Abstract
Background: ASPM is a newly reported stem cell marker and plays important roles in mitosis, cell cycle and tumorigenesis. It links with poor clinical prognosis in various tumors. However, the clinical significance of ASPM in colonic adenocarcinoma (CA) has not been fully studied. The purpose of this study was to investigate if ASPM is correlated with the clinicopathological features of CA. Methods: Primary CA tissue, adenoma and the matched normal mucosa from 99 patients, were detected using immunohistochemical analysis by primary antibodies against ASPM. Meanwhile, 20 CAs and 20 liver metastatic cases were examined by RNA in situ hybridization (RNAscope). To assess the clinical relevance of ASPM, we analyzed the survival follow-up information. Results: ASPM was found only in single cells in the base of normal colon mucosal crypts. But the expression of ASPM was detected high in colonic adenomas (49.5%, 49/99), and significantly higher in CA (56.6%, 56/ 99, P<0.001). In CAs, ASPM expression was more intense in stage III and IV than II and I stage patients (P=0.03), and positively correlated with lymph node metastasis (P=0.03), but not with the age at diagnosis, gender and histological grade (P>0.05). We also analyzed the survival follow-up information, the data showed that ASPM-positive expression was correlated with a shorter disease-free survival (DFS) time, the average DFS time of patients with ASPM positive and negative expression was 62.79±2.32 months and 71.30±2.72 months, respectively, and there was no statistical significance between the two groups (P>0.05). The results of ASPM mRNA measurement by RNAscope revealed ASPM mRNA expression was higher in primary CA than that in metastatic liver CA (P<0.001). Conclusions: ASPM might play an important role in colonic carcinogenesis and be a potential marker in predicting prognosis of CA.
Keywords: Colonic adenocarcinoma, immunohistochemistry, ASPM, RNAscope
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the United States [1]. The survival of patients with CRC has improved significantly because of advanced treatment. However, a significant proportion of patients experience recurrence and metastasis. The survival of patients in stage IV still remains low. The resistance to chemotherapeutic drugs is the key reason for the failure. Cancer stem cells have the ability of self-renew and escaping from the chemotherapeutic drugs, resulting in recurrence and metastasis of cancer [2]. Therefore, it is of great interest to identify novel biomarkers for estimation of recurrence and metastasis potential.
Abnormal spindle microcephaly associated (ASPM) was first known in autosomal recessive primary microcephaly (MCPH). Firstly, recent study found that ASPM played an important part in spindle organization, orientation and mitotic progression [3]. ASPM also might influence the division of neural progenitor by Wnt signaling pathway [4]. In recently years, more and more studies showed that ASPM highly expressed not only in central nervous neoplasms but also in many kinds of solid tumors. Meanwhile, ASPM is linked with poor clinical prognosis and early recurrence. However, the role of ASPM in CA remains unclear. In this study, primary cancer tissue, adenoma and the matched normal mucosa in 99 cases were examined by immunohistochemistry. Meanwhile, the ASPM expression at RNA level was examined by RNAscope. The aim is to investigate the expression of ASPM and its clinical significance in CA in a Chinese cohort.
Materials and methods
Patients and tissue samples
Ninety-nine cases of CA with adenoma undergoing operation during January 2010 to December 2012 at the Chinese People’s Liberation Army General Hospital (Beijing, China) were analyzed. Moreover, twenty cases staged as IV dating from 2014-2016 were also retrieved. Detailed clinical information including age, gender, tumor histological grade, TNM stage and lymph node metastasis status was collected. Patients who had been administered chemotherapy or radiotherapy prior to surgery were all excluded from this study.
Immunohistochemistry
The EnVision method was used for immumohistochemical staining. All the tissues were cut at 4-μm thickness and rehydrated through a graded ethanol series. The sections were autoclaved in EDTA (pH 8.0) at 120°C for 2.5 min for antigen retrieval. Endogenous peroxidase was quenched with aqueous 3% H2O2 for 10 minutes. The sections were incubated at 37°C for 1.5 hours (rabbit polyclonal antibody, 1:200, Lifespan, USA) and then washed with PBS×3. Next, the sections were incubated with secondary antibody (Dako) for 20 min at room temperature. Color development was visualized with diaminobenzidine (DAB; Dako).
