Abstract
Background: β-catenin and E-cadherin are adhesion molecules that promote metastatic potential through epithelial-mesenchymal transition (EMT). Although they have not been extensively studied in gastric cancers, they represent potential testable prognostic markers. Methods: We explored the association between the immunohistochemical expression of these markers and clinicopathologic parameters by retrospectively reviewing 205 cases of gastric cancer from tissue microarrays (TMA). A method was developed to evaluate for membranous staining of β-catenin and E-cadherin using grading criteria that characterized both the intensity of staining and the percentage of cells with loss of staining. Results: Weak membranous expression of E-cadherin and β-catenin were associated with worse overall survival (p<0.05). Abnormal expression of E-cadherin and β-catenin were significantly associated with each other (p<0.01). Loss of and/or weak membranous staining for both E-cadherin and β-catenin was significantly associated with advanced cancer stage T2-T4 (versus stage T1, p<0.05) and tumors that are negative for H pylori infection (p<0.05). In addition, loss of and/or weak membranous staining for β-catenin was significantly associated with poorly differentiated tumors (p<0.05), diffuse Lauren-type gastric tissue (p=0.02), and tumors with a significantly higher rate of lymphovascular invasion (p=0.02). Conclusion: Loss of/weak membranous expression of both E-cadherin and β-catenin was associated with poorer overall survival rates and clinicopathologic parameters that indicated an aggressive clinical behavior. β-catenin shows significant associations with more clinical parameters, making it a better biomarker than E-cadherin. In our multivariate analysis, weak intensity of staining of β-catenin was an independent prognostic factor for survival and may be a useful immunohistochemical prognostic marker for patients with gastric cancer.
Keywords: β-catenin, E-cadherin, gastric cancer, clinicopathology
Introduction
Gastric cancer is a major health issue, the fourth most common cancer, and the second leading cause of cancer death worldwide [1]. Early gastric cancers are often asymptomatic, and the patients typically have advanced incurable diseases at the time of presentation. The overall five year survival rate of patients with resectable gastric cancer is 32%, which is comparatively low to other malignancies [2]. Most patients die of a metastatic disease or recurrence even after surgical intervention, radiation and chemotherapy. The ability of the tumor to metastasize and to have invasive growth is a major impediment to effective treatment. The epithelial-mesenchymal transition (EMT) is the process in which epithelial cells lose cell polarity from the disappearance of differentiated junctions and instead gain mesenchymal phenotypic traits [3]. This process is normally seen in embryonic cells but is reactivated in cancers, enabling invasive growth and metastatic potential. Once the cells lose their junctions, they are able to disseminate locally or through the bloodstream and lymphatic system [4,5].
E-cadherin is a transmembrane glycoprotein that is expressed in normal gastric mucosal cells, where it mediates cell-to-cell adhesion and suppresses the cell’s ability to invade [4]. However, during carcinogenesis, the factor can be downregulated or inactivated, leading to redistribution of E-cadherin from the cell membrane to the cytoplasm [6]. Loss or downregulation of membranous E-cadherin is a major event during EMT and potentiates the cancer cell to have invasive growth and to metastasize. E-cadherin repression has been analyzed in several carcinomas, including those of colorectum [7], pancreas [8], bladder [9], prostate [10], endometrial, and breast where it has been associated with a more aggressive tumor phenotype.
β-catenin is a cytoplasmic protein that directly interacts with E-cadherin and plays a critical role in the E-cadherin mediated adhesion [11]. Truncation of the β-catenin protein has been reported to cause loss of E-cadherin-dependent intercellular adhesiveness [12]. In vitro, when β-catenin changes its expression pattern from membranous to cytoplasmic, a similar change is seen in E-cadherin localization, resulting in cell-cell junctional disorders and potential for metastasis [12]. Futhermore, immunohistochemical analysis of E-cadherin and β-catenin has demonstrated that abnormal E-cadherin and β-catenin expression is closely associated with metastasis of gastric carcinoma.
The goal of our study was to explore the association between the immunohistochemical expression patterns of these EMT markers and clinicopathologic parameters, including survival in patients with gastric cancer.
Methods
Patient selection
We retrospectively reviewed 205 cases of gastric cancer patients who were previously diagnosed at The University of Texas MD Anderson Cancer Center. There were 84 female patients and 121 male patients, with an overall mean age of 61 years (range 26 to 89 years) at the time of diagnosis. Of the 205 patients, 87 had moderately differentiated tumors and 118 had poorly differentiated tumors; 43 had early gastric cancer (T1), and 162 had advanced gastric cancer (T2-T4). Complete demographic and clinical data were collected. This study was approved by the Institutional Review Board of the University of Texas M.D. Anderson Cancer Center.
