Abstract
Background: Gastric cancer (GC) is one of the most common digestive malignancies worldwide. N-myc downstream-regulated gene 2 (NDRG2) is a differentiation-related gene which is considered to be a metastasis suppressor gene. The purpose of this study was to detect the serum expression of NDRG2 and its clinical significance in the early detection of patients with GC. Methods: Serum NDRG2 expression were examined in 107 patients with GC, 52 with benign gastric disease patients, and 64 healthy volunteers using reverse transcription quantitative real-time polymerase chain reaction (qRT-PCR) and western blot analysis at mRNA and protein level, respectively. The relationship between NDRG2 expression and clinicopathologic characteristics was analyzed by chi-square test. The diagnostic value of NDRG2 was estimated via establishing the receiver operating characteristic (ROC) curve. Results: the serum NDRG2 expression was lower in GC patients than that in patients with benign disease and healthy volunteers both at mRNA and protein level (P<0.05). And the low NDRG2 expression was significantly associated with tumor size, lymph node metastasis and TNM stage. ROC curve manifested that NDRG2 had a high diagnostic value with an AUC of 0.896 corresponding with a sensitivity of 85.9% and a specificity of 62.6%. Conclusion: The expression of NDRG2 was reduced in GC patients. Moreover, serum NDRG2 could be a potential diagnostic marker for GC.
Keywords: NDRG2, diagnosis, gastric cancer
Introduction
Gastric cancer (GC) is one of the most common malignant tumors with a high incidence rate and it is the second most common cause of cancer-related deaths in Asia [1]. Most patients who have been clinically diagnosed are already at middle or advanced stage because there were no obvious symptoms in the early stage of GC which lead to a poor prognosis. The 5-year survival rate is disappointingly less than 10% globally [2]. Moreover, the clinically diagnosed patients may miss the best treatment opportunity [3]. Conventional treatments with surgery, chemotherapies or radiation therapy play minor roles in improving the patients’ survival rates [4,5]. Therefore, it is urgent to find out a specific and sensitive bio-marker for the early diagnosis of GC.
N-myc downstream-regulated gene 2 (NDRG2), located on human chromosome 14q11.2, is a member of the NDRG family, a new family of differentiation-related genes which are associated with cell proliferation, differentiation, apoptosis, stress responses, and cell migration/metastasis [6-9]. In previous studies, NDRG2 is aberrant expressed and often act as a tumor suppressor in various types of cancers such as glioblastoma, colorectal cancer, gallbladder carcinoma, breast cancer, and prostatic carcinoma [10-14]. In addition, previous studies have also demonstrated that NDRG2 is down-regulated in gastric cancer tissues [15,16]. However, little is known about the association between NDRG2 expression and diagnosis in GC.
In this study, we detected the serum expression of NDRG2 in GC patients, gastric benign disease controls and healthy controls, and analyzed its relationship with clinical factors of patients. What’s more, we estimated the diagnostic value of NDRG2 via building a ROC curve in GC.
Materials and methods
Patients and samples
This study was approved by the Ethics Committee of the Chinese PLA General Hospital. All serum specimens and clinical materials were obtained and used after written informed consents from the patients and the Chinese PLA General Hospital was obtained. A total of 107 patients who were diagnosed with GC were enrolled in the study. None of them had received any radiotherapy or chemotherapy before sampling. Tumors were classified according to the TNM cancer staging system set by the Union of International Cancer Control. Besides, 52 patients with benign gastric diseases such as gastritis, gastrohelcosis, and gastric polyps, and 64 healthy volunteers were taken as benign controls and healthy controls, respectively. None of the control patients had formerly been diagnosed with any malignancy.
The blood specimens were severally obtained from 107 patients with GC and 116 controls and lasted for 30-60 min. Then the specimens were centrifuged at 2000 rpm for 15 min at room temperature. The supernatant was transferred to an EP tube and stored at -80°C for further use. The data of clinicopathological features of the GC patients including age, sex, tumor size, histological grade, lymph node metastasis, invasion depth, and TNM stage were recorded in a database.
