Abstract
Radiotherapy-induced lacrimal gland injury often causes dry eye. Oxidative stress and local inflammation are the primary consequences of radiotherapy-induced injury. The most recent research shows that the human-specific gene CHRFAM7A plays an important role in inflammation. However, the effect of CHRFAM7A on radiotherapy-induced lacrimal gland injury remains unclear. In this study, humanized mice were successfully generated via the transplantation of human peripheral blood mononuclear cells that expressed human-specific genes. After radiation, the CHRFAM7A gene was highly expressed in the lacrimal glands of humanized mice, in which it protected the function of the lacrimal gland after radiotherapy. CHRFAM7A down-regulated radiotherapy-induced inflammation by suppressing p38/JNK signalling. CHRFAM7A also inhibited oxidative stress in the haematopoietic system after radiotherapy. Further signalling pathway analyses indicated that CHRFAM7A suppressed Akt (protein kinase B, PKB) phosphorylation. CHRFAM7A may therefore be a therapeutic target in radiation-induced lacrimal gland injury.
Keywords: Radiotherapy, lacrimal gland, human-specific gene
Introduction
In head-and-neck tumours, radiotherapy (RT) tends to induce lacrimal gland injuries, which are clinically difficult to treat [1]. There is no effective treatment to repair RT-induced lacrimal gland injury because the precise mechanisms involved in its pathogenesis remain unknown. P38 pathway activation has been observed in cells and tissues in response to gamma-irradiation injury [2]. Inhibiting p38 attenuated irradiation-induced haematopoietic cell senescence [3]. In our previous study, we also found that suppressing the p38 pathway ameliorated RT-induced lacrimal gland injury [4]. Oxidative stress also plays a critical role in radiation-induced tissue injury by producing superoxide anion and other reactive oxygen species (ROS) in addition to reactive nitrogen species, which induce inflammation via the mitogen-activated protein kinase (MAPK) pathway [5,6]. Radiation-induced oxidative stress alters the expression of several genes that encode pro-oxidant and antioxidant proteins [7,8]. However, there are no reports showing that human-specific genes are involved in radiation-induced oxidative stress.
The human-specific gene CHRFAM7A arose from a rearrangement of the α7-N acetylcholine receptor gene (CHRNA7), which is located on human chromosome 15q13-14, during human evolution. The CHRFAM7A gene was originally found to be expressed in the central nervous system, where it is associated with mental illness [9]. It is also involved in inflammatory bowel disease and wound healing [10-13]. CHRFAM7A has been implicated in the cholinergic anti-inflammatory response [14]. CHRFAM7A is down-regulated by nicotine [15,16], and it inhibits the effects of α7nAChR/CHRNA7 because it encodes a functionally active channel that regulates the inflammatory response pathway [14,17,18].
Human-specific genes are likely to play important roles in the pathogenesis of lacrimal gland damage after RT. However, the role of the human-specific gene CHRFAM7A in RT-induced lacrimal gland injury remains poorly understood.
No wild-type animal models are well-suited for human-specific gene research, and this limits the study of human-specific genes in animal models. Humanized mice (HM) are induced to carry functional human genes via human haematopoietic stem cell transplantation. While they are often used to study human inflammatory responses to burns or sepsis [19,20], no studies have reported the results of exposing HM to radiation.
In this study, we hypothesized that the CHRFAM7A gene is a key factor in a process that relieves inflammation and oxidative stress in RT-induced lacrimal gland injury in HM. We determined that the CHRFAM7A suppressed p38/JNK signalling and Akt (protein kinase B, PKB) phosphorylation. Our findings may facilitate the development of effective human-specific gene therapies for lacrimal gland injury resulting from orbital disease RT.
Materials and methods
Animal preparation and irradiation
HM were constructed as previously described [21]. Ten healthy, 8-week-old female HM were used for this study. The mice underwent an initial lacrimal gland scintigraphy. The first group (n = 5) was irradiated with a dose of 15 Gy using a combination of 3 mg/kg (S)-ketamine-hydrochloride (Ketanest-S®, Parke-Davis, Hoofddorp, The Netherlands) and 0.1 mg/kg xylazine-hydrochloride (Rompun®, Bayer, Germany) while under general anaesthesia. Three days after irradiation, scintigraphy was performed a second time, and the left-side inferior lacrimal gland was then excised for histological examination. Seven days later, the same procedure was performed, and the contralateral lacrimal gland was removed. In the second group (n = 5), we performed sham surgeries, but the animals were not irradiated, and the excised tissues were used as control glandular tissues.
Ethics statement
The collection of lacrimal gland tissue at the Eye and ENT hospital of Fudan University (Shanghai, China) was approved by the ethics committee for human studies. The experimental procedures were approved by the Fudan University Animal Care and Use Committee, and all animals were housed under standard conditions according to institution-approved guidelines, as previously described [22].
Surgical harvesting of the inferior lacrimal gland
The inferior lacrimal gland was surgically exposed and excised, and the harvested glands were immediately fixed in 4% neutral phosphate-buffered formalin.
