Abstract
Lysosome Associated Membrane Protein-1 (LAMP1), expressed in several functional processes, is a heavily glycosylated lysosomal membrane protein which is able to protect the lysosomal membranes from intracellular proteolysis. Its pro-tumorigenic effects are involved in the development of several types of Malignancy. However, the role of LAMP1 in human Epithelial Ovarian Cancer (EOC) patients remains unclear. To demonstrate its prognostic significance in EOC, the methods of RT-PCR and IHC were used to evaluate LAMP1 expression in both EOC and nonmalignant tissues. What’s more, the relationship between LAMP1 and clinicopathological factors was investigated. Survival analysis was used to elaborate its prognostic value. Expression of LAMP1 in tumor cells in EOC was significantly higher than those in noncancerous tissues. LAMP1 over-expression in EOC was significantly related to FIGO stage, metastasis invasion, positive ascites cell and the level of serum CA125. The Kaplan-Meier plot and Cox regression method have shown that LAMP1 over-expression in EOC and FIGO stage was significantly related to malignant features in EOC and EOC patients’ unfavorable survival. It was strongly evidenced in our research that LAMP1 is a prognostic factor in EOC. Our results suggest that LAMP1 could be used as a poor prognostic factor and novel therapeutic target for EOC.
Keywords: LAMP1, epithelial ovarian cancer, RT-PCR, immunohistochemistry, prognosis
Introduction
Ovarian cancer is one of the most lethal malignancies among women in both the developed and the developing world, with exceedingly 204,000 new cases and 125,000 deaths occurring yearly, constituting entirely more than 4% of cancer cases and cancer deaths in women all over the world [1]. Concerning the treatment of EOC, necessary surgery for permitted situation of ovarian cancer patients and postsurgical chemotherapy has significantly promoted the survival in the past 25 years, yet 5-year survival rate is still below 50% [2].
Generally speaking, the malignant transformation in ovarian surface epithelial cells, a single continuous epithelium encircling the ovary, leads to the ovarian cancer. Owing to atypical symptoms and lack of sensitive detection at early stage, most EOC patients scan revealed advanced ovarian cancer when detected. A leading factor for poor outcome is our insufficient understanding of the events that initiate EOC and promote progresses of EOC. Therefore, novel and high sensitive and specific biomarkers with great clinicopathologic and prognostic significance are badly needed for the diagnosis and target therapies of EOC. And what is more, in the times of ‘precision medicine’, biomarkers play a significant role such as early detection and significant improvements in treatment outcomes [3-5].
Lysosome Associated Membrane Protein-1 (also known as CD107a) is a heavily glycosylated lysosomal membrane protein which is believed to protect the lysosomal membranes from intracellular proteolysis [6,7]. Although LAMP1 primarily was expressed in the endosome-lysosomal membrane of cells, it could be found in the plasma membrane [8,9]. Recently, several studies have demonstrated that LAMP1 is over-expressed in several cancers and implicated in carcinogenesis of various cancer types [6-8]. Furuta et al. described a high expression of LAMP1 in cancer cells with high metastatic potential in colorectal neoplasm, indicating an activity for LAMP1 in cell adhesion and migration [10]. Künzli et al. reported a positive association between LAMP1 expression and prognostic status of patients with pancreatic carcinoma [11]. What’s more, an elevated expression of LAMP1 at the surface of the cell has also been witnessed during platelet and granulocytic cell activation, including in metastatic tumor cells [12,13], suggesting that the cell-surface expressed portion of LAMP1 may serve as ligand for selectins and could be modulated by tumor cells [14]. It’s implicated that LAMP1 has facilitated cancer progression and tumor metastasis [13-15].
Nevertheless, as far as we know, LAMP1 expression and its clinical features, as well as its prognostic role, are yet to be detected in EOC. Here we reported the identification of LAMP1, as a novel therapeutic target for EOC, to investigate their prognostic significance in EOC.
