with thin linear staining); Grade 2: TBM, thickened basement membrane; Grade 3: G, gaps in the continuity of the basement membrane; Grade 4: DBM, disrupted basement membrane [14-16]. Based on previous studies, the grading of staining intensities observed in the epithelial and mesenchymal tissues was on a scale of 0 to 3 as follows: 0, 0-25%, negative; 1, 25-50%, mild; 2, 50-75%, moderate and 3, 75-100%, strong [1,4,14,17].
Statistical analysis
The statistical analysis was performed by using SPSS software version 13.0. The statistics of expression of Col IV, MMP-2, MMP-9 and TIMP-1 in normal oral mucosa, epithelial hyperplasia and epithelial dysplastic were described as the mean ± standard deviation. The associations between immunochemical results and clinic-pathologic parameters were analyzed by using χ2 test with the chi-square or Fisher’s exact test. Pearson Correlation coefficient test was applied for examining the correlations among the expressions of MMP-2, MMP-9, TIMP-1 and Col IV. P-values < 0.05 were regarded to be statistically significant.
Results
Histological analysis
The clinical manifestation of oral leukoplakia was presented as a white patch or plaque (Figure 1B), which is different from normal oral mucosa (Figure 1A). According to histopathological criteria, the number of epithelial cells were increasing in the spinous layer and basal layer in epithelial hyperplasia (Figure 1D). Epithelial cell atypia and the stratification disorder were shown in epithelial dysplasia (Figure 1E), compared to normal oral mucosa (Figure 1C).
Figure 1.
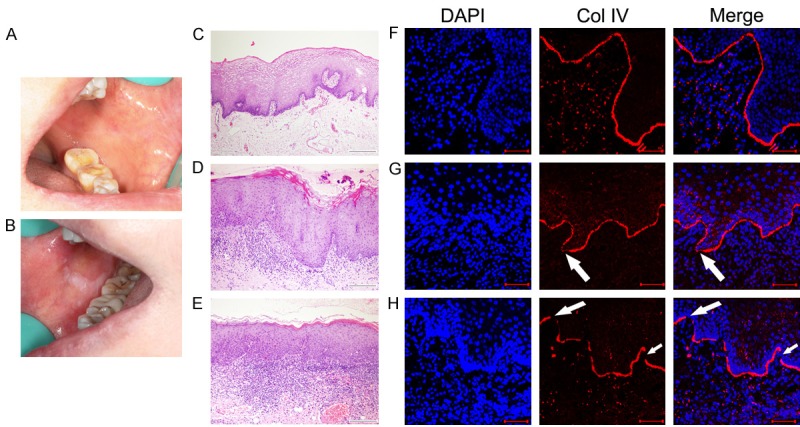
Clinical manifestations, histopathological features and expressions type IV collagen (Col IV) in normal oral mucosa and oral leukoplakia. Clinical manifestations of normal oral mucosa (A) and oral leukoplakia (B). Histopathologic features of normal oral mucosa (C) epithelial hyperplasia (D) and epithelial dysplasia (E) by H&E staining. Col IV expression in the basement membrane (BM), continuous in normal oral mucosa (F), gaps in epithelial hyperplasia (white arrows in G) and discontinuity in epithelial dysplasia (white arrows in H) by immunofluorescent staining. Scale bar = 100 um.
Immunochemical analysis
Col IV presented as a continuous linear structure below basal cells in most normal oral mucosa (Figure 1F). Association between Col IV expression and clinic-pathological parameters was shown in Table 1. Col IV in epithelial hyperplasia was observed as thickened BM (35%) or with gaps in the continuity (15%) of the BM (Figure 1G). No BM fragmented either in normal oral mucosa or epithelial hyperplasia. However, the expression of Col IV in the BM was thickened, with gaps in the continuity and non-continuous or fragmented (Figure 1H) in epithelial dysplasia (70%).
Table 1.