Lacrimal gland scintigraphy
The mice were placed in a prone position with the head projected to the front. After 3.7 MBq (1 mCi = 37 MBq, 100 µCi = 3.7 MBq) with Na99mTcO4 was intravenously administered to serve as a tracer, the mice underwent sequential scintigraphy using a four-head camera (Picker CX 250 compact, LEHR collimator and field-of-view of 25 cm; Nano SPECT/CT Plus, Bioscan Corporation). Time-activity curves were also registered and analysed.
Flow cytometry
Cell staining was optimized using isotype-matched antibodies and/or fluorescence minus one (FMO) analyses. Cells were processed for flow cytometry in PBS supplemented with 1.0% foetal bovine serum using a BD Biosciences Accuri flow cytometer, and the generated data were analysed using FlowJo software (TreeStar, Ashland, OR, USA). In all experiments, gating was set to a minimum of 10,000 viable cells by the results of staining with 7-AAD, as instructed by the manufacturer (BD Biosciences, San Jose, CA, USA), and the results were analysed using forward and side scatter.
Pharmacological inhibitor
A p38 inhibitor (5 mg/g SB203580; Sigma Aldrich, St Louis, MO, USA) was administered intraperitoneally 1 h before irradiation. This dose was based on dose-response studies that showed that 5 mg/g inhibited p38 MAPK activity [23,24].
Isolation of RNA from cultured cells and preparation of cDNA for PCR and qPCR
Total RNA was prepared from cell lysates using an RNeasy kit and quantified using a NanoDrop Spectrophotometer. One microgram of total RNA was reverse-transcribed using an iScript cDNA synthesis kit (Bio-Rad, San Diego, CA, USA) in a 20 μL reaction as instructed by the manufacturer, and 1 μL was used in RT-PCR or real-time qPCR analyses.
RT-PCR and quantitative RT-PCR analyses of CHRFAM7A
RT-PCR was performed using 50 μL reactions containing 45 μL of PCR blue mix (Invitrogen [Thermo Fisher Scientific]), 1 μL of each primer (10 μmol/L), 1 μL of cDNA and 2 μL of water. The cycling conditions were one cycle at 94°C for 4 min, 35 cycles at 94°C for 30 s, 60°C for 30 s and 72°C for 60 s, and a final extension at 72°C for 5 min. Ten microlitres of each PCR product was resolved on a 2% agarose gel, and images of the gels were acquired using an Alpha Innotech imaging system (Fisher Scientific [Thermo Fisher Scientific]). Real-time qPCR was performed in a 25 μL reaction containing 12.5 μL of 2 × SYBR Green PCR Master Mix (Bio-Rad), 0.5 μL of each primer (10 μmol/L), 1 μL of cDNA and 10.5 μL of water. The following qPCR cycling conditions were used: one cycle at 95°C for 10 min and 45 cycles at 94°C for 25 s, 60°C for 25 s and 72°C for 40 s. The primer efficiency for CHRFAM7A was 100%.
The primers used to amplify CHRFAM7A and human GAPDH are shown in Table 1. The mouse GAPDH primers used in this study were proprietary primers that were purchased from Qiagen (Cat #QT01658692).
Table 1.
Primers of the CHRFAM7A and human GAPDH gene
| Primers name | Sense (5’-3’) | Antisense (5’-3’) |
|---|---|---|
| CHRFAM7A | ATAGCTGCAAACTGCGATA | CAGCGTACATCGATGTAGCAG |
| hGAPDH | CATGAGAAGTATGACAACAGCCT | AGTCCTTCCACGATACCAAAGT |
Cell isolation and culture
Human primary haematopoietic CD34+ cells were provided by PromoCell (Heidelberg, Germany). Thawed CD34+ cells were cultured in serum-free Iscove’s Modified Dulbecco’s Medium (IMDM).
Cloning of CHRFAM7A and gene transfection
CHRFAM7A cDNA clones were placed in a pcDNA 3.1 backbone according to a previously described method [18]. Human CD34+ cells were cultured for 7 days and then transfected using Lipofectamine 2000 (Lipo2000) in conjunction with Nupherin (Biomol Research Laboratories, Plymouth Meeting, PA, USA) according to the manufacturer’s instructions.
Design of siRNA sequences and transfection of siRNA
The human CHRFAM7A gene sequence was obtained from GenBank (KJ899881.1). According to siRNA design principles, 4 siRNAs were designed to specifically target CHRFAM7A. The sequences that most effectively blocked CHRFAM7A expression and the sequence of the negative control are shown in Table 2. On the day before transfection, cells in the logarithmic growth phase were seeded in 6-well plates at a density of 6 × 104 cells/well. When cell confluency reached approximately 70%-80%, Opti-MEM I containing 0.5% foetal bovine serum was mixed with Lipo2000 and siRNA, and the mixture was then added to the cells. The cells were incubated at 37°C in 5% CO2 for 48 h. The number of transfected cells was determined using fluorescence microscopy. RT-PCR and western blot analyses were used to detect the protein expression level of CHRFAM7A and determine the best interference effect.