Materials and methods
Patient specimens
All samples were collected by specialized personnel during the surgery in the Department of Gynecology, Affiliated Hospital of Nantong University between July 2005 and July 2010. There were 15 cases of normal ovarian samples, 20 cases of ovarian benign tumor tissue samples and 115 cases of ECO tissue samples. Normal ovary tissues resected from non-ovarian disease were collected for control. Of the 115 cases of EOC, there were 86 serous carcinoma and 29 other types. As for FIGO stage, there were 55 stage I, and 60 stage II-IV cases. In view of histological grading, 93 cases were being high and 22 cases were being low. Detailed clinicopathological data were shown in Table 1.
Table 1.
Association of LAMP1 expression with clinical characteristics of EOC
| Groups | n=115 | LAMP1 expression | P value | ||
|---|---|---|---|---|---|
|
| |||||
| Low or no expression (n=32) | High expression (n=83) | Pearson χ2 | |||
| Age at diagnosis | 1.534 | 0.215 | |||
| ≤60 years | 80 | 25 (31.25) | 55 (68.75) | ||
| >60 years | 35 | 7 (20.00) | 28 (80.00) | ||
| FIGO Stage | 7.779 | 0.005* | |||
| 1 | 55 | 22 (40.00) | 33 (60.00) | ||
| 2~4 | 60 | 10 (16.67) | 50 (83.33) | ||
| Grade | 2.319 | 0.128 | |||
| Low | 22 | 9 (40.91) | 13 (59.09) | ||
| High | 93 | 23 (24.73) | 70 (75.27) | ||
| Single or double | 0.000 | 0.991 | |||
| Single | 54 | 15 (27.78) | 39 (72.22) | ||
| Double | 61 | 17 (27.87) | 44 (72.13) | ||
| Type | 0.001 | 0.973 | |||
| Serous | 86 | 24 (27.91) | 62 (72.09) | ||
| Other | 29 | 8 (27.83) | 21 (72.41) | ||
| lymph nodes | 3.526 | 0.060 | |||
| No | 84 | 28 (33.33) | 56 (66.67) | ||
| Yes | 18 | 2 (11.11) | 16 (88.89) | ||
| Unknown | 13 | 2 | 11 | ||
| Metastasis | 4.292 | 0.038* | |||
| No | 55 | 20 (36.36) | 35 (63.64) | ||
| Yes | 58 | 11 (18.97) | 47 (81.03) | ||
| Unknown | 2 | 1 | 1 | ||
| Ascites cell | 4.201 | 0.040* | |||
| 0 | 56 | 19 (33.93) | 37 (66.07) | ||
| 1 | 25 | 3 (12.00) | 22 (88.00) | ||
| Unknown | 34 | 10 | 24 | ||
| CA125 | 6.774 | 0.009* | |||
| 0 | 28 | 11 (39.29) | 17 (60.71) | ||
| 1 | 28 | 6 (13.04) | 40 (86.96) | ||
| Unknown | 41 | 15 | 26 | ||
P<0.05.
The clinical data of the patients’ (including age, tumor classification, tumor grade, follow-up including 5-year survival and other information) were recorded in detail which was obtained from the medical records of patients. All patients underwent standard surgery and none of the patients received any therapy such as chemotherapy, radiation or immunotherapy prior to the operation. After surgery, a chemotherapy based on platinum was adopted at least six times for each patient. All patients were diagnosed by at least 2 pathologists and followed up from the time of being diagnosed till December 31, 2015. All samples were obtained with medical-ethics approval from the Ethics Committee of Nantong University Affiliated Hospital and all patients gave informed consent.
LAMP1 mRNA isolation and real-time RT-PCR assay
45 fresh frozen tissue samples were used in the study, which included 15 cases of normal ovarian tissue, 20 cases of benign ovarian tumors, and 10 cases of EOC. Total mRNA was isolated from EOC and normal samples by using TRIZOL reagent (Life Technologies). The expression of LAMP1 was detected by qRT-PCR using the Light Cycler Fast Start DNA Master SYBR Green I Kit (Roche Diagnostics, Tokyo, Japan). Following primers were used: forward primer 5’-GTT TCT TCA TTC TTT ACT G-3’, reverse primer 5’-TCT CTA CTG TTG TAA TGT-3’. β-actin was employed as an internal control, and the primers for β-actin were shown as follows: forward 5’-TAA TCT TCG CCT TAA TAC TT-3’, reverse 5’-TAATCTTCGCCTTAATACTT-3’. One-step PCR was performed by using SyberGreen on an ABI 7500 thermal cycler (Applied Biosystems) as previously described [16]. Briefly, the PCR conditions were as follows: initial denaturation at 95°C for 10 min; denaturation at 95°C for 15 s, annealing and extension at 60°C for 1 min, 40 cycles.
