Abstract
Gastrointestinal stromal tumors (GISTs) are the most frequently mesenchymal tumors found in the gastrointestinal tract. These tumors are usually composed of spindle-shaped cells. More than 80% of GISTs harbor mutations of KIT gene which encodes for an important receptor tyrosine kinase (RTK) type III. Within KIT gene mutations, the mutation of exon 13 is very rare. Here we described a case of GIST in a 42-year-old male which carried two new KIT exon 13 mutations (R634W and N655T). This patient was diagnosed with upper digestive tract hemorrhage and later CT scan revealed a 2.2 cm × 4.0 cm soft-tissue mass on the posterior wall of the stomach. The patient went through a laparoscopic gastrectomy. Following pathological examination revealed this tumor to be a low-risk GIST. Gene sequencing analysis shown that the tumor had two mutations in KIT exon 13, which were not found in the literature. The postoperative course was uneventful and no recurrence was observed after 6 months. Furthermore, we also gave a short review of previously published papers describing KIT exon 13 mutations.
Keywords: Gastrointestinal stromal tumors (GISTs), KIT mutation, exon 13, receptor tyrosine kinase (RTK), digestive tract hemorrhage
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract [1,2]. The majority of GISTs are frequently localized in the stomach, then the small intestine, the colon-rectum, and the esophagus [3]. GISTs are often consisted of spindle cells, but can also be composed of epithelioid cells or mixed cell types. The majority of GISTs are characterized by KIT or PDGFRA gene mutations. Both KIT and PDGFRA are coding for two important receptor tyrosine kinase (RTK) type III which consist of five Ig-like domains, a transmembrane, a juxtamembrane and two tyrosine kinase domains separated by a kinase insert [4]. The KIT gene mutations are most frequently occurred in exon 11 (60-70% of cases), followed by exon 9 (10% of the cases); exon 13 (1%) and 17 (0.5-1%) mutations are very rare and generally not primary [1,5-8]. RTK inhibitor imatinib mesylate (Gleevec, Novartis Pharma AG, Basel, Switzerland) can target KIT and show very good effects on sensitive GISTs, therefore this drug serves as the first-line option in the medical treatment of GISTs [9,10]. Here, we described a case of gastric GIST with double new KIT exon 13 substitution mutations.
Clinical history
A 42-year old male had symptom of melena for three days and also with vomiting of brown-colored stomach contents. This patient went to the hospital and was diagnosed with upper digestive tract bleeding. Chest computed tomography (CT) scan revealed a 2.2 cm × 4.0 cm soft-tissue mass on the posterior wall of the stomach (Figure 1). Together with gastroscopy, both exams were all indicating GIST. After all the above symptoms were restrained, the patient underwent laparoscopic gastrectomy.
Figure 1.
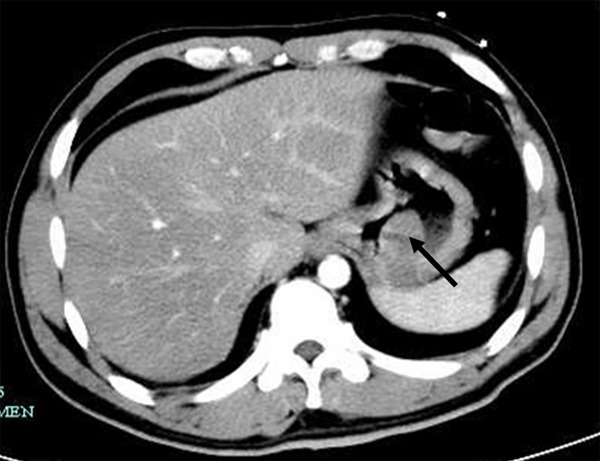
Chest computed tomography scan. CT scan demonstrating 2.2 cm × 4.0 cm mass on the posterior wall of the stomach, arrow indicated.
Pathologic findings
Grossly, a solid mass was observed, and this tumor had white cut surface measured in 4 cm × 2.5 cm × 2.2 cm size. Pathologically, this tumor had a clear boarder and was locating under the mucosa and muscular layer. The tumor mass was comprised of spindle-shaped cells with swirling and fence-like distribution (Figure 2A), and with focal necrosis. Additionally, superficial ulcer was observed on the tumor surface which invaded neighboring blood vessel wall, but the tumor did not rupture (Figure 2B). Specifically, there were many lymphocytes infiltrating in the mesenchyme (Figure 2C). The mitotic rate is lower than 5/50 HPF. Immunohistologically, the spindle-shaped cells of tumor sections were positive for CD117 (Figure 3A), DOG-1 (Figure 3B), CD34 and negative for S-100, desmin and smooth muscle actin (SMA). Moreover, the infiltrating lymphocytes were positive for CD3 and negative for CD20 and TdT, suggesting they were mainly the T lymphocytes. Besides, the overall Ki-67 index was around 8%. Histologic and immunohistochemical examinations of the surgically resected solid tissue revealed this lesion to be a low-risk gastrointestinal stromal tumor (GIST). KIT and PDGFRA triplicate DNA sanger sequencing analysis shown two point mutations in KIT gene exon 13, nucleotide sequence 1920 C→T and 1964 A→C, which resulted in amino acid substitution of amino acid sequence R634W (Figure 4A) and N655T (Figure 4B), respectively.
Figure 2.

Pathological analysis. (A) H+E × 100, the tumor was mainly consisted of spindle-shaped cells with swirling and fence-like distribution; (B) H+E × 100, superficial ulcer was observed on the tumor surface and invaded neighboring blood vessel wall, arrow indicated (C) H+E × 200, tumor cells shown no obvious atypia and rarely had mitosis. Many lymphocytes was observed infiltrating in the mesenchyme.
Figure 3.