Table 2.
siRNA sequence of the CHRFAM7A gene
| Sequence name | Sense (5’-3’) | Antisense (5’-3’) |
|---|---|---|
| siRNA-CHRFAM7A | AGUUUCAACCGUCUUAAUCAG | GAUUAAGACGGUUGAAACUAG |
| Negative control (siRNA-NC) | UUCTCCGAACGUGCUCACGUTT | ACCUGACACGUUCGGAGAATT |
Western blot assay
Primary antibodies against the following proteins were used: Akt, p-Akt, p38, p-p38, JNK, p-JNK, Erk and p-Erk. All antibodies were purchased from Cell Signalling Technology. The signals were detected using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA) after the cells were incubated with IR Dye 800-conjugated anti-rabbit (LI-COR, Lincoln, NE, USA) secondary antibodies. The data were quantified using Image J software (NIH, Bethesda, MD, USA).
ROS levels of the haematopoietic cells in HM exposed to irradiation
The irradiated HM were divided into the two following groups: RT+ and RT++CHRFAM7A. Additional HM were included in non-radiation control (RT- group) and RT-+CHRFAM7A groups. PBMCs were collected from animals in the RT+ and RT++CHRFAM7A groups at 7 d and 14 d after irradiation. The PBMCs were resuspended in PBS (Beyotime, China) containing 5 mM glucose, 1 mM CaCl2, 0.5 mM MgSO4, and 5 mg/mL bovine serum albumin. A Reactive Oxygen Species Assay Kit (Beyotime, China) was used according to the kit instructions. PBMCs (1 × 106/mL) were incubated with diluted DCFH-DA (at a final concentration of 10 μmol/L) in the dark at 37°C for 20 min. ROS-up was added to the positive control wells. After they were incubated, the cells were rinsed in PBS 3 times to fully remove any non-internalized DCFH-DA. To assess ROS levels, flow cytometry (Becton-Dickinson, USA) was used to measure the fluorescence intensity of DCF in the BMNCs (at 488-nm excitation and 524-nm emission).
Statistical analyses
Statistical analyses were performed using a two-tailed Student’s t test, and One-way analysis of variance test was performed by SAS 6.12 (Software, Inc., San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
CHRFAM7A expression was higher in the human lacrimal gland after RT than before RT
When normal human lacrimal glands were compared to human lacrimal glands that were exposed to RT, we found that CHRFAM7A was expressed at higher levels after RT. This increased expression was detected using immunohistochemical staining and RT-PCR (Figure 1).
Figure 1.
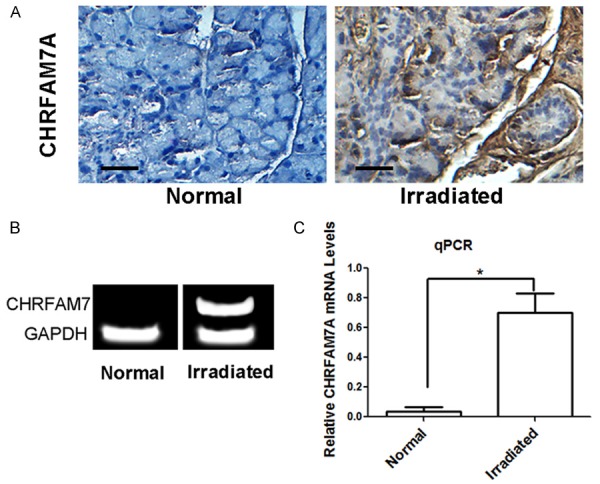
A. Immunohistochemical staining for CHRFAM7A in irradiated and normal lacrimal glands. Scale bar = 15 µm. B. Gel images of RT-PCR analysis of CHRFAM7A expression in normal and irradiated lacrimal gland tissues. C. Statistical analysis of the relative mRNA levels in the lacrimal gland.
Human-specific CHRFAM7A expression in constructed humanized mice
Human CD45-positive cells were detected in a variety of tissues in humanized mice, including the liver, spleen and peripheral blood (Figure 2A, 2B). The human-specific gene CHRFAM7A was also expressed in the liver, spleen, and peripheral blood (Figure 2C, 2D).
Figure 2.
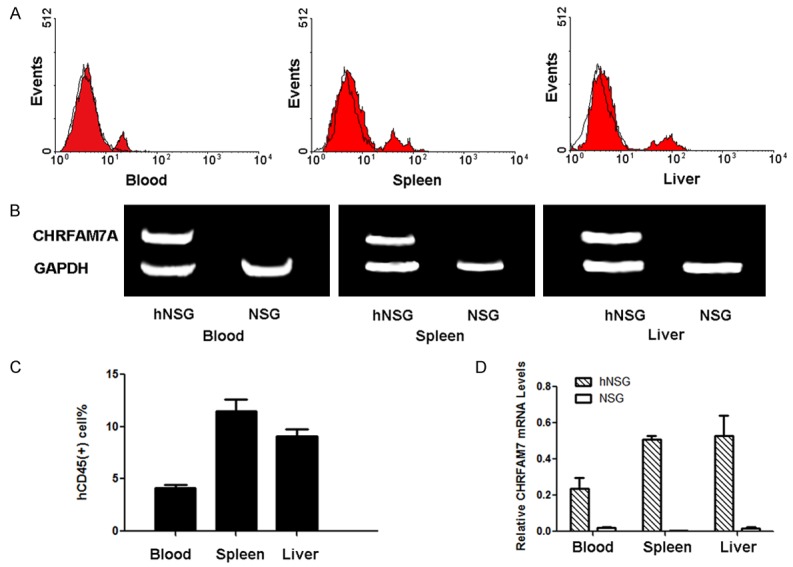
Human peripheral blood cells circulate in humanized mice. A. Histograms showing the results of flow cytometry for human CD45. B. Gel images of RT-PCR analyses of CHRFAM7A expression. C. Statistical analysis of the proportion of hCD45-positive cells in the blood, spleen and liver. D. Statistical analysis of relative mRNA levels in the blood, spleen and liver.
