Abstract
Purpose: To explore the effects of HMGB1 silence on cell apoptosis, inflammatory response and endothelial permeability barrier. Methods: Retinal tissues were isolated from 8 week-old SD rats and cells were cultured and identified. Effects of HMGB1 silence were detected by qRT-PCR and Western blot. Proliferation capability of cells was detected by MTT assay and LDH activity assays. Cell apoptosis was analyzed by flow cytometry, Hoechst staining and Caspase-3 activity assay. Furthermore, concentrations of VEGF, ICAM-1, VCAM-1, TNF-α and MCP-1 in the cell media were measured. Results: Results of our study showed that high concentration of glucose caused increased cell apoptosis and inflammatory response, and also influenced the endothelial permeability barrier. Whereas, these damaging effects of high concentration of glucose could be relieved by HMGB1 silence. Conclusion: The present study indicates that HMGB silence is a promising therapeutic option for diabetic retinopathy, and also provides theoretical basis for further exploration of diabetic retinopathy treatment.
Keywords: High mobility group box-1, retinal endotheliocyte injury, high concentration of glucose, MAPK, NF-κB, diabetic retinopathy
Introduction
Diabetes is a kind of metabolic diseases characterized by high blood sugar, due to the lack of insulin or insulin resistance. During diabetes, Long-termed high blood sugar caused injuries in a variety of tissues, especially in heart, kidney, eyes and nervous system in diabetes patients. Diabetic retinopathy is regarded as a disease of the retinal microvasculature as well as a consequence of vascular cell damage [1]. Diabetic retinopathy has been one of the leading causes of blindness worldwide and a serious threat to human vision [2].
High mobility group box-1 (HMGB1) is the most abundant in all HMG family members. HMGB1 is extremely conserved during evolution [3] and has a close relationship with proliferation, migration, invasion and differentiation of cells [4-7]. HMBG1 is also associated with various kinds of diseases, such as, diabetes, cancer and autoimmune diseases [8-10]. HMGB1 plays important roles both intracellularly and extracellularly. Intracellular HMGB1 acts as a DNA chaperon regulating a serious of gene events [11]. Extracellular HMGB1 is secreted by inflammatory cells or released by dead or injured cells [12]. HMGB1 is a multifunctional cytokine; it binds to receptors and regulates several vital cellular processes such as involving inflammation, immunity, antimicrobial and tissue regeneration [13].
HMGB1 has been reported to be a closely relationship related with diabetes pathogenesis [14]. Oxidative stress caused by diabetes hyperglycemia promotes HMGB1 expression [15] and loss of HMGB1 receptor inhibits diabetes onset [16]. HMGB1 also plays important roles in diabetic retinopathy [17] and is accepted to act as an alarming biomarker. HMGB1 is reported to be expressed highly in retina and vitreous fluid of diabetes [18]; however, whether silence of HMGB1 has effects on diabetic retinopathy is still unclear. In this study, we explored whether silence of HMGB1 by shRNA could attenuate injury of retina endotheliocyte induced by high concentration of glucose.
Materials and methods
Cell isolation, culture and identification
Eight week-old SD rats were purchased from the Laboratory Animal Center of China Medical University (Shenyang, China) and their retinal tissues were isolated in sterile condition. After washing with phosphate buffer saline (PBS), retinal tissues were cut into small pieces and re-suspended in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA). After passing through 200 mesh sieves, cells were cultured in DMEM media containing 10% FBS in a humidified atmosphere at 37°C with 5% CO2. The isolated cells were identified by immunofluorescence (IF) with antibody against CD31 (1:50, Santa Cruz, Dallas, TX, USA) and immunocytochemistry (ICC) with antibody against Factor VIII (1:100, Bioss, Beijing, China). All animal experiments were approved by China Medical University Animal Care and Use Committee.
Infection
Recombinant lentivirus containing shRNA for HMGB1 (Table 1) was purchased from Hanbio (Shanghai, China). 1×105 cells were seeded in 6-well plates. When cells grew to 70% confluence, suitable DMEM media containing lentivirus was added into each well according to the protocol. After incubation for 24 h, the media was changed to fresh DMEM media. The infection efficiency was detected by quantitative real time PCR and Western blot.
Table 1.