TMA construction and IHC analysis
The tissue microarray system (Quick-Ray, UT06, UNITMA, and Korea) was performed for IHC analysis. Tissue sections were deparaffinized and rehydrated in graded ethanol solutions. The sections were incubated in H2O2 with a concentration of 3% to block the activity of endogenous peroxidase. IHC analysis was performed as previously described [17]. Immunostaining was performed using the primary antibody against LAMP1 (1:200, Abcam, Cambridge, USA), and then incubated with the secondary antibody (Santa Cruz Biotechnology, SantaCruz, CA, USA). Hhosphate-buffered saline was used instead of the primary antibody as negative controls. Not knowing about the clinical background of the cases, two independent pathologists were conducted to evaluate and score the staining intensity of LAMP1 expression of each slide. The staining intensity of LAMP1 was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (intense staining). Staining area was scored as 0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%) on the basis of the percentage of positively stained cells. The final LAMP1 staining score was a product of the percentage and intensity score. The cutoff point for the LAMP1 expression score that was statistically significant in terms of survival was set using the X-tile software program (The Rimm Lab at Yale University; http://www.tissuearray.org/rimmlab) as described previously [18]. We defined the staining scores using a two-level grading system for LAMP1 staining: 0-90: low expression; 91-300: high expression.
Statistical analysis
We analyzed all data by using SPSS20 statistic software (SPSSInc, Chicago, IL, USA) and STATA 12.0 (Stata Corp, College Station, TX, USA). Statistical calculations of LAMP1 mRNA expression were performed using t test. χ2 tests were performed to evaluate relationships between LAMP1 expression and clinic-pathologic parameters. We used the Kaplane-Meier method to calculate patients’ outcome survival curves. Univariate and multivariate Cox regression models were adopted to evaluate prognostic significance. Concerning the above analyses, a p value <0.05 was regarded as statistical significance.
Results
LAMP1 mRNA expression by RT-PCR
To evaluate the LAMP1 mRNA expression between EOC and normal tissue, RNA were isolated from 10 cases of ovarian carcinoma, 15 normal ovary tissues and 20 benign ovarian tumor samples using real-time PCR. As is shown in Figure 1, the means of LAMP1 mRNA in carcinoma and in noncancerous tissues (ovary tissue and benign ovarian tumor samples) were 0.6119±0.1332, 0.1445±0.0687, 0.1359±0.0163 respectively. PCR results showed higher expression of LAMP1 mRNA in ovarian cancer samples than in noncancerous tissues (all P<0.001).
Figure 1.
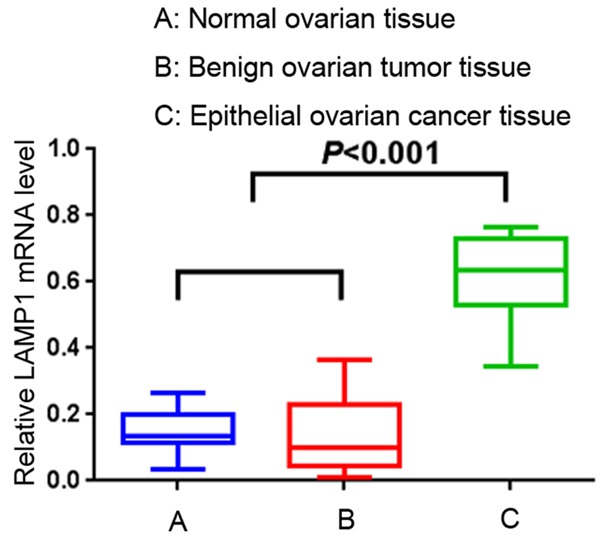
LAMP1 mRNA expression in EOC tissues and noncancerous tissues. Real-time PCR demonstrated that the expression of LAMP1 mRNA in carcinoma and in noncancerous tissues (ovary tissue and benign ovarian tumor samples) were 0.6119±0.1332, 0.1445±0.0687, 0.1359±0.0163 respectively. The expression of LAMP1 mRNA in ovarian cancer samples was significantly higher than in noncancerous tissues (all P<0.05).