Tissue microarray construction
Tissue microarrays were constructed using formalin-fixed, paraffin-embedded archival tissue blocks with a tissue microarrayer (Beecher Instruments, Sun Prairie, WI) [13]. Their matching hematoxylin and eosin-stained (H&E) slides were retrieved, reviewed, and screened for representative tumor regions. For each patient, three cores of tumor were sampled from representative areas using a 1.0-mm punch.
Immunohistochemical analysis
Immunohistochemical stains were performed on 4-µm unstained sections from the tissue microarray blocks using primary mouse monoclonal antibodies as follows: anti-E-cadherin (1:7000 dilution, HECD-1, Invitrogen, Carlsbad, CA, USA) and anti-β-catenin (1:1500 dilution, 14, BD Biosciences, San Jose, CA, USA). Antigen retrieval was performed on the tissue sections at 100°C in a steamer containing either Tris-EDTA buffer for 20 minutes (E-cadherin) or Citrate buffer for 10 min (β-catenin) after deparaffinization. The sections were then incubated with the primary antibody at 35°C for 15 min. Subsequently, they were immersed in 3.0% hydrogen peroxidase at 35°C for 5 min to block the endogenous peroxidase activity. A primary enhancer solution was then applied to the slides and incubated at 35°C for 8 minutes. The sections were then incubated with secondary anti-mouse immunoglobulin at 35°C for 8 min. Diaminobenzidine (DAB) was used as a chromogen and hematoxylin was used for counterstaining. The immunohistochemically stained slides of tissue microarrays were examined using standard light microscopy (Olympus BX40, Tokyo, Japan) with the appropriate positive and negative controls.
Scoring of immunohistochemical stains
The staining results were scored semiquantitatively by a pathologist (M.X.), who was blinded to the clinicopathologic data. The immunohistochemical results showed a heterogeneous pattern of staining in some tumors where there was only very focal strong staining or moderate but diffuse staining as shown in Figure 1. Therefore, slides were evaluated for membranous staining using two methods to account for the heterogeneity of the tumors and to more precisely characterize the staining patterns. The first method was a system similar to a HercepTest, and the second method was a test based on the percentage of cells with loss of membranous staining.
Figure 1.
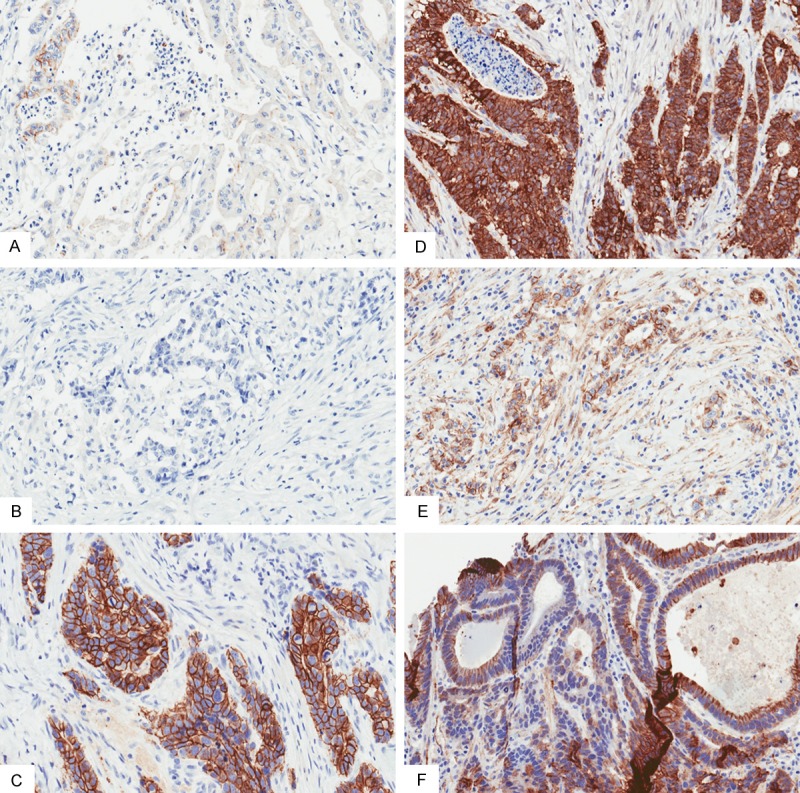
E-cadherin (A-C) and beta-catenin (D-F) staining patterns in a variety of gastric cancers showing staining heterogeneity (20× magnification). (A, B) Examples of tumors with loss of membranous staining in 10% of cells and weak intensity of staining, (C, D) Examples of preserved membranous staining with normal intensity of staining, (E, F) Note the heterogeneity in the staining of some tumors with weak intensity of staining throughout but no areas with loss of staining (E), while other tumors have focal strong intensity of staining but with large areas with loss of membranous staining (F).