CHRFAM7A gene expression and tear secretion in RT-injured lacrimal glands
CHRFAM7A was expressed a higher levels in the lacrimal glands of HM treated with irradiation than in the control groups (NSG mice and mice without irradiation) (Figure 3A, 3B). After 50 s and 120 s, tear secretion was also lower in HM that was treated with irradiation than was observed in the control group (Figure 3C).
Figure 3.
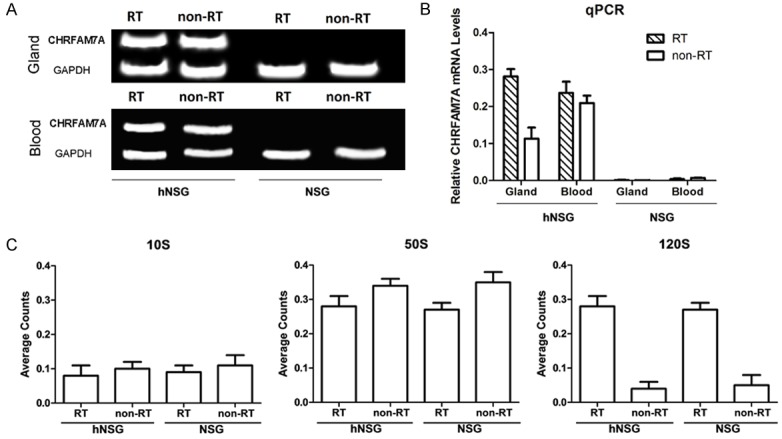
Human-specific gene expression in RT-injured glands. A. RT-PCR image of CHRFAM7A gene expression in the blood and lacrimal glands after irradiation. B. Real-time PCR analysis of CHRFAM7A gene expression in the blood and lacrimal glands in radiotherapy- and non-radiotherapy-treated mice. C. Tear secretion function analysis of the major lacrimal glands of mice in the radiotherapy and non-radiotherapy treatment groups.
CHRFAM7A siRNA construct and transfection
CHRFAM7A was expressed at significantly lower levels in cultured human primary haematopoietic CD34+ (hCD34+) cells after siRNA transfection, indicating that the siRNA we designed was effective (Figure 4). CHRFAM7A expression was also lower in the peripheral blood and lacrimal glands of HM that were treated with siRNA-transfected hCD34+ cells. CHRFAM7A expression was significantly lower in the HM that were injected with siRNA-transfected cells and treated with lacrimal gland irradiation (Figure 4).
Figure 4.
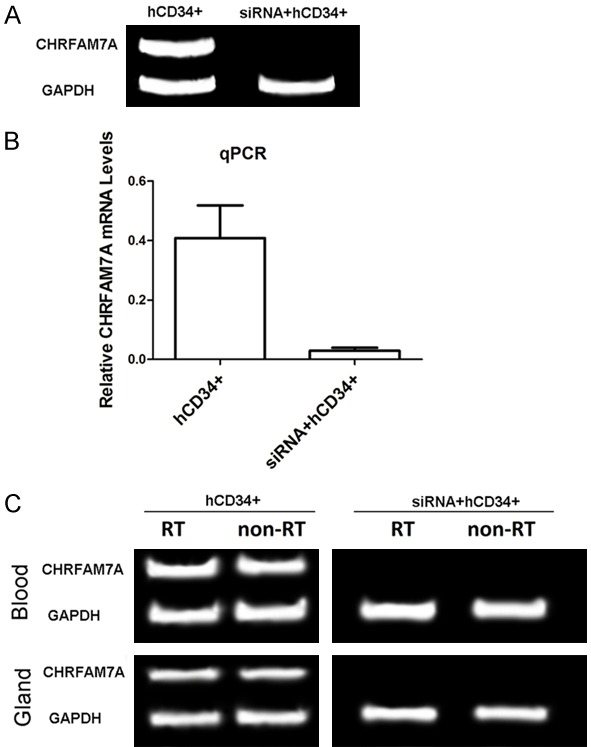
CHRFAM7A expression after siRNA transfection and screening. A. In vitro study, RT-PCR gel image of CHRFAM7A mRNA expression in human CD34+ cells (hCD34+) and human CD34+ cells that were transfected with siRNA (siRNA + hCD34+). B. Real-time PCR analysis of CHRFAM7A gene expression in cultured hCD34+ cells and human CD34+ cells that were transfected with siRNA (siRNA + hCD34+). C. RT-PCR gel image of CHRFAM7A mRNA expression in human CD34+ cells (hCD34+) and human CD34+ cells that were transfected with siRNA (siRNA + hCD34+) in vivo.