LAMP1 protein expression by IHC
IHC analyses were performed to evaluate expression of LAMP1 in EOC tissue specimens using TMA. High cytoplasmic expression of LAMP1 was observed in 83 (72.17%) cases of EOC tumors compared with only 4 (26.67%) cases of normal ovary tissues and 5 (33.33%) of the benign tumor samples. The data was of statistical significance using χ2 test analysis (χ2=18.3916, P<0.001) (Table 2). Representative IHC staining for LAMP1 in EOC and noncancerous tissue samples was shown in Figure 1.
Table 2.
LAMP1 immunohistochemical staining of protein in normal ovarian, benign ovarian tumor and EOC tissues
| Samples | n | LAMP1 expression | |||
|---|---|---|---|---|---|
|
| |||||
| Low or none expression | High expression | Pearson χ2 | P-value | ||
| Normal ovarian | 15 | 11 (73.33) | 4 (26.67) | 18.3916 | 0.000* |
| Benign ovarian tumor | 20 | 10 (66.67) | 5 (33.33) | ||
| EOC | 115 | 32 (27.83) | 83 (72.17) | ||
P<0.05.
LAMP1 expression association with clinic pathological parameters of EOC
In order to better understand the potential influences of LAMP1 in the development and progression of EOC, we observed the relationship between LAMP1 expression in EOC and various clinic pathological parameters of EOC. In Table 1 we analyzed LAMP1 expression and its relationship with clinic pathological factors, then, stratified clinical characteristics by the 2 LAMP1 expression groups. It was observed that LAMP1 protein positivity was significantly connected with ascites cells (P=0.04), FIGO stages (P=0.005), metastasis (P=0.038), and level of serum CA125 (P=0.009) (Table 1). The expression of LAMP1 in ovarian cancer, higher than those in normal tissues (Figure 2), was related to advanced cancer biology, which was implicated by FIGO stage, metastasis, and the serum CA125 level.
Figure 2.
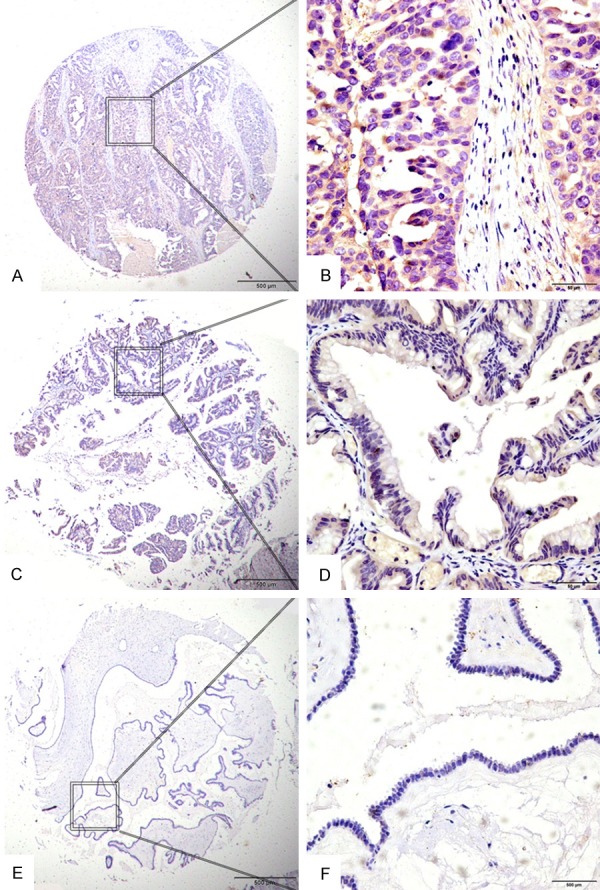
Expression of LAMP1 in EOC with tissue microarray (TMA). Positive staining was predominantly localized in the cytoplasm of EOC cells. LAMP1 protein expression in tumors from EOC patients showed three different levels. (A and B) Showed strong IHC staining of LAMP1 in EOC samples with advanced cancer, expressing high LAMP1 levels. (C and D) Showed weak IHC staining in EOC samples, (E and F) Showed negative IHC staining of LAMP1. Original magnification ×40 in (A, C, E) (scale bars 500 μm); ×400 in (B, D, F) (scale bars 50 μm).