Association between Col IV expression and clinic-pathological parameters
| Col IV | Grade 1 | Grade 2 | Grade 3 | Grade 4 | p |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 47.23±15.39 | 53.67±11.34 | 56.57±12.45 | 46.67±7.04 | 0.283 |
| Gender (%) | |||||
| Male | 16 (53.33%) | 6 (20%) | 4 (13.33%) | 4 (13.33%) | 0.982 |
| Female | 17 (56.67%) | 6 (20%) | 4 (13.33%) | 3 (10%) | |
| Sites (%) | |||||
| Tongue | 11 (42.3%) | 6 (23.08%) | 5 (19.23%) | 4 (15.38%) | 0.283 |
| Buccal and/or labial | 22 (66.67%) | 5 (15.15%) | 3 (9.09%) | 3 (9.09%) | |
| Others | 0 | 1 | 0 | 0 | |
| Parameters | |||||
| Oral mucosa (%) | 17 (85%) | 2 (10%) | 1 (5%) | 0 | < 0.01 |
| Epithelial hyperplasia (%) | 10 (50%) | 7 (35%) | 3 (15%) | 0 (%) | |
| Epithelial dysplasia (%) | 6 (30%) | 3 (15%) | 4 (20%) | 7 (35%) |
Positive expression of MMP-2 was observed in the cytoplasm of proliferating epithelial cells and in mesenchymal fibroblasts and microvascular endothelial in normal oral mucosa (Figure 2A), epithelial hyperplasia (Figure 2D) and epithelial dysplasia (Figure 2G). Association between MMP-2 expression and clinic-pathological parameters was shown in Table 2. The expression of MMP-2 in the epithelial and mesenchymal cells of normal oral mucosa was negative or weak positive (35%). Its expression was increased in epithelial hyperplasia (50%) and epithelial dysplasia (80%).
Figure 2.

Comparative immunolocalization of MMP-2, MMP-9 and TIMP-1 (E and S indicate the epithelium and subepithelial mesenchyme respectively). The expression of MMP-2, MMP-9 and TIMP-1 in normal oral mucosal epithelium and subepithelial mesenchyme were weak positive (A-C). MMP-2 expression was mainly located in the cytoplasm of mesenchymal cells and proliferating epithelial cells in epithelial hyperplasia and epithelial dysplasia (D and G). MMP-9 expression was increasing in epithelial hyperplasia and epithelial dysplasia, mainly in proliferating epithelial cells and mesenchymal cells around the blood vessels (asterisks in E and H). TIMP-1 expression are scattered in the cytoplasm of mesenchymal cells and epithelial cells in epithelial hyperplasia and epithelial dysplasia (F and I).
Table 2.
Association between MMP-2 expression and clinic-pathological parameters
| MMP-2 | Score 0 | Score 1 | Score 2 | Score 3 | p |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 46.56±15.594 | 51.18±11.98 | 46.3311±11.6 | 52.25±11.25 | 0.537 |
| Gender (%) | |||||
| Male | 10 (33.33%) | 8 (26.67%) | 7 (23.33%) | 5 (16.67%) | |
| Female | 15 (50%) | 3 (10%) | 5 (16.67%) | 7 (23.33%) | 0.296 |
| Sites (%) | |||||
| Tongue | 10 (38.46%) | 5 (19.23%) | 5 (19.23%) | 6 (23.08%) | |
| Buccal and/or labial | 14 (43.75%) | 5 (15.63%) | 7 (21.88%) | 6 (18.75%) | 0.96 |
| Others | 1 (50%) | 1 (50%) | 0 | 0 | |
| Parameters | |||||
| Oral mucosa (%) | 13 (65%) | 2 (10%) | 3 (15%) | 2 (10%) | |
| Epithelial hyperplasia (%) | 10 (50%) | 4 (20%) | 3 (15%) | 3 (15%) | 0.136 |
| Epithelial dysplasia (%) | 4 (20%) | 4 (20%) | 5 (25%) | 7 (35%) |
Positive expression of MMP-9 was observed in the cytoplasm of proliferating epithelial cells and in mesenchymal fibroblasts and microvascular endothelial in normal oral mucosa (Figure 2B), epithelial hyperplasia (Figure 2E) and epithelial dysplasia (Figure 2H). Association between MMP-9 expression and clinic-pathological parameters was shown in Table 3. The expression of MMP-9 in the epithelial and mesenchymal cells of normal oral mucosa was negative or weak positive (30%). Its expression was increased in epithelial hyperplasia (45%) and epithelial dysplasia (75%). The expression of MMP-9 in mesenchyme was mainly around the blood vessels.