When grading the intensity of staining, we used a system similar to the HercepTest modified for gastric cancer [14,15]. Normal intensity of staining was considered as strong, complete membranous staining or basolateral staining similar to a HercepTest result of 3+. Weak intensity of staining was considered as none, faint or moderate, incomplete membranous or incomplete basolateral staining similar a HercepTest result of 0-2+. Cases with strong but incomplete membranous or basolateral staining were considered to have weak intensity of staining.
The slides were then reevaluated based on the percentage of cells with loss of membranous staining. Cases considered to have no preserved membranous staining had >90% cells with evidence of at least trace membranous staining (this includes both complete and incomplete membranous and basolateral staining). Cases that were considered to have loss of membranous staining had ≥10% cells with complete negative membranous staining. The same grading criterion was used for both E-cadherin and β-catenin.
Statistical analysis
The patients’ follow-up information was extracted from the medical records. We associated the abnormal expression of E-cadherin and β-catenin with age (≤60 vs >60), sex, tumor depth (T1 vs T2-T4), histologic type (well to moderate vs. poor), Lauren type gastric tissue (intestinal vs. diffuse vs. mixed), presence of H pylori infection, lymphovascular invasion, lymph node metastasis, and distal metastasis. We also obtained information on the patients’ overall survival rates. The recurrence information was updated whenever patients came to the clinic for a follow-up visit.
Chi-square analysis or Fisher’s exact tests were used to compare categorical data. The survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. Overall survival (OS) was calculated as the time from the date of diagnosis to patient death or last follow-up. The prognostic significance of clinicopathologic characteristics was determined using multivariate Cox proportional hazards regression analysis. All statistical analyses were performed using Statistical Analysis System 9.1 software (for Windows, SAS Institute, Cary, North Carolina). We used a two-sided significance level of 0.05 for all statistical analyses.
Results
Abnormal expression of E-cadherin and β-catenin in gastric cancer
Weak membranous expression of E-cadherin and β-catenin was seen in 159 (77.6%) and 106 (51.7%) patients, respectively. In addition, when separately looking at tumors where ≥10% of cells had loss of membranous staining, 176 (85.9%) and 169 (82.4%) patients had loss of staining with E-cadherin and β-catenin respectively. When abnormal expression of E-cadherin was associated with abnormal expression of β-catenin, the results were significant both in terms of intensity and loss of membranous staining as shown in Tables 1 and 2 (p<0.01).
Table 1.
Association between E-cadherin and β-catenin expression intensity in gastric tumors
| β-catenin expression intensity | E-cadherin expression intensity | ||
|---|---|---|---|
|
| |||
| Weak | Normal | p-value | |
| Weak | 99 | 7 | <0.01 |
| Normal | 60 | 39 | |
Table 2.
Association between E-cadherin and β-catenin loss of membranous staining in gastric tumors
| β-catenin membranous staining | E-cadherin membranous staining | ||
|---|---|---|---|
|
| |||
| Loss | Preserved | p-value | |
| Loss | 158 | 11 | <0.01 |
| Preserved | 18 | 18 | |
Clinicopathologic association of E-cadherin and β-catenin in gastric cancer
Loss of and/or weak membranous staining for both E-cadherin and β-catenin was significantly associated with advanced cancer stage T2-T4 (versus stage T1, p<0.05) and tumors that are negative for H pylori infection (p<0.05). In addition, loss of and/or weak membranous staining for β-catenin was significantly associated with poorly differentiated tumors (p<0.05), diffuse Lauren-type gastric tissue (p=0.02), and tumors with a significant higher rate of lymphovascular invasion (p=0.02). We found no additional association of the abnormal expression of E-cadherin and β-catenin with any other clinicopathologic parameters including age, sex, lymph node and distal metastasis (p>0.05). This information is presented in Tables 3 and 4 with significant findings summarized in Table 5.
Table 3.