Over-expression of LAMP1 predicts poor prognosis
The Kaplan-Meier figure evaluated that patients had a poor OS when LAMP1 protein expression was higher in EOC tissues (log rank, P<0.001; Figure 3).
Figure 3.
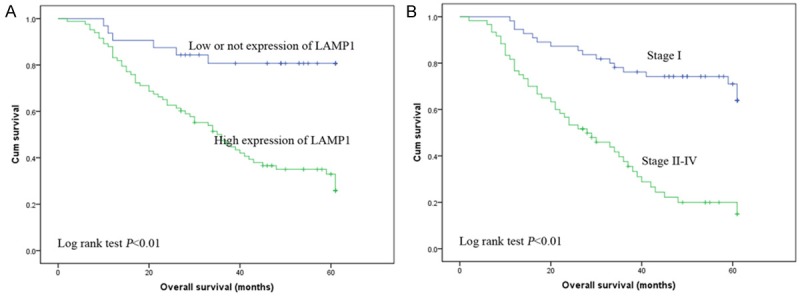
Kaplan-Meier plots using the log rank survival test. A. Overall survival rate in patients with high LAMP1 expression was significantly lower than that in patients with low and no LAMP1 expression. (P<0.01). B. Overall survival rate in patients with high FIGO stage was significantly lower than that in patients with low FIGO stage. (P<0.01).
In the univariate survival analysis, high-level of LAMP1 expression (HR 4.758; P<0.001), patient age (HR 2.156; P=0.003), FIGO stage (HR 4.354; P<0.001), lymph nodes (HR 2.313; P=0.006), metastasis (HR2.361; P=0.001), and ascites cell (HR 2.228; P=0.007) were associated with overall survival (Table 3).
Table 3.
Univariate and multivariable analysis of prognostic factors in cancer for 5-year survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | P | 95% CI | HR | P | 95% CI | |
| LAMP1 | ||||||
| Low vs High | 4.758 | 0.000* | 2.047-11.058 | 5.166 | 0.007* | 1.569-17.006 |
| Age (years) | ||||||
| <60 vs ≥60 | 2.156 | 0.003* | 1.289-3.605 | 0.324 | 0.194 | 0.059-1.772 |
| FIGO Stage | ||||||
| I vs II-IV | 4.354 | 0.000* | 2.461-7.703 | 3.687 | 0.000* | 2.019-6.733 |
| Grade | ||||||
| Low vs High | 1.971 | 0.074 | 0.937-4.150 | |||
| Single or double | ||||||
| Single vs double | 0.710 | 0.180 | 0.431-1.171 | |||
| Histological type | ||||||
| Serous vs others | 0.660 | 0.184 | 0.358-1.218 | |||
| lymph nodes | ||||||
| Yes vs No | 2.313 | 0.006* | 1.274-4.197 | |||
| Metastasis | ||||||
| Yes vs No | 2.361 | 0.001* | 1.395-3.995 | |||
| Ascites cell | ||||||
| Yes vs No | 2.228 | 0.007* | 1.246-3.985 | |||
| CA125 | ||||||
| <100 vs ≥100 | 1.367 | 0.552 | 0.488-3.829 | |||
P<0.05.
Univariate prognostic factors were included in the multivariable analysis together, but FIGO stage already included ascites cell, lymph nodes and metastasis, after the removal of these factors, the rest factors were included in the multivariable analysis. In the multivariable survival analysis, we found that LAMP1 overexpression (HR 5.166; P=0.007) and FIGO stage (HR 3.687; P<0.001) were identified as independent predictive factors for poor outcome (Table 3).