Table 3.
Association between MMP-9 expression and clinic-pathological parameters
| MMP-9 | Score 0 | Score 1 | Score 2 | Score 3 | p |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 47.73±15.51 | 47.75±11.68 | 48.36±12.49 | 53.29±7.27 | 0.801 |
| Gender (%) | |||||
| Male | 14 (46.67%) | 7 (23.33%) | 4 (13.33%) | 5 (16.67%) | 0.496 |
| Female | 16 (53.33%) | 5 (16.67%) | 7 (23.33%) | 2 (6.67%) | |
| Sites (%) | |||||
| Tongue | 13 (54.17%) | 5 (20.83%) | 4 (16.67%) | 2 (8.33%) | 0.105 |
| Buccal and/or labial | 16 (47.06%) | 6 (17.65%) | 7 (20.59%) | 5 (14.71%) | |
| Others | 1 (50%) | 0 | 1 (50%) | 0 | |
| Parameters | |||||
| Oral mucosa (%) | 14 (70%) | 3 (15%) | 3 (15%) | 0 | 0.048 |
| Epithelial hyperplasia (%) | 11 (55%) | 5 (25%) | 2 (10%) | 2 (10%) | |
| Epithelial dysplasia (%) | 5 (25%) | 4 (20%) | 6 (30%) | 5 (25%) |
Positive expression of TIMP-1 was scattered in the cytoplasm of epithelial and mesenchymal cells in normal oral mucosa (Figure 2C), epithelial hyperplasia (Figure 2F) and epithelial dysplasia (Figure 2I). Association between TIMP-1 expression and clinic-pathological parameters was shown in Table 4. The expression of TIMP-1 in the epithelial and mesenchymal cells of normal oral mucosa was negative or weak positive (30%). Its expression was increased in epithelial hyperplasia (40%) and epithelial dysplasia (55%).
Table 4.
Association between TIMP-1 expression and clinic-pathological parameters
| TIMP-1 | Score 0 | Score 1 | Score 2 | Score 3 | p |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean ± SD | 48.69±14.56 | 48.67±11.28 | 47.25±13.41 | 48.8±12.83 | 0.994 |
| Gender (%) | |||||
| Male | 15 (50%) | 7 (23.33%) | 3 (10%) | 5 (16.67%) | 0.091 |
| Female | 20 (66.67%) | 5 (16.67%) | 5 (16.67%) | 0 | |
| Sites (%) | |||||
| Tongue | 13 (50%) | 6 (23.08%) | 4 (15.38%) | 3 (11.54%) | 0.928 |
| Buccal and/or labial | 20 (62.5%) | 6 (18.75%) | 4 (12.5%) | 2 (6.25%) | |
| Others | 2 | 0 | 0 | 0 | |
| Parameters | |||||
| Oral mucosa (%) | 14 (70%) | 4 (20%) | 2 (10%) | 0 | 0.58 |
| Epithelial hyperplasia (%) | 12 (60%) | 4 (20%) | 2 (10%) | 2 (10%) | |
| Epithelial dysplasia (%) | 9 (45%) | 4 (20%) | 4 (20%) | 3 (15%) |
Association between the expression of MMP-2, MMP-9, TIMP-1 and Col IV Pearson correlation coefficient analysis revealed that the expression of MMP-9 was negatively correlated with Col IV expression in epithelial hyperplasia (P = 0.024, Figure 3B). The expression of MMP-2 and TIMP-1 was not correlated with Col IV expression in epithelial hyperplasia (P = 0.255 and P = 0.937, respectively; Figure 3A and 3C). The expression of MMP-2 and MMP-9 were negatively correlated with Col IV expression in epithelial dysplasia (P = 0.026 and P = 0.003, respectively; Figure 3D and 3E). There was no correlation between the expression of Col IV and TIMP-1 in epithelial dysplasia (p=0.717; Figure 3F).
Figure 3.

Association between expressions of MMP-2, MMP-9, TIMP-1 and Col IV in patients with oral leukoplakia by Pearson correlation test. The association between the expression of Col IV with MMP-2 (A), MMP-9 (B) or TIMP-1 (C) in epithelial hyperplasia. The association between the expression of Col IV with MMP-2 (D), MMP-9 (E) or TIMP-1 (F) in epithelial dysplasia. r represents the coefficient of correlation. P < 0.05 was regarded to be statistically.