Sequence for shRNA (5’-3’)
| Forward Primer | Reverse Primer | |
|---|---|---|
| shRNA | GATCCCCGAAGCACCCGGATGCTTCTTTCAAGAGAAGAAGCATCCGGGTGCTTCTTTTT | AGCTAAAAAGAAGCACCCGGATGCTTCTTCTCTTGAAAGAAGCATCCGGGTGCTTCGGG |
| NC | GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT | AGCTAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG |
Groups
High concentration of glucose medium contained 30 mM glucose. Control media contained 5 mM glucose and 25 mM mannitol. Cells were divided into five groups: Group A, cells without infection were cultured in control media; Group B, cells without infection were cultured in high concentration of glucose media; Group C, cells infected with negative control (NC) for shRNA were cultured in high concentration of glucose media; Group D, cells infected with shRNA were cultured in control media; Group E, cells infected with shRNA were cultured in high concentration of glucose media. After culture in different media for 24 h, cells were harvested by centrifugation for the subsequent experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from each sample using RNA Simple Total RNA Kit (TIANGEN, Beijing, China) and reverse transcribed to cDNA using Super M-MLV (BioTeke, Beijing, China) and oligo(dT)15 according to the protocols. Then mRNA levels of HMGB-1, vascular endothelial growth factor (VEGF), intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1) were measured by qRT-PCR with cDNA as template and primers in (Table 2). The relative mRNA level was calculated using 2-ΔΔCt method with β-actin as an internal reference.
Table 2.
Primers for quantitative real time PCR (5’-3’)
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HMGB1 | TGTAATGCCTTTGCCCTTCTATC | TTTATCCGCTTTCCTTGTATCTG |
| VEGF | CCCGACAGGGAAGACAAT | TCTGGAAGTGAGCCAACG |
| TNF-α | TGGCGTGTTCATCCGTTCT | CCACTACTTCAGCGTCTCGT |
| ICAM-1 | GGTTGGAGACTAACTGGATG | CGCTCTGGGAACGAATACA |
| VCAM-1 | ACCCAAACAAAGGCAGAGTA | CACTTGAGCAGGTCAGGTTC |
| MCP-1 | TGAGTCGGCTGGAGAACTACAAG | AGGTGCTGAAGTCCTTAGGGTTG |
| β-actin | GGAGATTACTGCCCTGGCTCCTAGC | GGCCGGACTCATCGTACTCCTGCTT |
Protein in each sample was extracted with NP-40 lysis buffer (Beyotime, Shanghai, China) and the concentration of protein was detected by Enhanced BCA Protein Assay Kit (Beyotime). After separating by SDS-PAGE, protein was transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat-milk or 5% BSA following by incubation with primary antibodies against HMGB-1 (1:100, Santa Cruz), ERK, p-ERK, JNK, p-JNK, P38, p-P38, P65, p-P65 and β-actin (1:1000, Wanleibio, Shenyang, China) at 4°C overnight. Then the membranes were incubated with corresponding secondary antibodies at 37°C for 45 min and detected with Enhanced ECL Detection System (Beyotime).
Immunofluorescence (IF)
Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 following by blockade with goat serum. Then cells were incubated with primary antibody against CD31 at 4°C overnight. Thereafter, Cy3 labeled secondary antibody was added to cells and incubated for 60 min in the dark. The nucleus was stained with DAPI after washing with PBS. Cells were observed under fluorescence microscope and the images were captured.
Immunocytochemistry (ICC)
After fixing and permeabilizing, the cells were treated with 3% H2O2 for 15 min to inactivate endogenous peroxidase. After blockade, cells were incubated with primary antibody against Factor VIII (1:100, Bioss), VEGF (1:200, Wanleibio), ICAM-1, VCAM-1 (1:50, Santa Cruz) at 4°C overnight followed by incubation with biotin labeled secondary antibody (1:200, Beyotime) and HRP labeled streptavidin (1:200, Beyotime) at 37°C. Thereafter, cells were incubated with DAB Detection System and stained with hematoxylin. After observation under microscopy, images were captured.
Proliferation assay
Proliferation capability of cells was detected by MTT assay and LDH activity assay. For MTT assay, 1×104 cells were seeded in 96-well plates after different treatment. After incubation for 24 h, 0.2 mg/ml MTT was added into each well and incubated for additional 4 h. After careful removal of the media, 200 μl DMSO was added into each well. Thereafter, the absorbance at 490 nm was measured. For LDH activity assay, media supernatant was collected after different treatment and the LDH activity in the media was measured using LDH Activity Detection Kit (Jianchengbio, Nanjing, China) according to the manufacturer’s protocol.