RNA extraction and qRT-PCR analysis
Total RNA was isolated from all samples by using mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA), respectively. cDNA was generated by reverse transcription using PrimeScript RT (Takara, Dalian, China) according to the manufacturer’s instructions. The generated cDNA was pooled and amplified by PCR. Then RT-PCR reaction was conducted at the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, California, USA). GAPDH was taken as the internal controls. The primers sequences of NDRG2 and GAPDH were as follows: NDRG2: forward-5’-ATCTCTGGACCAGCTTGCAG-3’, reverse-5’-TATCTCGCCAGGATGTAGGC-3’; GAPDH: forward-5’-CTGGGCTAC ACTGAGCACC-3’, reverse-5’-AAGTGGTCGTTGAGGGCAATG-3’. The relative mRNA expression of NDRG2 was calculated using the 2-ΔΔCt method. Each sample was in triplicate.
Western blot analysis
Total protein was extracted from the serum of patients with GC, benign controls and healthy controls, respectively. Then the protein was separated by SDS-PAGE and the brands were transferred onto nitrocellulose membranes (Amersham Pharmacia Biotech, China). Membranes were blocked in 5% non-fat milk and incubated with primary antibody at 4°C for overnight. After washing for 1 h, the membrane was incubated with HRP-conjugated secondary antibody (Sigma) for 1 h at room temperature. The resulting blot was visualized by ECL-Plus Western detection reagents (Amersham Biosciences).
Statistical analysis
All statistical analyses were carried out using Origin pro 9.0. Each experiment was repeated at least three times, and the data were presented as means ± SD. The differences between two groups were analyzed using Students’ t test. The relationship between serum NDRG2 expression and clinicopathological factors were assessed using x2 tests or Fisher’s exact tests. Receiver operating characteristic (ROC) curve was established to determine the diagnostic performance of serum NDRG2 levels in distinguishing patients with GC from healthy controls. All P values <0.05 were considered to be statistically significant.
Results
Expression of NDRG2was decreased in GC patients
QRT-PCR was used to evaluate the mRNA expression of serum NDRG2 while western blot was taken to measure the protein expression of this gene in 107 GC patients, 52 benign gastric disease patients and 64 healthy volunteers. As shown in Figure 1, the serum NDRG2 expression was significantly lower in patients with GC than that in benign gastric disease controls and healthy controls both at mRNA and protein level (P<0.05).
Figure 1.
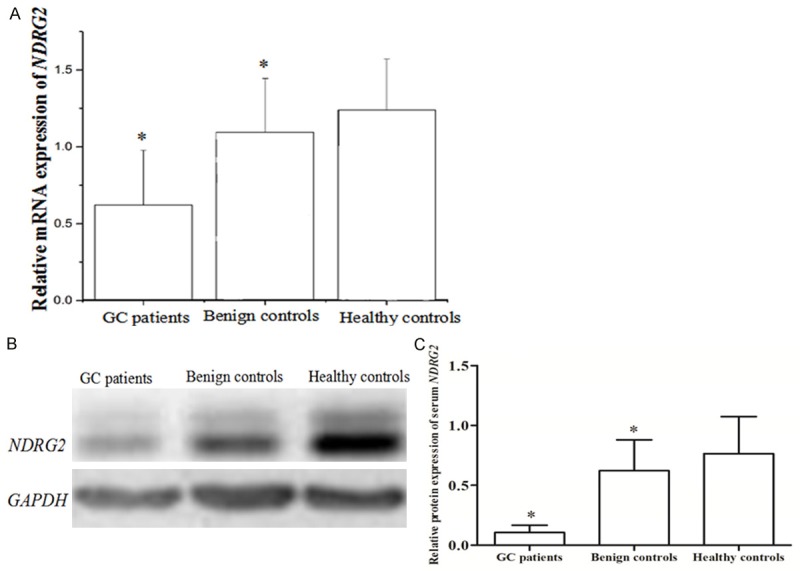
Serum NDRG2 expression in GC patients, benign gastric disease control and healthy controls. The expression of NDRG2 was lower in patients with GC compared to that in benign controls and healthy controls both at mRNA (A) and protein (B, C) level (P<0.05).