Discussion
Col IV attached to the BM can effectively prevent harmful substances penetrating to the lamina propria and restrain pathological stimulating factors acting on oral mucosa [18-20]. Previous study showed when Col IV reduced, fragmented and dissolved completely, cancer cells could invade to the lamina propria in oral squamous cell carcinoma (OSCC) [1,4], and about 17% to 35% of the oral leukoplakia cases could undergo malignant transformation into OSCC [2,21,22]. Therefore, we conducted this study to detect the integrity of Col IV in oral leukoplakia. We found that Col IV gradually reduced, fragmented or collapsed from epithelial hyperplasia to epithelial dysplasia with the development of oral leukoplakia. The reduction of Col IV may be an implication that the destruction of BM in the phase of oral leukoplakia. Thus it is necessary to analyze the cause for Col IV distribution in the BM of this disease.
MMPs play a direct role in the evolution and promotion modulation of the growth of primary tumor, accompany with the destruction of BM [23,24]. Gelatinase (MMP-2 and MMP-9) expression has been reported in various carcinoma [25,26]. To our knowledge, the clinical significance of MMP-2 and MMP-9 in the development of oral leukoplakia has not been demonstrated. Thus in this study, the expressions of MMP-2 and MMP-9 were evaluated in 40 oral leukoplakia tissue samples. They were observed in the cytoplasm and plasma membrane of proliferating epithelial cells, as well as in the mesenchymal cells. We found that MMP-2 expression was not correlated to Col IV expression in epithelial hyperplasia, while it was negatively correlated to Col IV in epithelial dysplasia. MMP-9 expression was negatively correlated to Col IV expression in epithelial hyperplasia and dysplasia.
Mesenchymal cells can act as ‘promoters’, with cancer cells as the ‘initiators’ of carcinogenesis [27-29]. The expression of MMP-2 and MMP-9 has been reported to be localized in the cytoplasm of tumor and stromal cells in carcinoma [1,4,30]. It’s necessary to pay an attention to the changes of MMP-2 and MMP-9 in precancerous lesions, which may be a risk factor in the progression of oral leukoplakia. MMP-9 has also been shown the implication for the invasion of cancer cells in previous research [31]. Taken together, with the negative correlation of MMP-9 and Col IV expression in epithelial hyperplasia and dysplasia, the results suggest that MMP-9 expression may be an early event in oral leukoplakia.
The effects of MMPs are regulated by the activities of the tissue inhibitors of metalloproteinases (TIMPs) [7,32,33]. TIMP-1 could form a 1:1 complex with all activated MMPs and with pro-MMP-9 [12]. Recent reports suggested that TIMP-1 primarily complexed with MMP-9 [34]. As the negative correlation of MMP-9 and Col IV expression was in both types of oral leukoplakia, we selected TIMP-1 to study. We found the expression of TIMP-1 was not correlated with Col IV expression neither in epithelial hyperplasia nor epithelial dysplasia. TIMPs could provide a stable level of anti-MMP activity in the extracellular matrix [32,34]. Their functions were depending on their concentration and the presence of extracellular matrix [35]. Therefore, it suggested that increasing TIMP-1 in oral leukoplakia may be helpful for preventing the destruction of gelatinase for BM in some aspect. However, in-depth studies are still warranted to determine the role of MMPs and their inhibitors in the genesis and development of oral leukoplakia.
Conclusions
In this study, the expression of Col IV in the BM of oral leukoplakia was found different from that in the normal oral mucosal tissues, particularly it was non-continuous or fragmented in epithelial dysplasia. Meanwhile there were more expressions of gelatinase (MMP-2 and MMP-9) in the oral leukoplakia than that in the normal oral mucosal tissues, which implied that gelatinase played an important role in the genesis and progression of oral leukoplakia. Specifically, the increased levels of gelatinase was related to the Col IV destruction of BM in epithelial dysplasia. It was found that the decreasing of Col IV was closely related to the increasing of MMP-9 in both epithelial hyperplasia and dysplasia, while MMP-2 expression was closely related to the increasing of Col IV expression in epithelial dysplasia. Thus monitoring changes in the expression of Col IV, gelatinase and TIMP-1 is a useful technique for assessing prognosis of oral leukoplakia. However, specific mechanisms of the Col IV, gelatinase and TIMP-1 actions merit further investigate and ascertain, and they will be adressed in our future research work.