Association between multiple clinicopathological factors and expression of both E-cadherin intensity of staining as well as loss of membranous staining in gastric tumors
| Clinicopathologic factor | Intensity of E-cadherin staining [cases (%)] | p value | Membranous E-cadherin staining [cases (%)] | p value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Weak | Normal | Loss | Preserved | |||
| Total | 159 | 46 | 176 | 29 | ||
| Age | ||||||
| ≤60 | 62 (39.0) | 20 (43.5) | 0.61 | 74 (42.1) | 8 (27.6) | 0.16 |
| >60 | 97 (61.0) | 26 (56.5) | 102 (58.0) | 21 (72.4) | ||
| Gender | ||||||
| Male | 97 (61.0) | 24 (52.2) | 0.31 | 105 (59.7) | 16 (55.2) | 0.69 |
| Female | 62 (39.0) | 22 (47.8) | 71 (40.3) | 13 (44.8) | ||
| Tumor Depth | ||||||
| Early cancer (T1) | 28 (17.6) | 15 (32.6) | 0.04 | 32 (18.2) | 11 (38.0) | 0.02 |
| Advanced cancer (T2-T4) | 131 (82.4) | 31 (67.4) | 144 (81.8) | 18 (62.1) | ||
| Histologic Type | ||||||
| Well to moderate | 67 (42.1) | 20 (43.5) | 0.86 | 72 (41.0) | 15 (51.7) | 0.31 |
| Poor and others | 92 (57.9) | 26 (56.5) | 104 (59.1) | 14 (48.3) | ||
| Lauren Type | ||||||
| Intestinal | 55 (34.6) | 17 (37.0) | 0.86 | 59 (33.5) | 13 (44.8) | 0.49 |
| Mixed | 14 (8.8) | 3 (6.5) | 15 (8.5) | 2 (6.9) | ||
| Diffuse | 90 (56.6) | 26 (56.5) | 102 (58.0) | 14 (48.3) | ||
| H pylori infection | ||||||
| Negative | 155 (97.5) | 40 (87.0) | 0.01 | 168 (95.5) | 27 (93.1) | 0.64 |
| Positive | 4 (2.5) | 6 (13.0) | 8 (4.6) | 2 (6.9) | ||
| Lymphovascular Invasion | ||||||
| Negative | 44 (27.7) | 10 (21.7) | 0.45 | 49 (27.8) | 5 (17.2) | 0.26 |
| Positive | 115 (72.3) | 36 (78.3) | 127 (72.2) | 24 (82.8) | ||
| Lymph Node Metastasis | ||||||
| Negative | 40 (25.2) | 14 (30.4) | 0.57 | 44 (25.0) | 10 (34.5) | 0.36 |
| Positive | 119 (74.8) | 32 (69.6) | 132 (75.0) | 19 (65.5) | ||
| Distal Metastasis | ||||||
| Negative | 129 (81.1) | 40 (87.0) | 0.51 | 144 (81.8) | 25 (86.2) | 0.8 |
| Positive | 30 (18.9) | 6 (13.0) | 32 (18.2) | 4 (13.8) | ||
Table 4.
Association between multiple clinicopathological factors and expression of both β-catenin staining intensity and loss of staining in gastric tumors
| Clinicopathologic factor | Intensity of β-catenin staining [cases (%)] | p-value | Membranous β-catenin staining [cases (%)] | p-value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Weak | Normal | Loss | Preserved | |||
| Total | 106 | 99 | 169 | 36 | ||
| Age | ||||||
| ≤60 | 48 (45.3) | 34 (34.3) | 0.12 | 73 (43.2) | 9 (25.0) | 0.06 |
| >60 | 58 (54.7) | 65 (65.7) | 96 (56.8) | 27 (75.0) | ||
| Gender | ||||||
| Male | 68 (64.2) | 53 (53.5) | 0.16 | 100 (59.2) | 21 (58.3) | 1 |
| Female | 38 (35.9) | 46 (46.5) | 69 (40.8) | 15 (41.7) | ||
| Tumor Depth | ||||||
| Early cancer (T1) | 13 (12.3) | 30 (30.3) | 0.001 | 29 (17.2) | 14 (38.9) | 0.006 |
| Advanced cancer (T2-T4) | 93 (87.7) | 69 (69.7) | 140 (82.8) | 22 (61.1) | ||
| Histologic Type | ||||||
| Well to moderate | 37 (35.0) | 50 (50.5) | 0.03 | 64 (37.9) | 23 (63.9) | 0.005 |
| Poor and others | 69 (65.1) | 49 (49.5) | 105 (62.1) | 13 (36.1) | ||
| Lauren Type | ||||||
| Intestinal | 31 (29.3) | 41 (41.4) | 0.13 | 53 (31.4) | 19 (52.8) | 0.02 |
| Mixed | 8 (7.6) | 9 (9.1) | 13 (7.7) | 4 (11.1) | ||
| Diffuse | 67 (63.2) | 49 (49.5) | 103 (61.0) | 13 (36.1) | ||
| H pylori infection | ||||||
| Negative | 105 (99.0) | 90 (90.9) | 0.008 | 161 (95.3) | 34 (94.4) | 0.69 |
| Positive | 1 (0.9) | 9 (9.1) | 8 (4.7) | 2 (5.6) | ||
| Lymphovascular Invasion | ||||||
| Negative | 20 (18.9) | 34 (34.3) | 0.02 | 42 (24.9) | 12 (33.3) | 0.3 |
| Positive | 86 (81.1) | 65 (65.7) | 127 (75.2) | 24 (66.7) | ||
| Lymph Node Metastasis | ||||||
| Negative | 22 (20.8) | 32 (32.3) | 0.08 | 42 (24.9) | 12 (33.3) | 0.3 |
| Positive | 84 (79.3) | 67 (67.7) | 127 (75.2) | 24 (66.7) | ||
| Distal Metastasis | ||||||
| Negative | 84 (79.3) | 85 (85.9) | 0.27 | 137 (81.0) | 32 (88.9) | 0.34 |
| Positive | 22 (20.8) | 14 (14.1) | 32 (19.0) | 4 (11.1) | ||
Table 5.