Apoptosis analysis
Cell apoptosis was analyzed by flow cytometry, Hoechst staining and Caspase-3 activity assay. For flow cytometry, cells were harvested by centrifugation after different treatment. After washing with PBS, cells were stained with Annexin V-FITC/PI Apoptosis Detection Kit (Wanleibio) and incubated at 37°C for 15 min in the dark. After incubation, cells were analyzed with a flow cytometer. For Hoechst staining, cells were fixed with fixing buffer after different treatment. After washing with PBS, cells were stained with Hoechst Staining Kit (Beyotime), then cells were observed under fluorescence microscope and the images were captured. For Caspase-3 activity assay, protein in each sample was extracted and the activity of Caspase-3 was measured using a Caspase-3 Activity Detection Kit (Beyotime) according to the manufacturer’s instruction.
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of VEGF, ICAM-1, VCAM-1, TNF-α and MCP-1 in the cell media were measured after different treatments with corresponding ELISA Kits (USCN, Wuhan, China) according to the manufacturer’s instruction. The concentration of each sample was analyzed using Curve Expert 1.3 software.
Electrophoretic mobility shift assay (EMSA)
After different treatment, cells were harvested and nucleoprotein in each sample was extracted using a Nucleoprotein and Cytoplasmic Protein Extract Kit (Beyotime). EMSA was performed using a NF-κB EMSA Kit (Viagene, Changzhou, China) according to the manufacturer’s protocol. Briefly, equal amount of nucleoprotein in each group was incubated with biotin labeled NF-κB probes and separated through EMSA gel. Then protein was transferred to PVDF membrane and cross-linked under a UV transilluminator. After incubation with HRP labeled streptavidin, the signal was detected with an Enhanced ECL Detection System (Manufacture, City, Country’s name).
Statistical analysis
All experiments were performed three times. The results were presented as mean ± SD. Differences between groups were analyzed with one-way ANOVA and Bonferroni’s Multiple Comparison. P<0.05 was considered to be significant.
Results
Infection with shRNA effectively silenced HMGB1
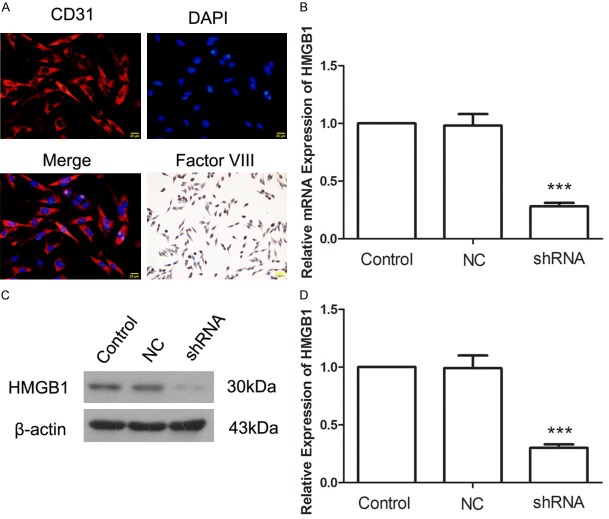
The isolated cells were identified by IF with CD 31 and ICC with Factor VIII. Data showed that the isolated cells were Factor VIII-positive and CD 31-positive (Figure 1A). qRT-PCR and western blot were used to detect the effect of HMGB1 silence. After infection with shRNA, the mRNA level of HMGB1 was decreased to 28±3% (Figure 1B) and the protein level of HMGB1 was decreased to 30±3% (Figure 1C and 1D). These results showed that infection with shRNA effectively silenced HMGB1 expression.
Figure 1.
A: The isolated cells were identified by immunofluorescence with antibody against CD31 and immunocytochemistry with antibody against Factor VIII. B: Histogram of HMGB1 mRNA expression in each group. C: Western blot assay for HMGB1. D: Histogram of HMGB1 protein expression in each group. ***P<0.001.