Acknowledgements
This work was supported by Beijing Natural Science Foundation (7164310). We thank School of Stomatology, Peking University for providing the tissue samples and experimental environment.
Disclosure of conflict of interest
None.
References
- 1.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–580. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 2.Reibel J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 3.Pindborg JJ, Daftary DK, Mehta FS. A follow-up study of sixty-one oral dysplastic precancerous lesions in Indian villagers. Oral Surg Oral Med Oral Pathol. 1977;43:383–390. doi: 10.1016/0030-4220(77)90325-5. [DOI] [PubMed] [Google Scholar]
- 4.Fan HX, Li HX, Chen D, Gao ZX, Zheng JH. Changes in the expression of MMP2, MMP9, and Col IV in stromal cells in oral squamous tongue cell carcinoma: relationships and prognostic implications. J Exp Clin Cancer Res. 2012;31:90. doi: 10.1186/1756-9966-31-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenghoefer M, Pantelis A, Najafi T, Deschner J, Allam JP, Novak N, Reich R, Martini M, Berge S, Fischer HP, Jepsen S, Winter J. Gene expression of oncogenes, antimicrobial peptides, and cytokines in the development of oral leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:351–356. doi: 10.1016/j.tripleo.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Brouns E, Baart J, Karagozoglu K, Aartman I, Bloemena E, van der Waal I. Malignant transformation of oral leukoplakia in a welldefined cohort of 144 patients. Oral Dis. 2014;20:e19–e24. doi: 10.1111/odi.12095. [DOI] [PubMed] [Google Scholar]
- 7.Emmert-Buck MR, Emonard HP, Corcoran ML, Krutzsch HC, Foidart JM, Stetler-Stevenson WG. Cell surface binding of TIMP-2 and pro-MMP-2/TIMP-2 complex. Febs Lett. 1995;364:28–32. doi: 10.1016/0014-5793(95)00345-a. [DOI] [PubMed] [Google Scholar]
- 8.Bosman FT. The borderline: basement membranes and the transition from premalignant to malignant neoplasia. Microsc Res Tech. 1994;28:216–225. doi: 10.1002/jemt.1070280306. [DOI] [PubMed] [Google Scholar]
- 9.Yang JS, Lin CW, Su SC, Yang SF. Pharmacodynamic considerations in the use of matrix metalloproteinase inhibitors in cancer treatment. Expert Opin Drug Metab Toxicol. 2016;12:191–200. doi: 10.1517/17425255.2016.1131820. [DOI] [PubMed] [Google Scholar]
- 10.Xia Z, Liu W, Li S, Jia G, Zhang Y, Li C, Ma Z, Tian J, Gong J. Expression of matrix metalloproteinase-9, type IV collagen and vascular endothelial growth factor in adamantinous craniopharyngioma. Neurochem Res. 2011;36:2346–2351. doi: 10.1007/s11064-011-0560-9. [DOI] [PubMed] [Google Scholar]
- 11.Nomura H, Fujimoto N, Seiki M, Mai M, Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69:9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg GI, Collier IE, Eisen AZ, Grant GA, Marmer BL, Wilhelm SM. Mosaic structure of the secreted ECM metalloproteases and interaction of the type IV collagenases with inhibitors. Matrix Suppl. 1992;1:25–30. [PubMed] [Google Scholar]
- 13.Abadie WM, Partington EJ, Fowler CB, Schmalbach CE. Optimal management of proliferative verrucous leukoplakia: a systematic review of the literature. Otolaryngol Head Neck Surg. 2015;153:504–511. doi: 10.1177/0194599815586779. [DOI] [PubMed] [Google Scholar]
- 14.Galateau-Salle FB, Luna RE, Horiba K, Sheppard MN, Hayashi T, Fleming MV, Colby TV, Bennett W, Harris CC, Stetler-Stevenson WG, Liotta L, Ferrans VJ, Travis WD. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in bronchial squamous preinvasive lesions. Hum Pathol. 2000;31:296–305. doi: 10.1016/s0046-8177(00)80242-7. [DOI] [PubMed] [Google Scholar]