Evaluation of IHC
All the immunohistochemical staining was assessed by two pathologists in a blind manner. German semi-quantitative scoring system was used in this study. Each tissue was assessed according to the intensity of staining in cytoplasm and the extent of stained cells. Each specimen was assigned a score according to the intensity of cytoplasmic staining (no staining as 0; weak staining as 1, moderate staining as 2, strong staining as 3) and the extent of stained cells (0%=0, 1-24%=1, 25-49%=2, 50-74%=3, 75-100% staining =4). The final score was got by the combined staining score (extent multiplies intensity). The scores ranged from 0 to 12. We defined negative expression as 0 score, weakly positive expression as 1-4 score, moderately positive expression as 5-8 score and strongly positive expression as 9-12, respectively.
RNA in situ hybridization (RNAscope)
The ASPM mRNA expression was detected by in situ hybridization with RNAscope assay (ACD, Hayward, CA) according to the manufacturer’ protocols. The tissues were deparaffinized, incubated with pretreat 1 reagent at room temperature for 10 min, then boiled with pretreat 2 reagent for 15 min, and next treated with pretreat 3 reagent at 40°C for 30 min. The tissues were hybridized with Hs-ASPM-probe (ACD, Cat.No.42788), positive control probe Hs-PPIB-probe (ACD, Cat. No.31-3901) and negative control probe DapB (ACD, Cat. No.310043) at 40°C for 2 hour. The signals were amplified and visualized with RNAscope 2.0 HD reagent kit-brown (ACD, Cat. No.310035). The staining was graded as follows: no punctate signal dots or <1/per 10 cells =0; 1-3/per 10 cells =1; 4-10/per 10 cells or a few clusters of dots =2; >10/per 10 cells or tufted dots <10%=3; 10 dots/per 10 cells, or with tufted spots >10%=4.
Statistical analysis
SPSS 22.0 software was used for all the statistical analysis. The correlation among ASPM expression in CA, adenoma and normal colonic mucosa was analyzed by chi-square test. The association between ASPM expression and gender, patient age, histopathological grade, TNM stage and lymph node status was also examined by chi-square test. The expression of ASPM at mRNA level between primary CA and metastasis in liver tissues was studied by Mann-Whitney test. Kaplan-Meier curve method was used for survival analysis. All statistical tests were two-sided and P value <0.05 was considered statistically significant.
Results
Clinicopathologic characterization
The mean age of 297 cases at the time of diagnosis was 64 years old (ranged from 35 to 87 years). Seventy-five cases were men and 24 cases were women. Fifteen cases were categorized into well-differentiated CA, 53 cases were moderately differentiated CA and 31 cases were poorly differentiated CA. Out of 99 cases, 63 cases were in early stage and 36 cases were in advanced stage. Thirty-three of 99 demonstrated lymph node metastasis. The follow-up data from 95 cases were recorded.
ASPM expression in colonic cancer by immunohistochemical analysis
The expression of ASPM was primarily located in the cytoplasm of cells (Figure 1). In normal colonic mucosa, the ASPM-positive cells were scattered and rarely in the crypt base (Figure 2). The ASPM positive rate was 56.6% (56/99) in CA, 49.5% (49/99) in adenoma and 17.2% (17/99) in normal mucosa, respectively (Table 1). In adenomas, the ASPM-positive cells increased, spread from the crypt base to the surface in a patchy distribution pattern. In CAs, the ASPM expression pattern was local or larger patches in cytoplasm (Figure 1).
Figure 1.