P38/JNK pathway regulation in humanized mice
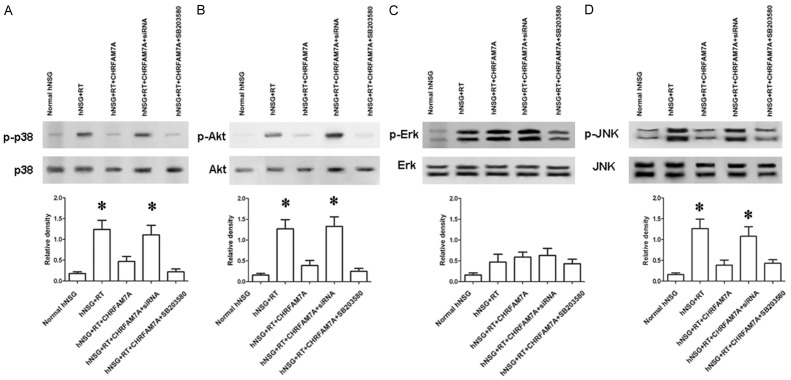
RT-induced lacrimal gland damage and local tissue inflammation are associated with Akt signalling and MAPK stress signalling and involve p38 and JNK. We performed western blot analyses of these signalling kinases to determine whether they are affected by the over-expression of CHRFAM7A in lacrimal gland tissue. We found that Akt, p38 and JNK phosphorylation were significantly promoted by CHRFAM7A siRNA transfection (Figure 5A, 5B, 5D) but that Erk1/2 was not significantly affected (Figure 5C).
Figure 5.
CHRFAM7A suppresses the p38/JNK pathway. (A) Western blot analysis (upper) and quantification (lower) of p38, Akt (B), Erk (C) and JNK (D) phosphorylation in irradiation-injured lacrimal glands. The results demonstrate the effects of administering hNSG + RT, hNSG + RT + CHRFAM7A, hNSG + RT + CHRFAM7A + siRNA, and hNSG + RT + CHRFAM7A + p38 inhibitor (SB203580, a p38 MAPK inhibitor with an IC50 of 0.3-0.5 μM) on p38 phosphorylation in irradiation-injured lacrimal glands. Lacrimal injury was induced in mice that received radiotherapy. The data are shown as the mean ± SD of five independent experiments. In (A), (B) and (D), *P<0.05 vs. hNSG, hNSG + RT + CHRFAM7A, hNSG + RT + CHRFAM7A + SB203580 (hNSG: non-irradiated humanized mice; hNSG + RT: radiotherapy-treated humanized mice; hNSG + RT + CHRFAM7A: radiotherapy-treated humanized mice after CHRFAM7A transfection; hNSG + RT + CHRFAM7A + siRNA: radiotherapy after CHRFAM7A and siRNA transfection; hNSG + RT + CHRFAM7A + SB203580: radiotherapy humanized mice after CHRFAM7A transfection and the administration of SB203580).
CHRFAM7A mitigates radiation-induced oxidative stress
As shown in Figure 6, ROS levels were 1.70 and 1.40 times higher in PBMCs at 7 d and 14 d, respectively, after radiation respectively, than the levels observed in the RT- group. CHRFAM7A slightly increased ROS levels in the non-irradiated mice, but the difference was not significant (P>0.05). In the RT++CHRFAM7A group, the ROS levels at 7 d and 14 d after radiation were 1.26 times and 0.97 times higher, respectively, than the levels observed in the RT- group. ROS levels were significantly lower in the RT++CHRFAM7A group than in the RT+ group (P<0.05). At 14 d after radiation, the ROS level in the PBMCs had returned to normal. There data indicate that CHRFAM7A reduced ROS production and inhibited radiation-induced oxidative stress in PBMCs.
Figure 6.
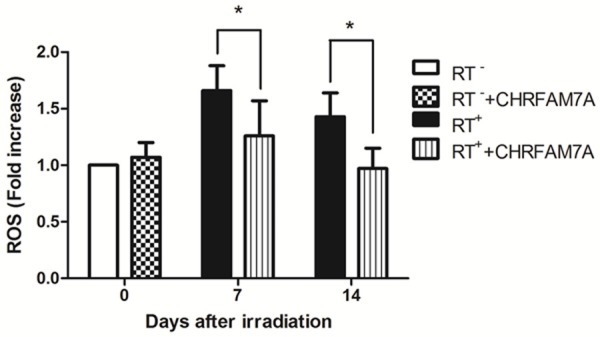
CHRFAM7A inhibited the RT-induced production of ROS in PBMCs.