Summary of significant associations between abnormal E-cadherin and β-catenin membranous expression and clinicopathologic parameters
| Staining Patterns | Clinicopathologic Factors |
|---|---|
| Weak β-catenin intensity | Advanced Cancer (T2-T4) > Early Cancer (T1) |
| Poorly Differentiated > Well or Moderate Differentiated | |
| Negative Helicobacter pylori Infection | |
| Lymphovascular Invasion | |
| Loss of β-catenin staining | Advanced Cancer (T2-T4) > Early Cancer (T1) |
| Poorly Differentiated > Well or Moderate Differentiated | |
| Diffuse Lauren Type > Intestinal or Mixed | |
| Weak E-cadherin intensity | Advanced Cancer (T2-T4) > Early Cancer (T1) |
| Negative Helicobacter pylori Infection | |
| Loss of E-cadherin staining | Advanced Cancer (T2-T4) > Early Cancer (T1) |
Survival analysis of E-cadherin and β-catenin in gastric cancer
Patients with tumors that had either weak membranous expression of E-cadherin or weak membranous expression of β-catenin had worse overall survival (p<0.05). In addition, loss of membranous staining of β-catenin was also associated with poorer overall survival (p<0.05). There was a trend towards poorer overall survival in patients with loss of membranous staining of E-cadherin; however, this was not statistically significant (p>0.05). The Kaplan-Meier curves for overall survival comparing patients with normal expression with those with abnormal expression of E-cadherin and β-catenin are shown in Figure 2. We then performed a multivariate analysis as shown in Table 6. The results showed that weak β-catenin expression was an independent predictor of short survival (p<0.05).
Figure 2.
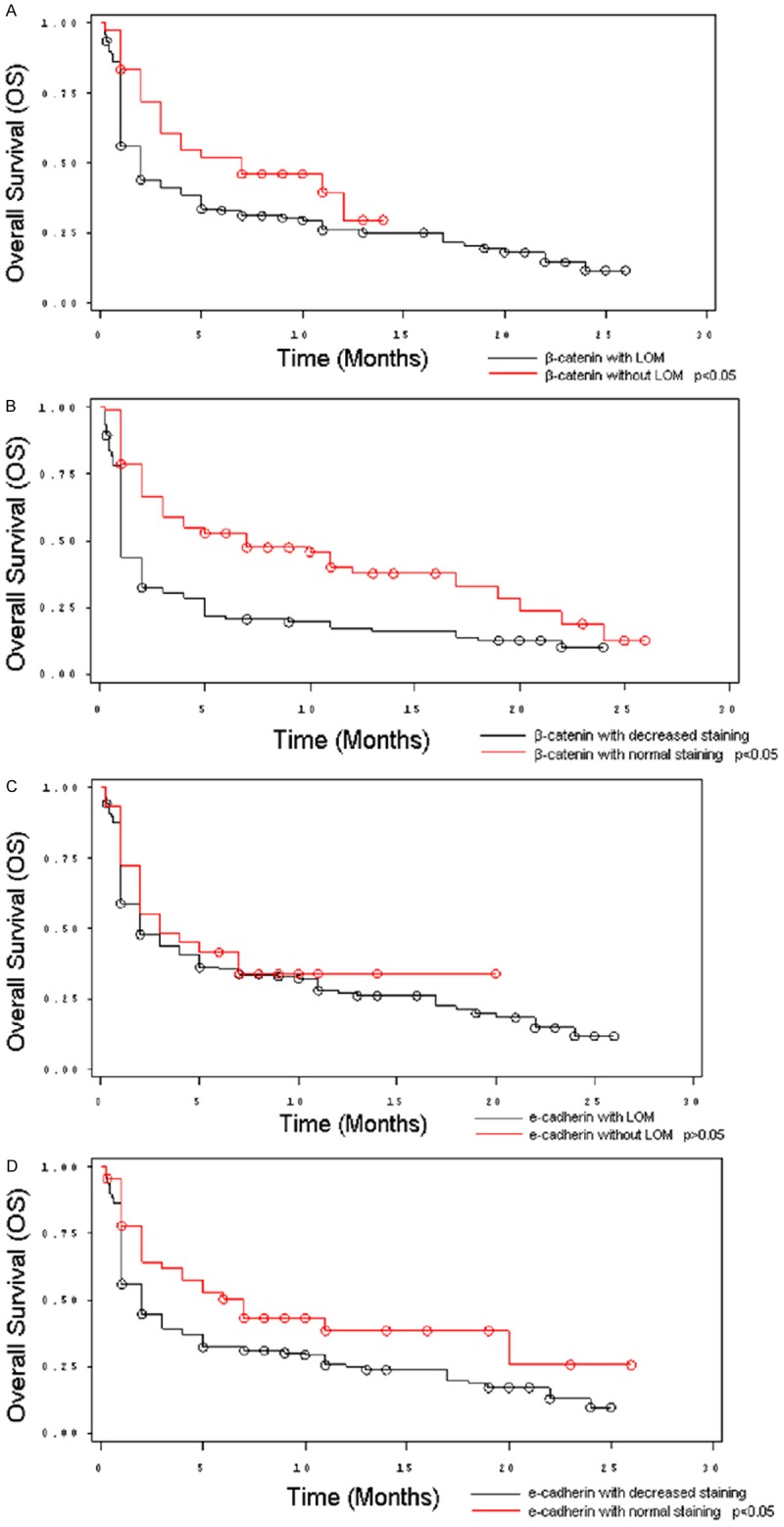
Kaplan-Meier Curves showing the overall survival of 205 gastric cancer patients based on E-cadherin and β-catenin expression patterns. A. Curve shows a significant association between loss of β-catenin staining and worse overall survival. B. Curve shows a significant association between weak β-catenin and worse overall survival. C. Curve shows a trend towards worse overall survival with loss of E-cadherin staining. D. Curve shows a significant association between weak E-cadherin staining and worse overall survival.