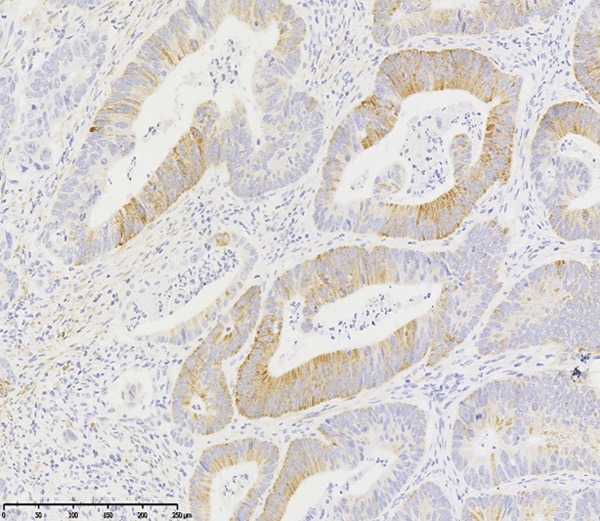
ASPM expression in CA. ASPM protein is mainly located in cytoplasm of cancer cells in the focal form or in a patchy distribution pattern (ASPM×200).
Figure 2.
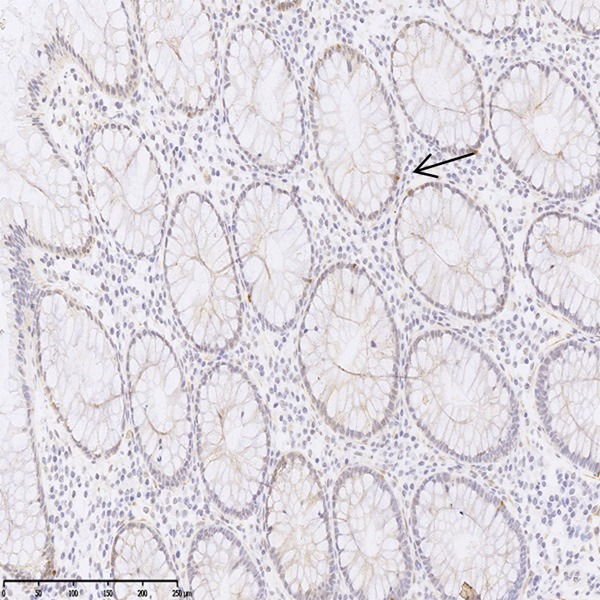
ASPM expression in normal colonic mucosa. ASPM-positive cells were scattered and rarely in the crypt base (arrow) (ASPM×200).
Table 1.
Expression of ASPM in CA, adenoma and normal colonic mucosa
| Group | Number | ASPM | Statistical value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Adenocarcinoma | 99 | 43 | 56 | χ2=36.085 |
| Adenoma | 99 | 50 | 49 | P<0.001 |
| Colonic tissue | 99 | 82 | 17 | |
Clinicopathologic significance of ASPM expression in CA
The expression of ASPM and its correlation with clinicopathologic characteristics in CA were shown in Table 2. We found that ASPM expression was not statistically correlated with patients’ ages at diagnosis, gender or histopathological grade. However, the expression of ASPM in III and IV stage (72.2%) was significantly higher than that in I and II stage (49.2%) (P<0.05). In addition, more samples with lymph node metastasis (72.7%) exhibited positive expression than those without lymph node metastasis (50%) (P<0.05).
Table 2.
Correlation between the expression of ASPM and clinicopathological features of CA
| Variable | Number | ASPM | Statistical value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Histologic grade | ||||
| Well differentiated | 15 | 5 | 10 (66.7%) | χ2=0.977 |
| Moderately differentiated | 53 | 22 | 31 (60.38) | P=0.62 |
| Poorly differentiated | 31 | 15 | 16 (51.6%) | |
| Age (year) | ||||
| <50 | 20 | 10 | 10 (50%) | χ2=0.264 |
| ≥50 | 79 | 32 | 47 (59.5%) | P=0.45 |
| Gender | ||||
| Male | 75 | 34 | 41 (54.7%) | χ2=0.637 |
| Female | 24 | 8 | 16 (66.7%) | P=0.349 |
| Stage | ||||
| I+II | 63 | 32 | 31 (49.2%) | χ2=4.07 |
| III+IV | 36 | 10 | 26 (72.2%) | P=0.03 |
| Lymph node metastasis | ||||
| Yes | 33 | 9 | 24 (72.7%) | χ2=3.76 |
| No | 66 | 33 | 33 (50%) | P=0.03 |
Expression of ASPM at RNA level by RNAscope
Positive staining was identified as dots in cytoplasm. We also found that ASPM mRNA was located beyond spindle pole during mitotic phage (Figure 3). The expression rate of ASPM by RNAscope was 60% (12/20) in primary CA, and 85% (17/20) in liver metastatic cases (P<0.05) (Table 3).