Discussion
More than 300 types of human-specific genes have been identified, although the exact number and the functions of these genes remain unclear [25,26]. Some human-specific genes are presumed to have formed as adaptions to human-specific behaviour during human evolution. For example, approximately 3 million years ago, when humans began to use and control fire, specific sequences in the human genome that control the post-burn inflammatory response first appeared [27,28]. CHRFAM7A is a human-specific gene that was produced as a result of a rearrangement of the CHRNA7 gene, which is located on human chromosome 15q13-14, during human evolution [18]. This gene was originally found to be expressed in the human central nervous system and is associated with neuropsychiatric-related diseases, such as schizophrenia [9,29]. CHRFAM7A is expressed in human leukocytes and regulates inflammatory reactions [9,12,14,18]. In human monocytes and macrophages, CHRFAM7A gene expression is regulated by LPS, burns and nicotine, which up-regulate CHRNA expression [11,12,27,30]. However, as a result of the lack of appropriate animal models, the effects of human-specific genes on human diseases are difficult to explore, and the function of CHRFAM7A in RT-induced lacrimal gland injury has not previously been investigated. In our experiments, HM provided a good model to investigate the role of CHRFAM7A in the RT-induced inflammatory reaction to lacrimal gland lesions.
Irradiation induces changes in the expression of inflammation-related genes in human peripheral blood [31]. The p38/MAPK/AKT signalling pathway regulates the radiation-induced inflammatory response and tissue repair [32-35]. In this study, we collected irradiated lacrimal gland tissues from patients and found that CHRFAM7A expression was increased in this tissue (Figure 1). We also detected the CHRFAM7A gene in a variety of HM tissues (i.e., the liver, spleen, and peripheral blood) (Figure 2). Irradiating the lacrimal glands of HM caused no significant change in CHRFAM7A expression in these tissues, but CHRFAM7A was expressed at higher levels in the lacrimal glands of irradiated animals than in those of the control groups (i.e., wild-type mice, non-irradiated mice). CHRFAM7A may represent a human-specific regulatory of the inflammatory response because it is detected in psychiatric diseases and during the intestinal epithelial cell injury response [30]. The incidences of mental illness, intestinal cancer and irritable bowel syndrome are positively correlated with CHRFAM7A mutations [10]. When we knocked down CHRFAM7A expression using siRNA, we found that the expression of CHRFAM7A was significantly lower in in vitro-cultured siRNA-transfected human leukocytes, suggesting that the siRNA we designed was effective (Figure 4). In addition, this reduction was also observed in the tissues (e.g., the peripheral blood and lacrimal gland) of HM that were generated by injecting siRNA-transfected cells. We also found that the expression of CHRFAM7A remained lower after irradiation (Figure 4) and that the p38/JNK signalling pathway was up-regulated (Figure 5). Though the local inflammatory response in with the expression of the p38/JNK signalling pathway, other regulatory mechanisms that are independent of NF-κB, p38 MAPK, and ERK phosphorylation could be involved [36]. The p38 MAPK pathway participates in UVB-irradiated skin tissue injury and inhibiting p38 confers a cytoprotective effect [37,38]. Inhibiting the p38 pathway markedly attenuated irradiation-induced damage and increased tissue repair [32]. Additionally, we previously reported that the expression levels of markers of the p38/JNK signalling pathway are higher in lacrimal gland tissue after radiation. In the present study, we showed that CHRFAM7A had similar effects on p38 inhibition in lacrimal gland tissue. In conclusion, we propose that CHRFAM7A expression protects lacrimal gland function in RTinduced injury by inhibiting the p38/JNK signalling pathway and oxidative stress. The data presented in this report supports the notion that CHRFAM7A has therapeutic potential for treating xerophthalmia in RT-induced lacrimal gland injury.
Our results demonstrate that CHRFAM7A exerts a protective effect in RT-injured lacrimal glands. We used a humanized mouse model to show that RT-induced lacrimal gland injury is associated with higher expression of the human-specific gene CHRFAM7A and the production of p38 and that the RT-induced overproduction of oxidative substances can be ameliorated by CHRFAM7A. Further investigations are needed to explore the effectiveness of CHRFAM7A as a therapeutic agent in different types of RT-induced lacrimal gland injury.
Acknowledgements
This work was supported by the National Natural Science Foundation for Young Scholars of China (31100703).
Disclosure of conflict of interest
None.