Relationship between NDRG2 expression and clinicopathological characteristics of patients with GC
To further investigate whether NDRG2 was correlated with the development of GC, we further analyzed the association between it with the clinicopathological data. And all the data of clinicopathological characteristics of the GC patients were showed in Table 1. The result showed that the low NDRG2 expression was closely associated with tumor size (P=0.003), lymph node metastasis (P=0.025) and TNM stage (P=002). However, there was no association between NDRG2 expression and patients’ age, gender, histological grade and invasion depth (P>0.05, Table 1).
Table 1.
Association between serum NDRG2 expression and clinicopathological features of patients with GC
| Variables | Cases (n=107) | NDRG2 expression | P values | |
|---|---|---|---|---|
|
| ||||
| Low (n=64) | High (n=43) | |||
| Age (years) | 0.971 | |||
| <60 | 50 | 30 | 20 | |
| ≥60 | 57 | 34 | 23 | |
| Gender | 0.273 | |||
| Male | 68 | 38 | 30 | |
| Female | 39 | 26 | 13 | |
| Tumor size | 0.003 | |||
| <5 cm | 46 | 20 | 26 | |
| ≥5 cm | 61 | 44 | 17 | |
| Histological grade | 0.190 | |||
| Well and moderate | 49 | 26 | 23 | |
| Poor | 58 | 38 | 20 | |
| Lymph node metastasis | 0.025 | |||
| Absent | 41 | 19 | 22 | |
| Present | 66 | 45 | 21 | |
| Invasion depth | 0.087 | |||
| T1 + T2 | 37 | 18 | 19 | |
| T3 + T4 | 70 | 46 | 24 | |
| TNM stage | 0.002 | |||
| I-II | 43 | 18 | 25 | |
| II-IV | 64 | 46 | 18 | |
Diagnostic value of NDRG2 in GC
To explore the diagnostic value of NDRG2 in GC, ROC curve was built. The outcome showed that NDRG2 had a high diagnostic value with an area under the curve (AUC) value of 0.896 combing with a sensitivity of 85.9% and a specificity of 62.6% (Figure 2). And the ideal cutoff value for NDRG2 expression was 0.8350.
Figure 2.
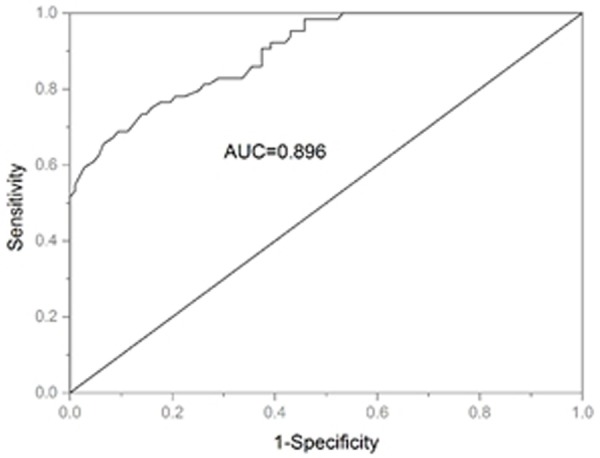
ROC curve for evaluation of the accuracy of serum NDRG2 to discriminate patients with GC from healthy controls.
Discussion
GC is one of malignant tumors threatening human health. Despite the application of varieties of techniques of imaging and endoscopic examinations have taken effect on the diagnosis of this tumor which make the survival time of GC patients protracted, it is rather complex and expensive of the examination process, especially difficult to realize the early detection [17,18]. Therefore, there is limitation in the early diagnosis, judgment of recurrence and evaluation of efficacy for tumors [19].