Discussion
Human ovarian cancer is one of the most common gynecologic malignancies and leads to the first cancer death among women. Currently, the standard therapy to EOC was standard surgery and preoperative or postoperative totally of 6-8 cycles’ regular Platinum-taxane based chemotherapy. For the past 30 years, 5-year survival has increased slightly, but still remains below 35%. Therefore, assessment of factors affecting behavior of EOC patients is indispensable in improving postoperative prognosis.
The lysosomal pathway is a novel concept in which lysosomal membrane permeabilization plays a significant role in leading to cell death pathways of cancers [19-21]. The outcome of the cell depends on the extent of lysosomal damage. Normally, limited lysosomal delivery leads to cell death by apoptosis while large number of lysosomal breakdown results in cytosolic necrosis [22]. The balance between apoptosis and necrosis is extremely crucial for cancer development because cancer cells usually acquire mutations to protect themselves from cell death by the classical apoptotic pathways [23]. LAMP1 is among the most abundant lysosomal membrane proteins which creates glycocalyx on the inner side of the lysosomal membrane to protect the membrane from the hydrolytic enzymes and degradation [7]. Recently, LAMP1 expression has been increasingly explored to correlate with cancer development and progression, including in astrocytoma, colorectal cancer, pancreatic carcinoma and various other cancer tissues [10,11,24,25]. However, we have not found any previous work indicating whether the evaluation of LAMP1 expression was related with clinical outcome of EOC patients.
In our present study, we detected mRNA and protein expression level of LAMP1 in both malignant and non-malignant tissues using RT-PCT and IHC analysis. LAMP1 mRNA level was significantly higher in EOC than that in non-malignant tissues. IHC analysis confirmed that the high LAMP1 protein expression, mainly located in the tumor cytoplasm, was detected in 83 (72.17%) cases of EOC patients’ tissue samples. In our studies, we have also confirmed that high cytoplasm staining of LAMP1 protein level was associated with higher FIGO stages, presence of metastasis, presence of ascites cell, high serum CA125 level. Furthermore, high LAMP1 expression in EOC is an independent factor for patient overall survival. Finally, our data clearly showed that high cytoplasmic expression of LAMP1 and higher FIGO Stage at diagnosis was associated with significantly poorer survival. However, our results are contrary to the previous study in which Künzli et al. reported that pancreatic carcinoma patients with higher LAMP1 expression had longer survival time [11]. It’s concluded that the inconsistency is in part due to the differences in the tumor types.
Although there are some limitations such as a limited number of patients and a relatively short follow-up time, and a lack of further studies in vitro and in vivo, the accurate function and therapeutic target of LAMP1 will be confirmed in our later studies.
In conclusion, to our knowledge, this is the first report demonstrating that LAMP1 plays an essential role as a prognostic marker of survival in EOC. Our results supported that LAMP1 plays an essential role in the progression and development of EOC.
Acknowledgements
This work is supported by the grants of the Technological Innovation and Demonstration of Social Undertakings Projects (MS32016023) of Nantong, China.
Disclosure of conflict of interest
None.
Authors’ contribution
Y.Q.Z. designed the study; Y.Z.X and X.M.C collected the tissue samples; S.Z. performed the IHC analysis; Y.Z.X collected clinical data; Y.Z.X and Z.J.S. drafted the manuscript; Y.Q.Z. supervised the study. All authors read and approved the final manuscript.
