Abstract
Sevoflurane (Sev) is a widely used anaesthetic agent in clinical patients. Growing evidences indicated that Sev resulted in cognitive impairment via inducing endoplasmic reticulum (ER) stress mediated neurons apoptosis in vivo. However, the underlying molecular mechanisms have not yet fully understood. In this study, we found that Sev exposure suppresses cell viability, and induces apoptosis by activating caspase-3 apoptotic signaling pathway. Our results further verified that Sevtriggers ER stress via upregulating its markers glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP), caspase-12 and cleaved-PARP proteins. Recently, microRNAs (miRNAs) have been proven to regulate ER stress in a variety of cells, especially neuronal cells. Therefore, we performed the microarray analysis to identified miRNA levels in HT22 cells after treatment with Sev. Our results showed that Sev induces miRNAs aberrant expression and miR-15b-5p was one of the miRNAs being most upregulated in HT22 cells. Furthermore, the Sev-induced apoptosis and ER stress were rescued by knockdown of miR-15b-5p. Additionally, we demonstrated that miR-15b-5p suppresses Rab1A, a regulator in inducing ER stress, by directly targeting its 3’-UTR in HT22 cells. These results suggested that Sev exposure induces ER stress mediated apoptosis in HT22 cells via regulating miR-15b-5p/Rab1A signaling pathway. These data may provide an important therapeutic strategy for fighting against Sev through ER stress mediated neuronal apoptosis in clinical patients.
Keywords: Sevoflurane, endoplasmic reticulum stress, neurons apoptosis, miR-15b-5p, Rab1A
Introduction
Volatile anaesthetics have been widely used in millions of young children every year during surgical procedures and imaging studies around the world [1]. Sevoflurane (Sev), which is one of the most commonly used volatile anesthetics, and is generally considered effective in pediatric anesthesia. However, increasing evidence demonstrated that Sev may cause cognitive impairment in both animals and humans [2-4]. Previous studies revealed that neural apoptosis, neuroinflammation and abnormal protein deposition may lead to cognitive impairment [5-7]. Additionally, previous study demonstrated that Sev anesthesia could lead to ER stress and apoptosis in hippocampal neurons of aging rats [8]. However, the underlying molecular mechanisms are still elusive.
Endoplasmic reticulum (ER) is a multi-functional organelle, involving synthesis, folding, and posttranslational modification of secretory and membrane proteins [9]. ER dysfunction induces an accumulation of unfolded or misfolded proteins in the ER lumen and triggers the ER stress and unfolded protein response (UPR), which affects complex signal transduction pathways designed to restore ER homeostasis [10]. The UPR signal has the dual-function of impairing the damage associated with ER stress or inducing cell death through apoptosis [11,12]. Increasing evidences reported that ER stress may play an important role in the pathogenesis of many acute and chronic neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, cerebral ischemia and amyotrophic lateral sclerosis [13-15]. Recent studies revealed that Sev induces ER stress and neuronal apoptosis in aging rats [8,16]. However, the precise mechanisms underlying Sev-induced ER stress are still elusive.
microRNAs (miRNAs) are a class of small (19-24 nucleotide) noncoding RNAs that mediate post-transcriptional regulation of target genes by translation repression or promoting RNA degradation and they are important in the regulation of various biological and pathological processes, such as cellular proliferation, differentiation, apoptosis and carcinogenesis [17]. A number of miRNAs were found in the mammalian central nervous system (CNS), such as the brain and spinal cord, where they play key roles in neuro development and are likely to be important mediators of plasticity [18-20]. Accumulating evidences confirmed that Sev anesthesia altered microRNAs expression in major organs such as liver, lung and brain of adult rats [21-23]. Moreover, Sev anesthesia may induce anxiety-disorder during adulthood through mediating Sev-induced miRNAs aberrant expression [24]. Recent study demonstrated that microRNAs acted as key regulators of ER homeostasis and important players in UPR-dependent signaling [25]. Therefore, we hypothesized that Sev induces ER stress and apoptosis via regulating miRNAs expression in mouse hippocampal neurons.
In this study, we investigated the effect of Sevexposure on mouse hippocampal neuronal HT22 cells and explored the underlying molecular mechanism. We found that Sev induced apoptosis, ER stress and miRNAs aberrant expression in HT22 cells. Meanwhile, we identified that miR-15b-5p is upregulated in HT22 cells after Sev exposure, and modulated the ER stress-induced apoptosis via targeting Rab1A. Taken together, our findings uncovered that Sev induced apoptosis and ER stress in HT22 cells via modulating miR-15b-5p/Rab1A signaling pathway.
Materials and methods
Cell culture and treatment
The mouse hippocampal neuronal HT22 cell line was obtained from the American Type Culture Collection (ATCC). HT22 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, USA) containing 10% fetal bovine serum (Sigma-Aldrich, USA) with 2 mM glutamine, 100 U/ml penicillin (Sigma-Aldrich, USA), and 100 μg/ml streptomycin (Sigma-Aldrich, USA) and maintained at 37°C in a humidified incubator with 5% CO2. Medium was replaced every 2 days.
Exposure to sevoflurane
Cell culture was exposed in an airtight plastic chamber with inlet and outlet connectors. The inlet port of the chamber was used to adjust the concentration of Sev (AbbVie Inc., North Chicago, IL, USA), which was connected to a Sev vaporizer. Subsequently, the chamber was gassed with different concentration of Sev (0%, 4.1% or 8%) in the carrier gas (95% air/5% CO2) for 15 min as described previously [26]. The chamber outlet of the chamber was used to monitor Sev concentration though a gas monitor (PM 8060, Drager, Lübeck, Germany) until the target concentration was reached. The chamber was then kept tightly sealed for 6 h at 37°C. The control cells grown in a humidified atmosphere with 5% CO2 at 37°C, and were not exposed to Sev.