Table 6.
Multivariate analysis with respect to overall survival (OS) in gastric cancer
| Parameter | Hazard Ratio | p-value |
|---|---|---|
| β-catenin intensity | ||
| Normal | 0.706 | 0.017 |
| Weak | 1.000 | |
| Gender | ||
| Female | 0.745 | 0.048 |
| Male | 1.000 | |
| Tumor Depth | ||
| Early cancer (T1) | 1.000 | 0.013 |
| Advanced cancer (T2-T4) | 1.612 | |
| Lymphovascular Invasion | ||
| Negative | 1.000 | 0.006 |
| Positive | 1.617 | |
| Distal Metastasis | ||
| Negative | 1.000 | 0.046 |
| Positive | 1.468 |
β-catenin intensity is an independent prognostic factor for these patients.
Discussion
The clinical significance of EMT has been reported in many diverse human tumors, where it enables invasive growth and increases metastatic potential [12]. Several studies have evaluated EMT phenotype in metastatic sites where EMT-derived migratory cells that established colonies at distant metastatic sites were shown to lose the mesenchymal phenotype via mesenchymal-epithelial transition (MET) during secondary tumor formation [5,16,17]. In addition, EMT has been linked to cancer progression and poorer patient survival through mechanisms such as evasion of apoptosis, resistance to chemotherapy, and acquisition of stem cell-like properties [18-21].
E-cadherin and β-catenin are important markers of EMT. In our study, we attempted to characterize the expression of these markers in gastric cancers. Initially, the grading was based on a combined grading system incorporating both the intensity of staining and loss of staining, with 10% cell staining representing the cutoff between 0 staining and 1-3+ staining. However, we felt that due to the large amount of heterogeneity in the tumor samples, separating the grading for intensity from the percentage of cells with staining was important. We therefore devised two separate grading systems.