- 15.Thorup AK, Reibel J, Schiodt M, Stenersen TC, Therkildsen MH, Carter WG, Dabelsteen E. Can alterations in integrin and laminin-5 expression be used as markers of malignancy? Apmis. 1998;106:1170–1180. doi: 10.1111/j.1699-0463.1998.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 16.Le BP, Piloquet P, Daniel A, Giumelli B. Immunohistochemical localization of type IV collagen and laminin (alpha1) in denture stomatitis. J Oral Pathol Med. 2001;30:98–103. doi: 10.1034/j.1600-0714.2001.300206.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990;21:607–612. doi: 10.1016/s0046-8177(96)90006-4. [DOI] [PubMed] [Google Scholar]
- 18.Krecicki T, Zalesska-Krecicka M, Jelen M, Szkudlarek T, Horobiowska M. Expression of type IV collagen and matrix metalloproteinase-2 (type IV collagenase) in relation to nodal status in laryngeal cancer. Clin Otolaryngol Allied Sci. 2001;26:469–472. doi: 10.1046/j.0307-7772.2001.00503.x. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Garcia A, Abad-Hernandez MM, Fonseca-Sanchez E, Julian-Gonzalez R, Galindo-Villardon P, Cruz-Hernandez JJ, Bullon-Sopelana A. E-cadherin, laminin and collagen IV expression in the evolution from dysplasia to oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2006;11:E100–E105. [PubMed] [Google Scholar]
- 20.Bar JK, Grelewski P, Popiela A, Noga L, Rabczynski J. Type IV collagen and CD44v6 expression in benign, malignant primary and metastatic ovarian tumors: correlation with Ki-67 and p53 immunoreactivity. Gynecol Oncol. 2004;95:23–31. doi: 10.1016/j.ygyno.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Thomson PJ, Goodson ML, Cocks K, Turner JE. Interventional laser surgery for oral potentially malignant disorders: a longitudinal patient cohort study. Int J Oral Maxillofac Surg. 2017;46:337–342. doi: 10.1016/j.ijom.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 22.van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–323. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Jakubowska K, Pryczynicz A, Januszewska J, Sidorkiewicz I, Kemona A, Niewiński A, Lewczuk Ł, Kędra B, Guzińska-Ustymowicz K. Expressions of matrix metalloproteinases 2, 7, and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis Markers. 2016;2016:9895721. doi: 10.1155/2016/9895721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen PS, Zhai WR, Zhou XM, Zhang JS, Zhang YE, Ling YQ, Gu YH. Effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cells. World J Gastroenterol. 2001;7:647–651. doi: 10.3748/wjg.v7.i5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–344. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A. La mala educacion of tumor-associated macrophages: diverse pathways and new players. Cancer Cell. 2010;17:111–112. doi: 10.1016/j.ccr.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Koyama H, Iwata H, Kuwabara Y, Iwase H, Kobayashi S, Fujii Y. Gelatinolytic activity of matrix metalloproteinase-2 and -9 in oesophageal carcinoma; a study using in situ zymography. Eur J Cancer. 2000;36:2164–2170. doi: 10.1016/s0959-8049(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki T, Kuwabara Y, Iwata H, Mitani M, Shinoda N, Sato A, Mitsui A, Sugiura M, Kato J, Fujii Y. Role of matrix metalloproteinase-9 in in vitro invasion of esophageal carcinoma cells. J Surg Oncol. 2002;81:80–86. doi: 10.1002/jso.10134. [DOI] [PubMed] [Google Scholar]
- 32.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 33.Corcoran ML, Hewitt RE, Kleiner DE, Stetler-Stevenson WG. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49:7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Dai H, Ding K, Xu X, Pang B, Wang C. Rapamycin attenuates bleomycin-induced pulmonary fibrosis in rats and the expression of metalloproteinase-9 and tissue inhibitors of metalloproteinase-1 in lung tissue. Chin Med J (Engl) 2014;127:1304–1309. [PubMed] [Google Scholar]
- 35.Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]