NDRG2, a newly discovered gene, contains an acyl carrier protein-like domain and can be harvested from human brain tissue by clone technology [20]. An increasing number of studies had revealed that the expression of NDRG2 was abnormal in variety of tumors. For instance, lorentzen et al., found NDRG2 was decreased in patients with colorectal carcinoma and related to the progression of the cancer [21]. NDRG2 was considered to be reduced and involved in the development of liver cancer [22]. In the study of Li et al., NDRG2 was confirmed to be down-regulated and could predict the prognosis of patients with astrocytomas [23]. Hu et al. reported that NDRG2 expression was significantly decreased in human liver and pancreatic cancers, without relationship to mutations in its coding region [24]. In our study, we found that the expression level of NDRG2 was lower in GC patients than that in gastric benign disease controls and healthy controls. These results are in agreement with previous studies [25]. Our findings revealed NDRG2 was a tumor suppressor.
NDRG2 was reported to be involved in many physiological and pathophysiological processes of diseases including differentiation, stress injury and organ formation via different ways. It was found that NDRG2 could suppress cell proliferation possibly by regulating the expression of cyclin D1 and T-cell factor (TCF)/β-catenin activity [26,27]. Kim et al. showed that NDRG2 could suppress tumor invasion by inhibiting MMP activities which were regulated by nuclear factor kappa B (NF-kB) signaling [28]. In breast cancer, NDRG2 could induce BMP-4 and suppress MMP-9 activity, thereby inhibits the metastatic potential of breast cancer cells [29]. Dake et al. reported that decreased expression of NDRG2 was significantly correlated with differentiation status, lymph node metastasis, and tumor node metastasis stage in patients with colorectal cancer [30]. Ma et al. found that low NDRG2 expression was significantly correlated with advanced TNM stage in breast carcinoma [31]. Li et al. reported that the expression level of NDRG2 was decreased in human lung cancer tissues, and positively correlated with tumor grade and tumor size [32]. Therefore, we estimated its relationship with clinical factors to see whether NDRG2 was involved in the development of GC. The result showed that the expression of NDRG2 was closely correlated with the clinical factors which indicated it participated in the progression of GC.
The prognostic value of NDRG2 had been verified in some cancers such as esophageal squamous cell carcinoma, glioblastoma multiforme, prostate cancer and pancreatic cancer [33-36]. However, its diagnostic value was rarely reported. In the present study, we explored its diagnostic value in GC. The outcome exhibited that NDRG2 could act as an independent diagnostic marker in GC for its high sensitivity and specificity. This was the first study showing the diagnostic value of NDRG2 expression in GC.
In conclusion, these findings provides the convincing evidence for the first time that the down-regulation of NDRG2 may serve as a novel molecular marker for the diagnosis of GC, and it is involved in the development and progression of this cancer. However, there are still some limitations. Firstly, the sample size is small. To solve this problem, further studies and more samples will be required to be done. Secondly, the current study has not elucidated the exact molecular mechanisms of NDRG2 acting on GC, which is also worth to be further investigated.
Disclosure of conflict of interest
None.
References
- 1.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 2.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A, Lieto E, Ciardiello F, De Vita F. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell MC, Mansfield PF. Surgical approaches to gastric cancer. J Surg Oncol. 2013;107:250–258. doi: 10.1002/jso.23180. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Wang B, Gao W, Huang S, Liu Z, Li W, Jia J. SIRT1 is downregulated in gastric cancer and leads to G1-phase arrest via NFkappa B/Cyclin D1 signaling. Mol Cancer Res. 2013;11:1497–1507. doi: 10.1158/1541-7786.MCR-13-0214. [DOI] [PubMed] [Google Scholar]