Figure 3.
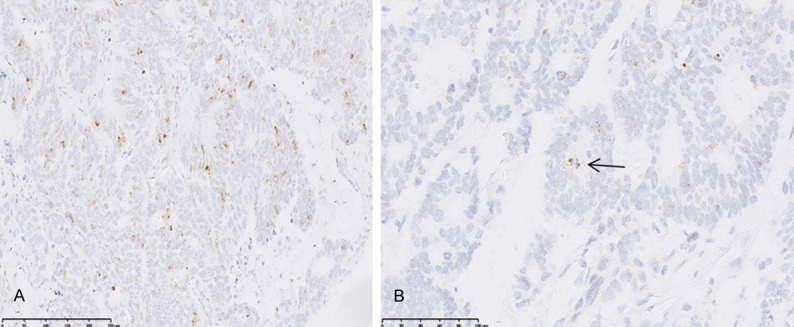
ASPM expression at mRNA level in CA. ASPM was stained as dots in the cytoplasm of cancer cells (A) (ASPM×200), which located to spindle poles during metaphase (arrow) (B) (ASPM×400).
Table 3.
Expression of ASPM at RNA level in primary CA and metastasis in liver
| Variable | Number | ASPM mRNA (score) | Statistical value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1 | 2 | 3 | 4 | |||
| Primary tumor | 20 | 8 | 10 | 1 | 0 | 1 | U=39 |
| Liver metastasis | 20 | 3 | 6 | 5 | 5 | 1 | P<0.001 |
High expression of ASPM predicts poor prognosis of human colonic cancers
Ninety-five out of 99 patients were followed up for survival analysis. Kaplan-Meier survival curves and Log-rank test demonstrated that ASPM positive patients showed a shorter average survival time compared with ASPM negative patients (62.79±2.32 months vs 71.30±2.72 months, P>0.05) as shown in Figure 4.
Figure 4.
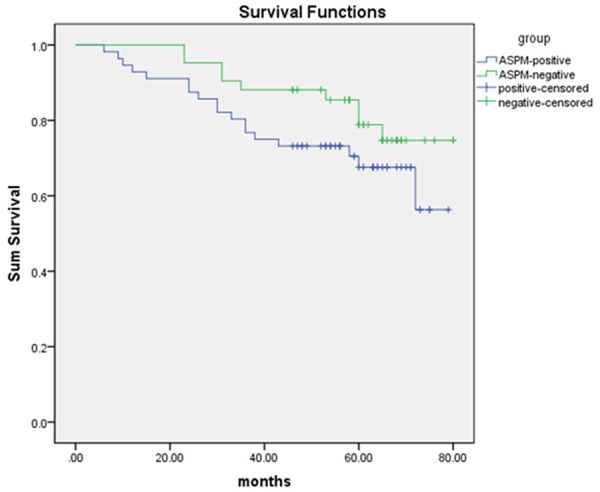
Kaplan-Meier survival analysis by ASPM status. The y-axis represents the percentage of patients; the x-axis represents their survival in months.
Discussion
Autosomal recessive primary microcephaly (MCPH) is a nervous system disease with a smaller but architecturally normal brain, which included 12 MCPH loci (MCPH1-MCPH12) [5]. ASPM, a homologous Asp of Drosophila melanogaster, plays an important part in the mitosis of embryonic nerve cells harboring MCPH5 locus [6]. The ASPM gene is composed of a 10434 bp long coding sequence, including 28 exons and spans 65 kb of genomic DNA at 1q31 [7]. ASPM gene encodes a protein of 3477 amino acids with a putative amino-terminal microtubule binding domain, two calponin homology domains and 81 repeat motifs, which may be bind to calmodulin and without any identified region in C-terminal domain [8]. This gene is mostly expressed in the cerebral cortical ventricular zone and proliferation zones of the medial and lateral ganglion [6]. However, its expression reduced greatly at birth [9]. The data explained that ASPM was possible a marker of stem cell. Besides, ASPM was widely expressed at low level in various tissues in healthy adults, but over-expression in tumor tissues [7].