In our study, 77.6% and 51.7% of patients had weak expression of E-cadherin and β-catenin respectively. In addition, 85.9% and 82.4% of patients had loss of membranous staining of E-cadherin and β-catenin respectively. This number may appear to be elevated, which is likely due to the fact that MD Anderson is a secondary referral center for patients who tend to have more aggressive tumors. Nevertheless, we found that a significant proportion of patients had abnormal expression of these markers.
There is strong evidence that abnormal expression of both E-cadherin and β-catenin are significantly correlated with EMT and malignant phenotype in gastric cancer cell lines in vitro [22-25]. However, to the best of our knowledge, there are only two published studies on the association between survival and the immunohistochemical expression pattern of these markers in fixed tissue, with conflicting results [26,27]. In the study from Jawhari et al. [26], 89 gastric cancer cases were evaluated, and the retention of membranous expression of β-catenin was significantly associated with a survival advantage (p<0.05). However, although the E-cadherin staining pattern showed a trend toward worse survival, it was not statistically significant (p>0.05). In the study from Yoon et al. [27], 251 cases were examined, and no association was found between survival and the expression of β-catenin and E-cadherin.
The results of our study are similar to the results from Jawhari et al. [26], as we found a significant association between weak intensity of staining of β-catenin and E-cadherin with poorer overall survival. In addition, loss of membranous staining of β-catenin was significantly associated with poorer overall survival (p<0.05), and a trend towards poorer overall survival was observed when evaluating for loss of membranous staining of E-cadherin (p>0.05). Our study showed that weak membranous expression of β-catenin appeared to be the best marker of survival compared to E-cadherin as our multivariate analysis indicated that it was an independent prognostic factor. Although there was a strong association between abnormal expression levels of E-cadherin and β-catenin, (p<0.05), our data suggests that β-catenin may be important in the process of EMT through other mechanisms other E-cadherin mediated adhesion.
Association of abnormal membranous staining of E-cadherin and β-catenin with other clinicopathologic parameters is also poorly understood with a limited number of published articles [26-32]. We found a significant association of abnormal expression of either β-catenin and/or E-cadherin with advanced stage cancers, poorly differentiated tumors, diffuse Lauren-type gastric tissue, lymphovascular invasion, and tumors with negative H pylori infection status. An important observation was that abnormal expression of E-cadherin and β-catenin was more frequently seen in tumors with a diffuse morphology. This further supports the theory that the E-cadherin-β-catenin complex is essential in mediating cell adhesion. It is reasonable to postulate that with abnormal membranous expression of these proteins, the gastric epithelium becomes disorganized and loses its normal glandular architecture, and that EMT might be a key process involved in the development of diffuse gastric adenocarcinomas. Since H pylori infection is mostly associated with intestinal type adenocarcinomas, this can present as a confounding variable in our examination of the H pylori infection status in our patients. Nevertheless, given our results, it is likely that tumorigenesis by H pylori (chronic atrophic gastritis leading to the formation of intestinal type adenocarcinomas) does not depend on changes in expression of E-cadherin-β-catenin complex.
Conclusion
In summary, loss of and/or weak membranous expression of both E-cadherin and β-catenin may be associated with poorer overall survival and clinicopathologic parameters that indicate a more aggressive clinical behavior. β-catenin appears to be a better biomarker than E-cadherin, because it shows a significant association with more clinical parameters. In our multivariate analysis, the intensity of staining of β-catenin was an independent prognostic factor for survival. It may be a useful immunohistochemical prognostic marker for patients with gastric cancer.
Disclosure of conflict of interest
None.
Authors’ contribution
DFT and MX participated in the conception of the study, research design, study management, review of the pathology slides, data interpretation and manuscript writing. YX was involved in the data collection, analysis and data interpretation. LZ, SR, CT, JL, GL, DZ were involved in the provision of study materials. All authors contributed to the drafting of the manuscript and all authors have read and approved the final manuscript.