Silence of HMGB1 inhibited apoptosis induced by high concentration of glucose
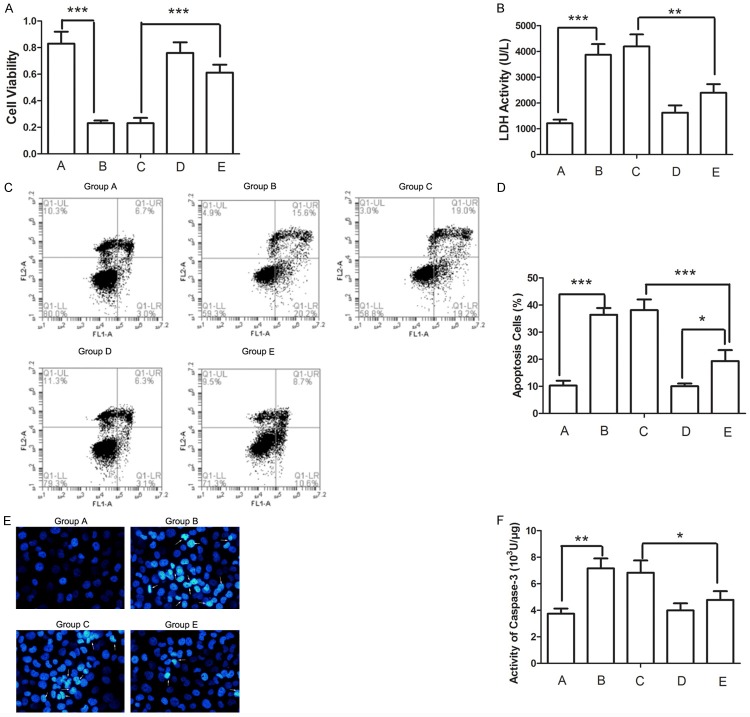
We detected the influence of HMGB1 silence on injury of retinal endotheliocytes induced by high concentration of glucose. The cell viability was inhibited by high concentration of glucose (Figure 2A, Group A vs Group B) and LDH activity in the media was up-regulated (Figure 2B, Group A vs Group B). Compared with Group C, the cell viability of the cells in Group E was increased significantly (P<0.001) and the LDH activity in the media was decreased significantly (P<0.01) (Figure 2A and 2B, Group E vs Group C). These results demonstrated that high concentration of glucose injured retinal endotheliocytes, but this damaging effect of high concentration of glucose could be reversed by silence of HMGB1.
Figure 2.
A. Cell viability was detected by MTT assay after different treatment. B. LDH activity in cell media was detected after different treatment. C, D. Cell apoptosis was detected by flow cytometry after different treatment. The percent of cells in each phase was calculated. E. Cell nucleus was stained with Hoechst. Arrows indicate pyknosis of chromatin. F. Activity of Caspase-3 was detected after different treatment. *P<0.05, **P<0.01, ***P<0.001.
As apoptosis is an important factor which impacts the proliferation of cells, an apoptosis assay was performed. After treatment with high concentration of glucose, the percentage of apoptosis cells was increased significantly (Figure 2C and 2D, Group A vs Group B, P<0.001), pyknosis of chromatin was discovered (Figure 2E, Group A vs Group B), and activity of Caspase-3 was increased significantly (Figure 2F, Group A vs Group B). Whereas, these damaging effects of high concentration of glucose can be reversed by HMGB1 silence (Figure 2C-F, Group E vs Group C). Together, these results demonstrated that silence of HMGB1 attenuated retinal endotheliocyte apoptosis induced by high concentration of glucose. No significant differences were observed between Groups A and Group D. This result indicates indicating that silence of HMGB1 has no effect on cell growth in normal condition. We ignored Group D in the following experiments.
Silence of HMGB1 decreased inflammatory cytokines induced by high concentration of glucose
Inflammatory responses are common in diabetic retinopathy, and changes in inflammatory cytokines TNF-α and MCP-1 were detected by qRT-PCR and ELISA. After treatment with high concentration of glucose, significant increase of TNF-α and MCP-1 was found, both in cells and in cell media (Figure 3A-D, Group A vs Group B). However, the upheaval of TNF-α and MCP-1 induced by high concentration of glucose can could be inhibited by silence of HMGB1 (Figure 3A-D, Group E vs Group C). These results suggested that silence of HMGB1 inhibited the inflammatory response induced by high concentration of glucose.
Figure 3.

A, B: The mRNA levels of TNF-α and MCP-1 were detected by qRT-PCR. C, D: The concentrations of TNF-α and MCP-1 in cell media were measured using ELISA. *P<0.05, **P<0.01, ***P<0.001.