- 6.Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, He F. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem. 2002;229:35–44. doi: 10.1023/a:1017934810825. [DOI] [PubMed] [Google Scholar]
- 7.Shimono A, Okuda T, Kondoh H. N-mycdependent repression of ndr1, a gene identified by direct subtraction of whole mouse embryo cDNAs between wild type and N-myc mutant. Mech Dev. 1999;83:39–52. doi: 10.1016/s0925-4773(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 9.Zhao W, Tang R, Huang Y, Wang W, Zhou Z, Gu S, Dai J, Ying K, Xie Y, Mao Y. Cloning and expression pattern of the human NDRG3 gene. Biochim Biophys Acta. 2001;1519:134–138. doi: 10.1016/s0167-4781(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 10.Tepel M, Roerig P, Wolter M, Gutmann DH, Perry A, Reifenberger G, Riemenschneider MJ. Frequent promoter hypermethylation and transcriptional downregulation of the NDRG2 gene at 14q11.2 in primary glioblastoma. Int J Cancer. 2008;123:2080–2086. doi: 10.1002/ijc.23705. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li X, Yang G, Liu X, Yao L, Zhang J, Shen L. Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression. Oncotarget. 2015;6:26161–26176. doi: 10.18632/oncotarget.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DG, Lee SH, Kim JS, Park J, Cho YL, Kim KS, Jo DY, Song IC, Kim N, Yun HJ, Park YJ, Lee SJ, Lee HG, Bae KH, Lee SC, Shim S, Kim YM, Kwon YG, Kim JM, Lee HJ, Min JK. Loss of NDRG2 promotes epithelial-mesenchymal transition of gallbladder carcinoma cells through MMP-19-mediated Slug expression. J Hepatol. 2015;63:1429–39. doi: 10.1016/j.jhep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Lorentzen A, Lewinsky RH, Bornholdt J, Vogel LK, Mitchelmore C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer. 2011;11:14. doi: 10.1186/1471-2407-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu C, Wu G, Dang N, Zhang W, Zhang R, Yan W, Zhao Y, Gao L, Wang Y, Beckwith N, Yuan J, Yao L. Inhibition of N-myc downstream-regulated gene 2 in prostatic carcinoma. Cancer Biol Ther. 2011;12:304–313. doi: 10.4161/cbt.12.4.16382. [DOI] [PubMed] [Google Scholar]
- 15.Choi SC, Yoon SR, Park YP, Song EY, Kim JW, Kim WH, Yang Y, Lim JS, Lee HG. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fasmediated cell death. Exp Mol Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 16.Assamaki R, Sarlomo-Rikala M, Lopez-Guerrero JA, Lasota J, Andersson LC, Llombart-Bosch A, Miettinen M, Knuutila S. Array comparative genomic hybridization analysis of chromosomal imbalances and their target genes in gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2007;46:564–576. doi: 10.1002/gcc.20439. [DOI] [PubMed] [Google Scholar]
- 17.Chen XJ, Li N, Huang YD, Ren S, Liu F, Chen L, Wang Y, Chen M. Factors for postoperative gallstone occurrence in patients with gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:877–881. doi: 10.7314/apjcp.2014.15.2.877. [DOI] [PubMed] [Google Scholar]
- 18.Pan Z, Pang L, Ding B, Yan C, Zhang H, Du L, Wang B, Song Q, Chen K, Yan F. Gastric cancer staging with dual energy spectral CT imaging. PLoS One. 2013;8:e53651. doi: 10.1371/journal.pone.0053651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park K, Jang G, Baek S, Song H. Usefulness of combined PET/CT to assess regional lymph node involvement in gastric cancer. Tumori. 2014;100:201–206. doi: 10.1177/030089161410000214. [DOI] [PubMed] [Google Scholar]
- 20.Boulkroun S, Fay M, Zennaro MC, Escoubet B, Jaisser F, Blot-Chabaud M, Farman N, Courtois-Coutry N. Characterization of rat NDRG2 (N-Myc downstream regulated gene 2), a novel early mineralocorticoid-specific induced gene. J Biol Chem. 2002;277:31506–31515. doi: 10.1074/jbc.M200272200. [DOI] [PubMed] [Google Scholar]