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Msika S, Benhamiche AM, Jouve JL, Rat P, Faivre J. Prognostic factors after curative resection for gastric cancer. A population-based study. Eur J Cancer. 2000;36:390–396. doi: 10.1016/s0959-8049(99)00308-1. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Vleminckx K, Vakaet L, Mareel M, Fiers W, van Roy F. Genetic manipulation of e-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 5.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Nawrocki B, Polette M, Van Hengel J, Tournier JM, Van Roy F, Birembault P. Cytoplasmic redistribution of e-cadherin-catenin adhesion complex is associated with down-regulated tyrosine phosphorylation of e-cadherin in human bronchopulmonary carcinomas. Am J Pathol. 1998;153:1521–1530. doi: 10.1016/s0002-9440(10)65740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigam AK, Savage FJ, Boulos PB, Stamp GW, Liu D, Pignatelli M. Loss of cell-cell and cellmatrix adhesion molecules in colorectal cancer. Br J Cancer. 1993;68:507–514. doi: 10.1038/bjc.1993.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Klöppel G, Lemoine NR. Loss of membranous e-cadherin expression in pancreatic cancer: Correlation with lymph node metastasis, high grade, and advanced stage. J Pathol. 1994;174:243–248. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 9.Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA. Decreased e-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res. 1993;53:3241–3245. [PubMed] [Google Scholar]
- 10.Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, Debruyne FM, Isaacs WB. Expression of the cellular adhesion molecule e-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104–5109. [PubMed] [Google Scholar]
- 11.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherincatenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 12.Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F, et al. A truncated betacatenin disrupts the interaction between E-cadherin and alpha-catenin: a cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res. 1994;54:6282–6287. [PubMed] [Google Scholar]
- 13.Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allred DC. Issues and updates: Evaluating estrogen receptor-alpha, progesterone receptor, and her2 in breast cancer. Mod Pathol. 2010;23(Suppl 2):S52–59. doi: 10.1038/modpathol.2010.55. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a her2 scoring system for gastric cancer: Results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 16.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 20.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The emt-activator zeb1 promotes tumorigenicity by repressing stemness-inhibiting micrornas. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 21.Gal A, Sjöblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained tgf beta exposure suppresses smad and non-smad signalling in mammary epithelial cells, leading to emt and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 22.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- 23.Gayther SA, Gorringe KL, Ramus SJ, Huntsman D, Roviello F, Grehan N, Machado JC, Pinto E, Seruca R, Halling K, MacLeod P, Powell SM, Jackson CE, Ponder BA, Caldas C. Identification of germ-line E-cadherin mutations in gastric cancer families of european origin. Cancer Res. 1998;58:4086–4089. [PubMed] [Google Scholar]
- 24.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 25.Grady WM, Willis J, Guilford PJ, Dunbier AK, Toro TT, Lynch H, Wiesner G, Ferguson K, Eng C, Park JG, Kim SJ, Markowitz S. Methylation of the cdh1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 26.Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing MJ. Abnormal immunoreactivity of the e-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997;112:46–54. doi: 10.1016/s0016-5085(97)70218-x. [DOI] [PubMed] [Google Scholar]
- 27.Yoon CS, Hyung WJ, Lee JH, Chae YS, Won NH, Yeom BW, Choi JS. Expression of s100a4, e-cadherin, alpha- and beta-catenin in gastric adenocarcinoma. Hepatogastroenterology. 2008;55:1916–1920. [PubMed] [Google Scholar]
- 28.Matsuura K, Kawanishi J, Fujii S, Kobayashi O, Kameda Y, Ohkawa S. Altered expression of e-cadherin in gastric cancer tissues and carcinomatous fluid. Br J Cancer. 1992;66:1122–1130. doi: 10.1038/bjc.1992.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micu G, Stăniceanu F, Zurac S, Bastian A, Gramadă E, Popp C, Andrei R, Tudorică L, Slavnea A, Olariu M, Tebeică T, Ene A, Mateescu R, Rimbaş M, Voiosu R. E-cadherin and betacatenin expression in gastric neoplastic and non-neoplastic lesions--correlations with H. pylori infection. Rom J Intern Med. 2010;48:271–280. [PubMed] [Google Scholar]
- 30.Ougolkov A, Yamashita K, Bilim V, Takahashi Y, Mai M, Minamoto T. Abnormal expression of E-cadherin, beta-catenin, and c-erbb-2 in advanced gastric cancer: its association with liver metastasis. Int J Colorectal Dis. 2003;18:160–166. doi: 10.1007/s00384-002-0427-2. [DOI] [PubMed] [Google Scholar]
- 31.Yoshii T, Miyagi Y, Nakamura Y, Kobayashi O, Kameda Y, Ohkawa S. Pilot research for the correlation between the expression pattern of e-cadherin-β-catenin complex and lymph node metastasis in early gastric cancer. Tumori. 2013;99:234–238. doi: 10.1177/030089161309900219. [DOI] [PubMed] [Google Scholar]
- 32.Joo YE, Rew JS, Choi SK, Bom HS, Park CS, Kim SJ. Expression of e-cadherin and catenins in early gastric cancer. J Clin Gastroenterol. 2002;35:35–42. doi: 10.1097/00004836-200207000-00009. [DOI] [PubMed] [Google Scholar]