Silence of HMGB1 attenuated changes in endothelial permeability barrier induced by high concentration of glucose
Diabetic retinopathy is a disease of the retinal microvasculature damage. The growth and barrier of blood vessel are very important to the pathology of diabetic retinopathy. Effects of HMGB1 silence on factors associated with vessel endothelial permeability barrier were detected. Data showed that the levels of VEGF, ICAM-1 and VCAM-1 were increased in high concentration of glucose condition, both in cells and in cell media (Figure 4A-C, Group A vs Group B). However, increases of VEGF, ICAM-1 and VCAM-1 induced by high concentration of glucose can be reversed by HMGB1 silence (Figure 4A-C, Group E vs Group C), indicating that silence of HMGB impacted the endothelial permeability barrier of retinal blood vessel.
Figure 4.
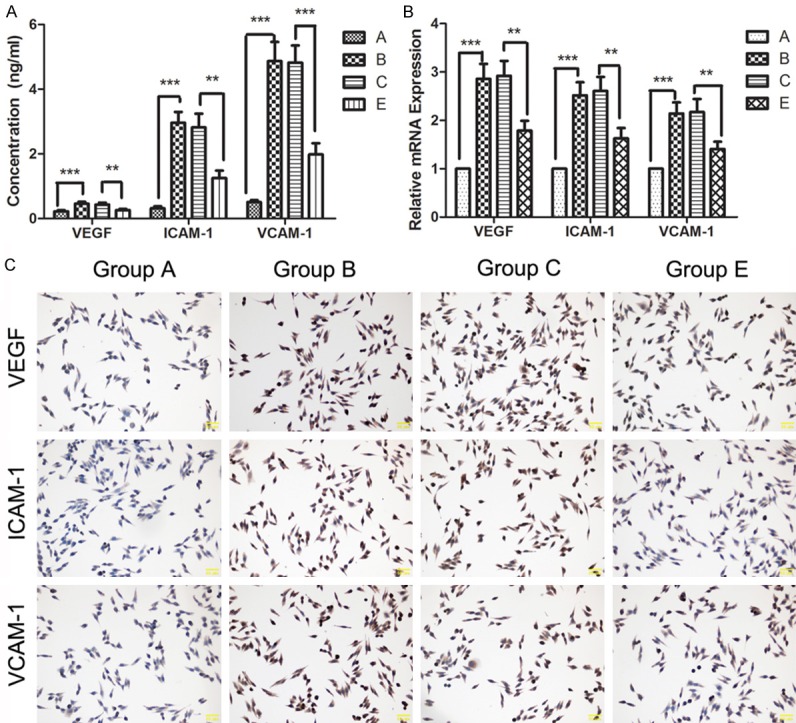
A: Levels of VEGF, ICAM-1 and VCAM-1 in the cell media were detected by ELISA after different treatment. B: mRNA levels of VEGF, ICAM-1 and VCAM-1 were detected by qRT-PCR. C: The expression of VEGF, ICAM-1 and VCAM-1 was detected by ICC. **P<0.01, ***P<0.001.
Silence of HMGB1 inhibited the activation of MAPK and NF-κB signaling pathways
MAPK and NF-κB signaling pathways are important to the cell growth and inflammation. To further explore the effect of HMGB1 silence, MAPK and NF-κB signaling pathways were detected. After treatment with high concentration of glucose, the phosphorylation levels of ERK, JNK and P38 were significantly increased, with no changes in ERK, JNK and P38 (Figure 5A-F, Group A vs Group B). These results illustrated that high concentration of glucose might injure retinal endotheliocytes through the activation of MAPK signaling pathway. Whereas, data of our study also showed that the increase of phosphorylation levels of ENK, JUK and P38 can could be relieved by HMGB1 silence (Figure 5A-F, Group E vs Group C). These results indicated evidenced that silence of HMGB1 inhibited that activation of MAPK signaling pathway induced by high concentration of glucose.
Figure 5.

A, B: Protein levels of ERK and phosphorylated ERK (p-ERK) were detected by western blot after different treatment. C, D: JNK and phosphorylated JNK (p-JNK) were detected by Western blot. E, F: P38 and phosphorylated P38 (p-P38) were detected by Western blot. **P<0.01, ***P<0.001.
Similar changes were also found in NF-κB signaling pathway. The phosphorylation level of NF-κB P65 was increased by high concentration of glucose condition (Figure 6A and 6B, Group A vs Group B), but relieved by silence of HMGB1 (Figure 6A and 6B, Group E vs Group C). The DNA binding activity of NF-κB was also detected by EMSA. After treatment with high concentration of glucose condition, the DNA binding activity of NF-κB was increased dramatically (Figure 6C and 6D, Group A vs Group B), but silence of HMGB reversed this apophysis (Figure 6C and 6D, Group E vs Group C). These results demonstrated that silence of HMGB1 inhibited the activation of NF-κB signaling pathway induced by high concentration of glucose.