References
- 1.Bhandare N, Moiseenko V, Song WY, Morris CG, Bhatti MT, Mendenhall WM. Severe dry eye syndrome after radiotherapy for head-andneck tumors. Int J Radiat Oncol Biol Phys. 2012;82:1501–1508. doi: 10.1016/j.ijrobp.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Xiao L, Liu W, Li J, Xie Y, He M, Fu J, Jin W, Shao C. Irradiated U937 cells trigger inflammatory bystander responses in human umbilical vein endothelial cells through the p38 pathway. Radiat Res. 2014;182:111–121. doi: 10.1667/RR13736.1. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Liu L, Zhou D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res. 2011;176:743–752. doi: 10.1667/rr2727.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Deng C, Qian J, Zhang M, Li X. Improvement of radiotherapy-induced lacrimal gland injury by induced pluripotent stem cellderived conditioned medium via MDK and inhibition of the p38/JNK pathway. Int J Mol Sci. 2014;15:18407–18421. doi: 10.3390/ijms151018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobuhara Y, Kawano S, Kageyama G, Sugiyama D, Saegusa J, Kumagai S. Is SS-A/Ro52 a hydrogen peroxide-sensitive signaling molecule? Antioxid Redox Signal. 2007;9:385–391. doi: 10.1089/ars.2006.1480. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 7.Si J, Zhang H, Wang Z, Wu Z, Lu J, Di C, Zhou X, Wang X. Effects of (12) C (6 +) ion radiation and ferulic acid on the zebrafish (Danio rerio) embryonic oxidative stress response and gene expression. Mutat Res. 2013;745-746:26–33. doi: 10.1016/j.mrfmmm.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Tyrrell RM. Modulation of gene expression by the oxidative stress generated in human skin cells by UVA radiation and the restoration of redox homeostasis. Photochem Photobiol Sci. 2012;11:135–147. doi: 10.1039/c1pp05222e. [DOI] [PubMed] [Google Scholar]
- 9.Sinkus ML, Graw S, Freedman R, Ross RG, Lester HA, Leonard S. The human CHRNA7 and CHRFAM7A genes: a review of the genetics, regulation, and function. Neuropharmacology. 2015;96:274–288. doi: 10.1016/j.neuropharm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird A, Coimbra R, Dang X, Eliceiri BP, Costantini TW. Up-regulation of the humanspecific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 2016;5:66–71. doi: 10.1016/j.bbacli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benfante R, Antonini RA, De Pizzol M, Gotti C, Clementi F, Locati M, Fornasari D. Expression of the alpha7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J Neuroimmunol. 2011;230:74–84. doi: 10.1016/j.jneuroim.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Costantini TW, Dang X, Coimbra R, Eliceiri BP, Baird A. CHRFAM7A, a human-specific and partially duplicated alpha7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J Leukoc Biol. 2015;97:247–257. doi: 10.1189/jlb.4RU0814-381R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R, Shields DC, Abrahams BS, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolton PF, Bourgeron T, Brennan S, Cali P, Correia C, Corsello C, Coutanche M, Dawson G, de Jonge M, Delorme R, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Foley S, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Green J, Guter SJ, Hakonarson H, Holt R, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Lamb JA, Leboyer M, Le Couteur A, Leventhal BL, Lord C, Lund SC, Maestrini E, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Merikangas A, Miller J, Minopoli F, Mirza GK, Munson J, Nelson SF, Nygren G, Oliveira G, Pagnamenta AT, Papanikolaou K, Parr JR, Parrini B, Pickles A, Pinto D, Piven J, Posey DJ, Poustka A, Poustka F, Ragoussis J, Roge B, Rutter ML, Sequeira AF, Soorya L, Sousa I, Sykes N, Stoppioni V, Tancredi R, Tauber M, Thompson AP, Thomson S, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Vorstman JA, Wallace S, Wang K, Wassink TH, White K, Wing K, Wittemeyer K, Yaspan BL, Zwaigenbaum L, Betancur C, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Geschwind DH, Haines JL, Hallmayer J, Monaco AP, Nurnberger JI Jr, Pericak-Vance MA, Schellenberg GD, Scherer SW, Sutcliffe JS, Szatmari P, Vieland VJ, Wijsman EM, Green A, Gill M, Gallagher L, Vicente A, Ennis S. A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum Genet. 2012;131:565–579. doi: 10.1007/s00439-011-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lucas-Cerrillo AM, Maldifassi MC, Arnalich F, Renart J, Atienza G, Serantes R, Cruces J, Sanchez-Pacheco A, Andres-Mateos E, Montiel C. Function of partially duplicated human alpha77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606. doi: 10.1074/jbc.M110.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Zanden EP, Hilbers FW, Verseijden C, van den Wijngaard RM, Skynner M, Lee K, Ulloa L, Boeckxstaens GE, de Jonge WJ. Nicotinic acetylcholine receptor expression and susceptibility to cholinergic immunomodulation in human monocytes of smoking individuals. Neuroimmunomodulation. 2012;19:255–265. doi: 10.1159/000335185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Severance EG, Dickerson FB, Stallings CR, Origoni AE, Sullens A, Monson ET, Yolken RH. Differentiating nicotine- versus schizophreniaassociated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J Neural Transm (Vienna) 2009;116:213–220. doi: 10.1007/s00702-008-0164-y. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Xiao C, Indersmitten T, Freedman R, Leonard S, Lester HA. The duplicated alpha7 subunits assemble and form functional nicotinic receptors with the full-length alpha7. J Biol Chem. 2014;289:26451–26463. doi: 10.1074/jbc.M114.582858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, Leonard S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function. Biochem Pharmacol. 