- 21.Lorentzen A, Vogel LK, Lewinsky RH, Saebo M, Skjelbred CF, Godiksen S, Hoff G, Tveit KM, Lothe IM, Ikdahl T, Kure EH, Mitchelmore C. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer. 2007;7:192. doi: 10.1186/1471-2407-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DC, Kang YK, Kim WH, Jang YJ, Kim DJ, Park IY, Sohn BH, Sohn HA, Lee HG, Lim JS, Kim JW, Song EY, Kim DM, Lee MN, Oh GT, Kim SJ, Park KC, Yoo HS, Choi JY, Yeom YI. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–4220. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Wang J, Shen X, Wang L, Li X, Liu Y, Shi M, Zhao G, Deng Y. Expression and prognostic value of NDRG2 in human astrocytomas. J Neurol Sci. 2011;308:77–82. doi: 10.1016/j.jns.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Hu XL, Liu XP, Lin SX, Deng YC, Liu N, Li X, Yao LB. NDRG2 expression and mutation in human liver and pancreatic cancers. World J Gastroenterol. 2004;10:3518–3521. doi: 10.3748/wjg.v10.i23.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang X, Li Z, Ma J, Deng P, Zhang S, Zhi Y, Chen J, Dai D. DNA methylation of NDRG2 in gastric cancer and its clinical significance. Dig Dis Sci. 2013;58:715–723. doi: 10.1007/s10620-012-2393-z. [DOI] [PubMed] [Google Scholar]
- 26.Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS, Kim JH, Song EY, Lee HG, Choi I, Kim JW. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int J Cancer. 2009;124:7–15. doi: 10.1002/ijc.23945. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Yoon SY, Kim JT, Song EY, Lee HG, Son HJ, Kim SY, Cho D, Choi I, Kim JH, Kim JW. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598–605. doi: 10.1093/carcin/bgp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim A, Kim MJ, Yang Y, Kim JW, Yeom YI, Lim JS. Suppression of NF-kappaB activity by NDRG2 expression attenuates the invasive potential of highly malignant tumor cells. Carcinogenesis. 2009;30:927–936. doi: 10.1093/carcin/bgp072. [DOI] [PubMed] [Google Scholar]
- 29.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 30.Chu D, Zhang Z, Li Y, Wu L, Zhang J, Wang W. Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Mol Cancer Ther. 2011;10:47–56. doi: 10.1158/1535-7163.MCT-10-0614. [DOI] [PubMed] [Google Scholar]
- 31.Ma J, Liu W, Guo H, Li S, Cao W, Du X, Lei S, Hou W, Xiong L, Yao L, Li N, Li Y. N-myc downstream-regulated gene 2 expression is associated with glucose transport and correlated with prognosis in breast carcinoma. Breast Cancer Res. 2014;16:R27. doi: 10.1186/bcr3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SJ, Wang WY, Li B, Chen B, Zhang B, Wang X, Chen CS, Zhao QC, Shi H, Yao L. Expression of NDRG2 in human lung cancer and its correlation with prognosis. Med Oncol. 2013;30:421. doi: 10.1007/s12032-012-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao W, Yu G, Lu Q, Zhang J. Low expression of N-myc downstream-regulated gene 2 in oesophageal squamous cell carcinoma correlates with a poor prognosis. BMC Cancer. 2013;13:305. doi: 10.1186/1471-2407-13-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou B, Tang Z, Deng Y, Hou S, Liu N, Lin W, Liu X, Yao L. Tumor suppressor candidate gene, NDRG2 is frequently inactivated in human glioblastoma multiforme. Mol Med Rep. 2014;10:891–896. doi: 10.3892/mmr.2014.2237. [DOI] [PubMed] [Google Scholar]
- 35.Ren GF, Tang L, Yang AQ, Jiang WW, Huang YM. Prognostic impact of NDRG2 and NDRG3 in prostate cancer patients undergoing radical prostatectomy. Histol Histopathol. 2014;29:535–542. doi: 10.14670/HH-29.10.535. [DOI] [PubMed] [Google Scholar]
- 36.Yamamura A, Miura K, Karasawa H, Morishita K, Abe K, Mizuguchi Y, Saiki Y, Fukushige S, Kaneko N, Sase T, Nagase H, Sunamura M, Motoi F, Egawa S, Shibata C, Unno M, Sasaki I, Horii A. Suppressed expression of NDRG2 correlates with poor prognosis in pancreatic cancer. Biochem Biophys Res Commun. 2013;441:102–107. doi: 10.1016/j.bbrc.2013.10.010. [DOI] [PubMed] [Google Scholar]