Immunohistochemical analysis. A. × 200, the spindle-shaped cells of the tumor were strongly positive for CD117; B. × 200, the spindle-shaped cells of the tumor shown positivity of DOG-1.
Figure 4.

Sanger sequence analysis of KIT exon 13. A. Nucleotide substitution of C to T at position 1920, leading to R to W in amino acid position 634. B. Substitution of A to C in nucleotide sequence of 1964, resulting in amino acid substitution of N to T at position 655. Mutations were arrow indicated.
Discussion
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and this tumor is characterized by KIT or PDGFRA gene mutations. After surgical resection, locally recurrence, abdomen spread and/or liver metastasis can often be seen in GISTs. Unresectable or metastasis GISTs are fatal due to their inherent resistance to both chemotherapy and radiation therapy [11]. The median survival duration of patients with metastasis is around 20 months and for those with local recurrence is only 9-12 months [12]. Imatinib mesylate is now the first-line option in the medical treatment of GISTs which targeting KIT and PDGFRA [9,10].
Our patient went to the hospital due to digestive tract hemorrhage and later was found to have GIST on his stomach wall. The patient underwent laparoscopic gastrectomy and remained health in the 6 follow-up months, no disease recurrence or metastasis was reported.
Based on the pathological analysis, hemorrhage was most likely caused by ulcer on the tumor surface that invaded neighboring blood vessel wall. Notably, digestive tract bleeding was rarely occurring in GISTs. Another characteristic of this case was many T lymphocyte infiltrating in the tumor mesenchyme. Moreover, the tumor in this case was absent of cytoplasmic vacuoles, which are generally present in most stomach GISTs. Whether the specific gene mutations we found influence the tumor pathological and morphological features remained unclear, and needed further research.
Based on literature, mutation rate of KIT gene exon 13 is very rare (1%) and mutations that have been reported are K642E, V654A and T670I [13-15]. GISTs having mutation of K642E were responsive to imatinib therapy. Whereas the V654A and T670I mutations are secondary mutations that caused secondary resistance. All the KIT exon 13 mutations found in the GISTs had poorer prognosis than other KIT exon mutations [16]. In this case, we detected two novel point mutations R634W and N655T. Moreover, the N655T mutation was next to the reported mutation of V654A and they were all in ATP-binding section. Whether the N655T had same effect as V654A as well as the consequence of the mutation of R634W still needed careful study of molecular mechanisms.
In conclusion, the present study is the first to report a case of GIST with double KIT exon 13 mutations. GISTs have been considered as the most frequent mesenchymal tumors of the gastrointestinal tract and can be identified based on its typical histological and immunohistochemical features. Patients diagnosed with medium and high-risk GISTs carrying responsive mutations will receive tyrosine kinase inhibitors (TKIs) therapy. Therefore finding new KIT gene mutations and understanding drug response of new gene mutations are very necessary. Whether these two new mutations we found are resistant to Gleevec treatment still require further study.
Disclosure of conflict of interest
None.
References
- 1.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 2004;22:3813–25. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CH, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 3.Miettinern M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumors: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 5.Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal tromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091–1095. doi: 10.1016/S0002-9440(10)64623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lux ML, Rubib BP, Biase TL, Chen CJ, Xiao S, Singer S, Fletcher CD, Fletcher JA. KIT extracellular and kinase mutations in gastrointerstinal stromal tumors. Am J Pathol. 2000;156:791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vu HA, Xinh PT, Kikushima M, Zhu Y, Tokuhara M, Shimizu T, Saito K, Tokunaga K, Sato Y. A recurrent duodenal gastrointestinal stromal tumor with a frameshift mutation resulting in a stop codon in KIT exon 13. Genes Chromosomes Cancer. 2005;42:179–183. doi: 10.1002/gcc.20110. [DOI] [PubMed] [Google Scholar]
- 8.Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505–510. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH818>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, von Mehren M, Antonescu CR, Dematteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN task force report: update on the management of patients with gastrointerstinal stromal tumors. J Natl Compr Canc Netw. 2010;8:S1–41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agulnik M, Giel JL. Understanding rechallenge and resistance in the tyrosine kinase inhibitor era: imatinib in gastrointestinal stromal tumors. Am J Clin Oncol. 2014;37:417–22. doi: 10.1097/COC.0b013e31824be3d6. [DOI] [PubMed] [Google Scholar]
- 11.DeMatteo RP, Heinrich MC, el-Rifai W, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 12.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognosis factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasota J, Corless CL, Heinrich MC, Debiec-Rychter M, Sciot R, Wardelmann E, Merkelbach-Bruse S, Schildhaus HU, Steigen SE, Stachura J, Wozniak A, Antonescu C, Daum O, Martin J, Del Muro JG, Miettinen M. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: a multicenter study on 54 cases. Mod Pahtol. 2006;21:476–489. doi: 10.1038/modpathol.2008.2. [DOI] [PubMed] [Google Scholar]
- 14.Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, Fletcher CD, Corless CL, Fletcher JA. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, Mckinley A, Ou WB, Fletcher JA, Fletcher C, Huang X, Cohen DP, Baum CM, Demetri GD. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008;26:5352–5259. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tornillo L. Gastrointestinal stromal tumor-an evolving concept. Front Med (Lausanne) 2014;1:1–12. doi: 10.3389/fmed.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]