2011;82:904–914. doi: 10.1016/j.bcp.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billerbeck E, Mommersteeg MC, Shlomai A, Xiao JW, Andrus L, Bhatta A, Vercauteren K, Michailidis E, Dorner M, Krishnan A, Charlton MR, Chiriboga L, Rice CM, de Jong YP. Humanized mice efficiently engrafted with fetal hepatoblasts and syngeneic immune cells develop human monocytes and NK cells. J Hepatol. 2016;65:334–343. doi: 10.1016/j.jhep.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. Humanized mouse models of clinical disease. Annu Rev Pathol. 2017;12:187–215. doi: 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baird A, Deng C, Eliceiri MH, Haghi F, Dang X, Coimbra R, Costantini TW, Torbett BE, Eliceiri BP. Mice engrafted with human hematopoietic stem cells support a human myeloid cell inflammatory response in vivo. Wound Repair Regen. 2016;24:1004–1014. doi: 10.1111/wrr.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang Y, Qian J, Zhang M, Wang X. Radiotherapyinduced Gadd45a impairs lacrimal gland epithelial cell migration and proliferation. Mol Med Rep. 2013;8:1049–1054. doi: 10.3892/mmr.2013.1636. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Wu Y, Dai Q, Fang B, Jiang L. p38-MAPK signaling pathway is not involved in osteogenic differentiation during early response of mesenchymal stem cells to continuous mechanical strain. Mol Cell Biochem. 2013;378:19–28. doi: 10.1007/s11010-013-1589-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LH, Jia YL, Lin XX, Zhang HQ, Dong XW, Zhao JM, Shen J, Shen HJ, Li FF, Yan XF, Li W, Zhao YQ, Xie QM. AD-1, a novel ginsenoside derivative, shows anti-lung cancer activity via activation of p38 MAPK pathway and generation of reactive oxygen species. Biochim Biophys Acta. 2013;1830:4148–4159. doi: 10.1016/j.bbagen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Stahl PD, Wainszelbaum MJ. Human-specific genes may offer a unique window into human cell signaling. Sci Signal. 2009;2:pe59. doi: 10.1126/scisignal.289pe59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YE, Long M. New genes contribute to genetic and phenotypic novelties in human evolution. Curr Opin Genet Dev. 2014;29:90–96. doi: 10.1016/j.gde.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costantini TW, Dang X, Yurchyshyna MV, Coimbra R, Eliceiri BP, Baird A. A human-specific alpha7-nicotinic acetylcholine receptor gene in human leukocytes: identification, regulation and the consequences of CHRFAM7A expression. Mol Med. 2015;21:323–336. doi: 10.2119/molmed.2015.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper DN, Kehrer-Sawatzki H. Exploring the potential relevance of human-specific genes to complex disease. Hum Genomics. 2011;5:99–107. doi: 10.1186/1479-7364-5-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE, Wang KH, Lipska BK. CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry. 2015;172:1122–1130. doi: 10.1176/appi.ajp.2015.14080978. [DOI] [PubMed] [Google Scholar]
- 30.Dang X, Eliceiri BP, Baird A, Costantini TW. CHRFAM7A: a human-specific alpha7-nicotinic acetylcholine receptor gene shows differential responsiveness of human intestinal epithelial cells to LPS. FASEB J. 2015;29:2292–2302. doi: 10.1096/fj.14-268037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P, Guo F, Han L, Wang X, Li J, Guo Y, Lu Y. X-ray-induced changes in the expression of inflammation-related genes in human peripheral blood. Int J Mol Sci. 2014;15:19516–19534. doi: 10.3390/ijms151119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan NM, Poduval TB. Bilirubin augments radiation injury and leads to increased infection and mortality in mice: molecular mechanisms. Free Radic Biol Med. 2012;53:1152–1169. doi: 10.1016/j.freeradbiomed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardi M, Caelles C, Serrano AL, Munoz-Canoves P. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D, Wang Y, Wu H, Lu L, Zhang H, Chang J, Zhai Z, Zhang J, Wang Y, Zhou D, Meng A. Mitigation of ionizing radiation-induced bone marrow suppression by p38 inhibition and G-CSF administration. J Radiat Res. 2011;52:712–716. doi: 10.1269/jrr.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segreto HR, Oshima CT, Franco MF, Silva MR, Egami MI, Teixeira VP, Segreto RA. Phosphorylation and cytoplasmic localization of MAPK p38 during apoptosis signaling in bone marrow granulocytes of mice irradiated in vivo and the role of amifostine in reducing these effects. Acta Histochem. 2011;113:300–307. doi: 10.1016/j.acthis.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Furusawa Y, Wei ZL, Sakurai H, Tabuchi Y, Li P, Zhao QL, Nomura T, Saiki I, Kondo T. TGFbeta-activated kinase 1 promotes cell cycle arrest and cell survival of X-ray irradiated HeLa cells dependent on p21 induction but independent of NF-kappaB, p38 MAPK and ERK phosphorylations. Radiat Res. 2012;177:766–774. doi: 10.1667/rr2792.1. [DOI] [PubMed] [Google Scholar]
- 37.Lan CC, Yu HS, Huang SM, Wu CS, Chen GS. FK506 induces interleukin-6 secretion from UVB irradiated cultured human keratinocytes via p38 mitogen-activated protein kinase pathway: implication on mechanisms of tacrolimus-induced skin irritation. J Dermatol Sci. 2007;48:225–228. doi: 10.1016/j.jdermsci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Lee ER, Kim JH, Choi HY, Jeon K, Cho SG. Cytoprotective effect of eriodictyol in UV-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 MAPK and Akt signaling pathways. Cell Physiol Biochem. 2011;27:513–524. doi: 10.1159/000329973. [DOI] [PubMed] [Google Scholar]