References
- 1.Xu Y, Wang C, Zhang Y, Jia L, Huang J. Overexpression of MAGE-A9 is predictive of poor prognosis in epithelial ovarian cancer. Sci Rep. 2015;5:12104. doi: 10.1038/srep12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH, Hermeking H, Nikitin AY. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–1128. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal SK, Figlin RA, Reckamp K. Targeted therapies for non-small cell lung cancer: an evolving landscape. Mol Cancer Ther. 2010;9:1931–1944. doi: 10.1158/1535-7163.MCT-10-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly K, Huang C. Biological agents in nonsmall cell lung cancer: a review of recent advances and clinical results with a focus on epidermal growth factor receptor and vascular endothelial growth factor. J Thorac Oncol. 2008;3:664–673. doi: 10.1097/JTO.0b013e3181758141. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson DR, Johnson BE, Sledge GW. Making personalized cancer medicine a reality: challenges and opportunities in the development of biomarkers and companion diagnostics. Clin Cancer Res. 2012;18:619–624. doi: 10.1158/1078-0432.CCR-11-2017. [DOI] [PubMed] [Google Scholar]
- 6.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem. 1999;274:31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 7.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson-Lawrence EJ, Dean CJ, Chang M, Hopwood JJ, Meikle PJ, Brooks DA. Immunochemical analysis of CD107a (LAMP-1) Cell Immunol. 2005;236:161–166. doi: 10.1016/j.cellimm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Kannan K, Stewart RM, Bounds W, Carlsson SR, Fukuda M, Betzing KW, Holcombe RF. Lysosome-associated membrane proteins h-LAMP1 (CD107a) and h-LAMP2 (CD107b) are activation-dependent cell surface glycoproteins in human peripheral blood mononuclear cells which mediate cell adhesion to vascular endothelium. Cell Immunol. 1996;171:10–19. doi: 10.1006/cimm.1996.0167. [DOI] [PubMed] [Google Scholar]
- 10.Furuta K, Ikeda M, Nakayama Y, Nakamura K, Tanaka M, Hamasaki N, Himeno M, Hamilton SR, August JT. Expression of lysosome-associated membrane proteins in human colorectal neoplasms and inflammatory diseases. Am J Pathol. 2001;159:449–455. doi: 10.1016/S0002-9440(10)61716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunzli BM, Berberat PO, Zhu ZW, Martignoni M, Kleeff J, Tempia-Caliera AA, Fukuda M, Zimmermann A, Friess H, Buchler MW. Influences of the lysosomal associated membrane proteins (Lamp-1, Lamp-2) and Mac-2 binding protein (Mac-2-BP) on the prognosis of pancreatic carcinoma. Cancer. 2002;94:228–239. doi: 10.1002/cncr.10162. [DOI] [PubMed] [Google Scholar]
- 12.Andrejewski N, Punnonen EL, Guhde G, Tanaka Y, Lullmann-Rauch R, Hartmann D, von Figura K, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274:12692–12701. doi: 10.1074/jbc.274.18.12692. [DOI] [PubMed] [Google Scholar]
- 13.Sarafian V, Jadot M, Foidart JM, Letesson JJ, Van den Brule F, Castronovo V, Wattiaux R, Coninck SW. Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int J Cancer. 1998;75:105–111. doi: 10.1002/(sici)1097-0215(19980105)75:1<105::aid-ijc16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Sawada R, Jardine KA, Fukuda M. The genes of major lysosomal membrane glycoproteins lamp-1 and lamp-2. The 5’-flanking sequence of lamp-2 gene and comparison of exon organization in two genes. J Biol Chem. 1993;268:13010. [PubMed] [Google Scholar]
- 15.Saitoh O, Wang WC, Lotan R, Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- 16.Ni S, Xu L, Huang J, Feng J, Zhu H, Wang G, Wang X. Increased ZO-1 expression predicts valuable prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2887–2895. [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J. FoxQ1 overexpression influences poor prognosis in non-small cell lung cancer, associates with the phenomenon of EMT. PLoS One. 2012;7:e39937. doi: 10.1371/journal.pone.0039937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhai X, Zhu H, Wang W, Zhang S, Zhang Y, Mao G. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31:970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]
- 19.Groth-Pedersen L, Jaattela M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 2013;332:265–274. doi: 10.1016/j.canlet.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23:2746–2756. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- 21.Kirkegaard T, Jaattela M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Jensen SS, Aaberg-Jessen C, Christensen KG, Kristensen B. Expression of the lysosomalassociated membrane protein-1 (LAMP-1) in astrocytomas. Int J Clin Exp Pathol. 2013;6:1294–1305. [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki K, Nagata M, Suzuki M, Fujiwara T, Ueda K, Miyoshi Y, Takahashi E, Nakamura Y. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: significantly increased expression in cancers of various tissues. Cancer Res. 1998;58:3499–3503. [PubMed] [Google Scholar]