Figure 6.

A, B: Protein levels of NF-κB P65 and phosphorylated NF-κB P65 (p-P65) were detected by Western blot after different treatment. C, D: After different treatment, the DNA binding activity of NF-κB was detected using EMSA. *P<0.05, **P<0.01.
Discussion
Diabetic retinopathy is a serious complication of diabetes as well as a threat to human health [19]. In this study, we explored the effects of HMGB1 silence on retina endotheliocyte injury induced by high concentration of glucose and found that high concentration of glucose promoted cell apoptosis, changed endothelial permeability barrier and induced inflammatory response, but silence of HMGB1 relieved these damaging effects of high concentration of glucose. These results indicated that silence of HMGB1 might be a novel means for the treatment of diabetic retinopathy.
Diabetes is characterized by high blood sugar, which will cause cell death and inflammatory response, thus leading to serious complications, such as diabetic retinopathy [20]. In our study, high concentration of glucose was found to inhibit the proliferation of retinal endotheliocyte and induce cell apoptosis, which mimics the influence of diabetes. The mitigatory effects of HMGB1 silence on retinal endotheliocyte injury induced by high concentration of glucose indicated that HMGB1 silence may be a therapeutic for diabetic retinopathy. Effects of HMGB1 silence also implied the important role of HMGB1 in the diabetes retinopathy, coincidently with previous study studies [21-23].
Inflammatory responses are usually seen in diabetes and diabetic retinopathy [24,25]. HMGB1 has a close relationship with inflammation [26]. Extracellular HMGB1 binds to its receptors regulating the expression and secretion of many inflammatory factors through the regulation of several signaling pathways [27,28]. Consistently, in our study, we found that silence of HMGB1 can effectively attenuate inflammatory response induced by high concentration of glucose.
Diabetic retinopathy is a microangiopathy, changes in vascular permeability is a hallmark of diabetic retinopathy [29]. VEGF is a cytokine and is very important to the growth of vessel [30]. VEGF is firstly called vascular permeability factor (VPF) which indicates its important role in blood retinal barrier [31]. ICAM-1 and VCAM-1 play important roles in intercellular adhesion and vascular endotheliocyte activation [32]. Changes in VEGF, ICAM-1 and VCAM-1 levels indicate that endothelial permeability is influenced by high concentration of glucose but can be reversed by HMGB1 silence. ICAM-1 also mediates the adhesion of leukocytes to the endothelium as well as its extravasation into the retinal tissues [33] and changes in ICAM-1 also forebode the results of inflammation.
Both MAPK signaling pathway and NF-κB signaling pathway are typical signaling pathways which are associated with the growth of cells as well as inflammatory response [34]. MAPK and NF-κB signaling pathways are also reported to be in the downstream signaling pathway of HMGB1 pathway through which HMGB1 plays important roles in diabetic retinopathy [35]. Results of our study showed that the activation of MAPK and NF-κB signaling pathways induced by high concentration of glucose was inhibited by HMGB1 silence. These results prompt us that silence of HMGB1 may inactivate MAPK and NF-κB signaling pathways, and thus relieve inflammatory response and revise endothelial permeability barrier induced by high blood sugar.
As HMGB1 plays essential roles in diabetic retinopathy, silence of HMGB1 may be a suitable method for the treatment of diabetic retinopathy. As shown in our study, silence of HMGB1 can effectively attenuate cell apoptosis of retinal endotheliocyte, changes in endothelial permeability and inflammatory response induced by high concentration of glucose. These results indicate that silence of HMGB1 might be a promising therapeutic for the treatment of diabetic retinopathy. At the meanwhile, we found that there was no significant difference in cell viability and cell apoptosis between Group D and Group A, indicating that HMGB1 shRNA has no effect on cells in normal condition, and that if HMGB1 shRNA was were used as a therapeutic for the treatment of diabetes retinopathy, it would have less side-effect. Other report also showed that inhibition of HMGB1 has an excellent therapeutic effect against diabetic complications [36].
In the present study, we discovered that silence of HMGB1 inhibited the activation of MAPK and NF-κB signaling pathway, attenuated cell apoptosis, blood retinal barrier damage and inflammatory response induced by high concentration of glucose. These results indicate that silence of HMGB1 could become a promising therapeutic of the treatment of diabetic retinopathy, but while with less side-effect. This study also provides theoretical foundation for further exploration of diabetic retinopathy therapeutic.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (Grant No. 81570866 and No. 81371045).