In previous study, ASPM was found to be peripheral to the nucleus in prophase and concentrated to spindle poles in prometaphase and metaphase [8]. This was also well verified in our data. The ASPM mutation made the spindle pole orientation perpendicular to the spindle pole from parallel to the spindle pole, resulting in chromosome separation failure, making cells from asymmetric division into asymmetric division, which leads to aneuploidy [3].
Originally, ASPM was observed that it possibly involved in the progression of malignant gliomas through expansion of cancer stem cell compartment [10]. Other studies showed that ASPM also played an important role in many kinds of cancer. For example, Lin et al. reported that ASPM could predict invasive or metastatic potential of hepatocellular carcinoma [11]. Wang et al. conducted that the expression of ASPM was associated with pancreatic epithelial tubulogenesis and positively correlated with patient clinical outcome [12]. Rawiah A et al. indicated that in endometrioid subtype of ovarian cancer, ASPM was correlated with the increasing tumor invasion and lymph node involvement [13]. Vange et al. certified that ASPM was over-expressed in gastric cancer and might be a possible progenitor cell marker [14]. However, the role of ASPM in CA has not been reported.
The Wnt signaling pathway was important in promoting neural progenitor proliferation. A study by Buchman JJ et al. found that ASPM regulated the Wnt signaling pathway in the developing brain [4]. As we know, Wnt/β-catenin signaling was over activated in the tumorigenicity of CA. A large proportion of CA patients demonstrate dysregulated Wnt signaling pathway [15]. Thus we speculated that ASPM might be a oncogenic factor in the CA development by Wnt signaling pathway. In previous reports, colorectal cancer (CRC) harbor ASPM mutation in 4.35%, but the differences in clinicopathologic features between the CRCs with or without ASPM gene mutation related intra-tumor heterogeneity had not been found [16]. Choi EJ et al. showed ASPM was present at low levels in 10 out of 11 colorectal carcinoma cell lines and immunohistochemistry staining of CA tissues revealed there was no difference between the expression of ASPM in CA and matching normal tissues just only from four cases [17].
To the best of our knowledge, this is the first study to examine ASPM expression in CA. we found 56 out of 99 (56.6%) CA samples were positive in ASPM expression. We further found that the expression of ASPM was closely related to TNM stage, and also in lymph node or liver metastasis. We also analyzed the survival follow-up information, and showed that ASPM-positive expression had a shorter disease-free survival time but without statistical significance.
In conclusion, our data shows that ASPM is over-expressed in human colonic cancer, especially in advanced pathological stage. The results suggest that ASPM might be a potentially prognostic biomarker for colonic cancer and a therapeutic target. However, further mechanism regarding influence of ASPM on the progression and prognosis of CA needs detailed research.
Acknowledgements
The authors thank all colleagues in the Department of Molecular Biology, Chinese People’s Liberation Army General Hospital for their help and support with this study. All authors have contributed significantly, and all authors are in agreement with the content of the manuscript. This research was supported by the grants from the National Natural Science Foundation of China [81230061, 81472612], Beijing Natural Science Foundation [5162027].