Disclosure of conflict of interest
None.
References
- 1.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, Sun X, Wang H, Wang Q, Tsung A, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall LL, Feng YQ, Cheng YH. Effect on proliferation and apoptosis of retinoblastoma cell by RNA inhibiting high mobility group protein box-1 expression. Int J Ophthalmol. 2017;10:30–34. doi: 10.18240/ijo.2017.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang X, Zhang Y, Zhang S. High-mobility group box 1 is overexpressed in cervical carcinoma and promoyts cell invasion and migration in vitro. Oncol Rep. 2017;37:831–840. doi: 10.3892/or.2016.5317. [DOI] [PubMed] [Google Scholar]
- 6.Lin F, Zhang W, Xue D, Zhu T, Li J, Chen E, Yao X, Pan Z. Signaling pathways involved in the effects of HMGB1 on mesenchymal stem cell migration and osteoblastic differentiation. Int J Mol Med. 2016;37:789–97. doi: 10.3892/ijmm.2016.2479. [DOI] [PubMed] [Google Scholar]
- 7.Pan C, Wang Y, Qiu MK, Wang SQ, Liu YB, Quan ZW, Ou JM. Knockdown of HMGB1 inhibits cell proliferation and induces and apoptosis in hemangioma via downregulation of AKT pathway. J Biol Regul Homeos Agents. 2017;31:41–49. [PubMed] [Google Scholar]
- 8.Li Y, Wang P, Zhao J, Li H, Liu D, Zhu W. HMGB1 attenuates TGF-β-induced epithelial-mesenchymal transition of FaDu hypopharyngeal carcinoma cells through regulation of RAGE expression. Mol Cell Biochem. 2017;431:1–10. doi: 10.1007/s11010-017-2968-2. [DOI] [PubMed] [Google Scholar]
- 9.Fu D, Tian X. Effect of high mobility group box 1 on the human retinal pigment epithelial cell in high-glucose condition. Int J Clin Exp Med. 2016;8:17796–803. [PMC free article] [PubMed] [Google Scholar]
- 10.Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, Tracey KJ, Ostrand-Rosenberg S. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–5733. doi: 10.1158/0008-5472.CAN-13-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scovell WM. High mobility group protein 1: a collaborator in nucleosome dynamics and estrogen-responsive gene expression. World J Biol Chem. 2016;7:206–222. doi: 10.4331/wjbc.v7.i2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agalave NM, Svensson CI. Extracellular highmobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol Med. 2014;20:569–578. doi: 10.2119/molmed.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ugrinova I, Pasheva E. HMGB1 protein: a therapeutic target inside and outside the cell. Adv Protein Chem Struct Biol. 2017;107:37–76. doi: 10.1016/bs.apcsb.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Boteanu RM, Uyy E, Suica VI, Antohe F. Highmobility group box 1 enhances the inflammatory process in diabetic lung. Arch Biochem Biophys. 2015;583:55–64. doi: 10.1016/j.abb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol. 2015;6:93. doi: 10.3389/fphys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szasz T, Wenceslau CF, Burgess B, Nunes KP, Webb RC. Toll-like receptor 4 activation contributes to diabetic bladder dysfuncion in a murine model of type 1 diabetes. Diabetes. 2016;65:3754–3764. doi: 10.2337/db16-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu EI-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM. High-mobility group box-1 modulates the expression of inflammatory and angiogenic signaling pathways in diabetic retina. Curr Eye Res. 2015;40:1142–52. doi: 10.3109/02713683.2014.982829. [DOI] [PubMed] [Google Scholar]
- 18.Santos AR, Dvoriantchikova G, Li Y, Mohammad G, Abu El-Asrar AM, Wen R, Ivanov D. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS One. 2014;9:e87574. doi: 10.1371/journal.pone.0087574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wykoff CC. Impact of intravitreal pharmacotherapies including antivascular endothelial groeth factor and corticosteroid agents on diabetic retinopathy. Curr Opin Ophthalmol. 2017;28:213–218. doi: 10.1097/ICU.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 20.Hwang DJ, Lee KM, Park MS, Choi SH, Park JI, Cho JH, Park KH, Woo SJ. Association between diabetic foot ulcer and diabetic retinopathy. Plos One. 2017;12:e0175270. doi: 10.1371/journal.pone.0175270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Song L, Gao X, Chang W, Qin X. Tolllike receptor 4 on islet beta cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Exp Mol Med. 2012;44:260–267. doi: 10.3858/emm.2012.44.4.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi: 10.1155/2012/697489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen XL, Zhang XD, Li YY, Chen XM, Tang DR, Ran RJ. Involvement of HMGB1 mediated signalling pathway in diabetic retinopathy: evidence from type 2 diabetic rats and ARPE-19 cells under diabetic condition. Br J Ophthalmol. 2013;97:1598–1603. doi: 10.1136/bjophthalmol-2013-303736. [DOI] [PubMed] [Google Scholar]
- 24.Waugh K, Snell-Bergeon J, Michels A, Dong F, Steck AK, Frohnert BI, Norris JM, Rewers M. Increased inflammation is associated with islet autoimmunity and type 1 diabetes in the diabetes autoimmunity study in the young (DAISY) PLoS One. 2017;12:e0174840. doi: 10.1371/journal.pone.0174840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rezzola S, Corsini M, Chiodelli P, Cancarini A, Nawaz IM, Coltrini D, Mitola S, Ronca R, Belleri M, Lista L, Rusciano D, De Rosa M, Pavone V, Semeraro F, Presta M. Inflammation and Nformyl peptide receptors mediate the angiogenic activity of human vitreous humour in proliferative diabetic retinopathy. Diabetoligia. 2017;60:719–728. doi: 10.1007/s00125-016-4204-0. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen AH, Detty SQ, Agrawal DK. Clinical implications of high-mobility group box-(HMGB1) and the receptor for advanced glycation endproducts (RAGE) in cutaneous mlignancy: a systematic review. Anticancer Res. 2017;37:1–7. doi: 10.21873/anticanres.11282. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y, Xiong J, Chen Q, Xia J, Zhang Y, Yang X, Tao K, Zhang S, He S. Hypoxia/reoxygenation-induced HMGB1 translocation and release promotes islet proinflammatory cytokine production and early islet graft failure through TLRs signaling. Biochim Biophys Acta. 2017;1863:354–364. doi: 10.1016/j.bbadis.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Wang D, Wang B, Li H, Xiong J, Xu S, Chen Q, Tao K, Yang X, Zhu Y, He S. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway. Mol Biol Cell. 2017;28:201–209. doi: 10.1091/mbc.E16-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arima M, Cui D, Kimura T, Sonoda KH, Ishibashi T, Matsuda S, Ikeda E. Basigin can be a therapeutic target to restore the retinal vascular barrier function in the mouse model of diabetic retinopathy. Sci Rep. 2016;6:38445. doi: 10.1038/srep38445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–132. doi: 10.1016/j.phrs.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 32.Hocaoglu-Emre FS, Saribal D, Yenmis G, Guvenen G. Vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and cluster of differentiation 146 levels in patients with type 2 diabetes with complications. Endocrinol Metab (Seoul) 2017;32:99–105. doi: 10.3803/EnM.2017.32.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jafarian M, Mozhgani SH, Patrad E, Vaziri H, Rezaee SA, Akbarin MM, Norouzi M. Evaluation of INOS, ICAM-1, and VCAM-1 gene expression: a study of adult T cell leukemia malignancy associated with HTLV-1. Arch Virol. 2017;162:1009–1015. doi: 10.1007/s00705-016-3213-0. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Xu B, Xu M, Chen D, Xiong Y, Lian M, Sun Y, Tang Z, Wang L, Jiang C, Lin Y. 6-gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol Res. 2017;119:137–148. doi: 10.1016/j.phrs.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Li W, Tu H, Chen N, Zhong Z, Yan P, Dong J. Curcumolide reduces diabetic retinal vascular leukostasis and leakage partly via inhibition of the p38MAPK/NF-κB signaling. Bioorg Med Chem Lett. 2017;27:1835–1839. doi: 10.1016/j.bmcl.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 36.Wang WK, Wang B, Lu QH, Zhang W, Qin WD, Liu XJ, Liu XQ, An FS, Zhang Y, Zhang MX. Inhibition of high-mobility group box 1 improves myocardial fibrosis and dysfunction indiabetic cardiomyopathy. Int J Cardiol. 2014;172:202–12. doi: 10.1016/j.ijcard.2014.01.011. [DOI] [PubMed] [Google Scholar]