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Higgins J, Midgley C, Bergh AM, Bell SM, Askham JM, Roberts E, Binns RK, Sharif SM, Bennett C, Glover DM, Woods CG, Morrison EE, Bond J. Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol. 2010;11:85. doi: 10.1186/1471-2121-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman JJ, Durak O, Tsai LH. ASPM regulates Wnt signaling pathway activity in the developing brain. Genes Dev. 2011;25:1909–1914. doi: 10.1101/gad.16830211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faheem M, Naseer MI, Rasool M, Chaudhary AG, Kumosani TA, Ilyas AM, Pushparaj P, Ahmed F, Algahtani HA, Al-Qahtani MH, Saleh Jamal H. Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics. 2015;8(Suppl 1):S4. doi: 10.1186/1755-8794-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, Walsh CA, Woods CG. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. doi: 10.1038/ng995. [DOI] [PubMed] [Google Scholar]
- 7.Kouprina N, Pavlicek A, Collins NK, Nakano M, Noskov VN, Ohzeki J, Mochida GH, Risinger JI, Goldsmith P, Gunsior M, Solomon G, Gersch W, Kim JH, Barrett JC, Walsh CA, Jurka J, Masumoto H, Larionov V. The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum Mol Genet. 2005;14:2155–2165. doi: 10.1093/hmg/ddi220. [DOI] [PubMed] [Google Scholar]
- 8.Paramasivam M, Chang YJ, LoTurco JJ. ASPM and citron kinase co-localize to the midbody ring during cytokinesis. Cell Cycle. 2007;6:1605–1612. doi: 10.4161/cc.6.13.4356. [DOI] [PubMed] [Google Scholar]
- 9.Luers GH, Michels M, Schwaab U, Franz T. Murine calmodulin binding protein 1 (Calmbp1): tissue-specific expression during development and in adult tissues. Mech Dev. 2002;118:229–232. doi: 10.1016/s0925-4773(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 10.Horvath S, Zhang B, Carlson M, Lu KV, Zhu S, Felciano RM, Laurance MF, Zhao W, Qi S, Chen Z, Lee Y, Scheck AC, Liau LM, Wu H, Geschwind DH, Febbo PG, Kornblum HI, Cloughesy TF, Nelson SF, Mischel PS. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc Natl Acad Sci U S A. 2006;103:17402–17407. doi: 10.1073/pnas.0608396103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SY, Pan HW, Liu SH, Jeng YM, Hu FC, Peng SY, Lai PL, Hsu HC. ASPM is a novel marker for vascular invasion, early recurrence, and poor prognosis of hepatocellular carcinoma. Clin Cancer Res. 2008;14:4814–4820. doi: 10.1158/1078-0432.CCR-07-5262. [DOI] [PubMed] [Google Scholar]
- 12.Wang WY, Hsu CC, Wang TY, Li CR, Hou YC, Chu JM, Lee CT, Liu MS, Su JJ, Jian KY, Huang SS, Jiang SS, Shan YS, Lin PW, Shen YY, Lee MT, Chan TS, Chang CC, Chen CH, Chang IS, Lee YL, Chen LT, Tsai KK. A gene expression signature of epithelial tubulogenesis and a role for ASPM in pancreatic tumor progression. Gastroenterology. 2013;145:1110–1120. doi: 10.1053/j.gastro.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 13.Alsiary R, Bruning-Richardson A, Bond J, Morrison EE, Wilkinson N, Bell SM. Deregulation of microcephalin and ASPM expression are correlated with epithelial ovarian cancer progression. PLoS One. 2014;9:e97059. doi: 10.1371/journal.pone.0097059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vange P, Bruland T, Beisvag V, Erlandsen SE, Flatberg A, Doseth B, Sandvik AK, Bakke I. Genome-wide analysis of the oxyntic proliferative isthmus zone reveals ASPM as a possible gastric stem/progenitor cell marker over-expressed in cancer. J Pathol. 2015;237:447–459. doi: 10.1002/path.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng X, Luo Z, Kang Q, Deng D, Wang Q, Peng H, Wang S, Wei Z. FOXQ1 mediates the crosstalk between TGF-beta and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol Ther. 2015;16:1099–1109. doi: 10.1080/15384047.2015.1047568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi EJ, Kim MS, Yoo NJ, Lee SH. Frameshift mutation of ASPM gene in colorectal cancers with regional heterogeneity. Pathol Oncol Res. 2016;22:877–879. doi: 10.1007/s12253-016-0108-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhong X, Liu L, Zhao A, Pfeifer GP, Xu X. The abnormal spindle-like, microcephaly-associated (ASPM) gene encodes a centrosomal protein. Cell Cycle. 2005;4:1227–1229. doi: 10.4161/cc.4.9.2029. [DOI] [PubMed] [Google Scholar]