Cell viability analysis
Cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. Cells were seeded in 96-well plates at a cellular density of 5 × 103 cells/well. The cell monolayer was rinsed with PBS three times after treatment with Sev, and then 1:10 diluted CCK-8 reagent in DMEM was added to the cells and incubated for 2 h at 37°C. The absorbance rate at 450 nm were measured by Microplate Reader (Bio-Rad, USA). All experiments were performed in quintuplicate on three separate occasions.
Apoptosis analysis
After exposure to Sev, 1 × 106 cells were harvested and washed twice with cold PBS. Then Cells were resuspended in binding buffer and stained with 5 μl of AnnexinV-FITC (BD, Mountain View, CA, United States) and 1 μl of propidium iodide (PI, 50 μg/ml) (BD, Mountain View, CA, United States). Flow cytometric evaluation was performed within 5 min. Stained cells were analyzed by flow cytometry (BD, FACSCalibur, CA, United States). The measurements were performed independently for at least three times with similar results.
Western blot analysis
After Sev treatment or transfection, total protein of cultured cells was extracted using RIPA buffer with protease inhibitor Cocktail (Pierce, Rockford, IL, USA). BCA protein assay kit (Beyotime, Haimen, China) was used to detect the concentration. Total proteins (20 μg) were separated on 10% SDS-PAGE (SigmaAldrich, St. Louis, MO) and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk at 4°C overnight, the membranes were incubated with primary antibodies against cleaved-caspase-3 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), total-cleaved-caspase-3 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), GRP78 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), CHOP (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), cleaved-caspase-12 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), cleaved-PARP (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) and Rab1A (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight, β-actin (1:1000, Sigma, St. Louis, MO) was used as an internal control for protein loading. Horseradish peroxidase-conjugated (HRP) antibodies were used as the secondary antibodies. The bands were scanned using the ChemiDocXRS + Imaging System (Bio-Rad) and quantified using Quantity One v4.6.2 software (Bio-Rad).
Caspase-3 activity
The colorimetric activity assay kit was used to measure caspase-3 activity. After exposure to Sev, the HT22 cells were collected by centrifugation and incubated in lysis buffer on ice for 15 min. Then the lysate was centrifuged at 15,000 rpm and 4°C for 15 min, and the protein concentration was determined using the BCA Protein Assay Kit (Beyotime, Haimen, China) according to the manufacturer’s instructions. The lysates (10 μl) were incubated with 10 μl of 0.2 mM Ac-DEVD-pNA in 80 μl of reaction buffer for 2 h at 37°C. The samples were measured at 405 nm using a microplate reader (Model 680, Bio-Rad, Hercules, CA, USA). The data are expressed as a fold increase in caspase-3 activity compared with the control group.
miRNA microarray analysis
After exposure to Sev, the cells of total RNA were extracted using TRIzol (Invitrogen, CA) and miRNeasy mini kit (Qiagen, West Sussex, UK) according to manufacturer’s instructions. The NanoDrop 1000 (Youpu Scientific Instrument Co., Ltd., Shanghai, China) was used to measure the quantity of RNA, the samples were labeled using the miRCURY™Hy3™/Hy5™ Power labeling kit (Exiqon, Vedbaek, Denmark) and hybridized on a miRCURY™ LNA Array (version 18.0, Exiqon, Vedbaek, Denmark). After washing, the slides were scanned using an Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, CA, USA). Scanned images were then imported into the GenePix Pro6.0 program (Axon Instruments) for grid alignment and data extraction. Replicated miRNAs were averaged, and miRNAs with intensities ≥50 in all samples were used to calculate a normalization factor. Expressed data were normalized by median normalization. After normalization, the miRNAs that were significantly differentially expressed were identified by Volcano Plot filtering. Finally, hierarchical clustering was performed to determine the differences in the miRNA expression profiles among the samples by using MEV software (version 4.6; TIGR, Microarray Software Suite 4, Boston, United States).
RT-PCR analysis
Total RNA was isolated from culture cells using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA synthesis was conducted by the High Capacity cDNA Synthesis Kit (Applied Biosystems) with miRNA-specific primers. The miR-522, miR-505, miR-15b-5p and miR-218-1 primers were purchased from Ribobio (Guangzhou, China). The U6 gene was used as a reference control for miRNAs. Real-time qRT-PCR was carried out on an Applied Biosystems 7500 Real-Time PCR machine with miRNA-specific primers by TaqMan Gene Expression Assay (Applied Biosystems). All reactions were performed in triplicate. The 2-ΔΔCt method was performed to analyze the miRNAs relative expression.
Luciferase reporter assay
The potential binding site between Rab1A and miR-15b-5p was searched in TargetScan (http://www.targetscan.org). The wild-type Rab1A-3’-UTR (wt) and mutant Rab1A-3’-UTR (mut) containing the putative binding site of miR-15b-5p were established (Figure 5A) and cloned in the firefly luciferase expressing vector pMIR-REPORT (Ambion, USA). The HT22 cells were seeded into 24-well plates the day before transfection, and transfected with either the pMIR-REPORT-Rab1A-3’-UTR wt or the pMIR-REPORT-Rab1A-3’-UTR mut reporter vector, together with miR-15b-5p mimics/inhibitor or mimics/inhibitor NC (RiboBio, Guangzhou, China) using Lipofectamine 2000 (Invitrogen). 48 h after transfection, the luciferase activity was measured using the Dual-Light luminescent reporter gene assay system (Applied Biosystems). All experiments were repeated three times in triplicate. The ratio of Renilla luciferase to Firefly luciferase was calculated for each well.
Figure 5.
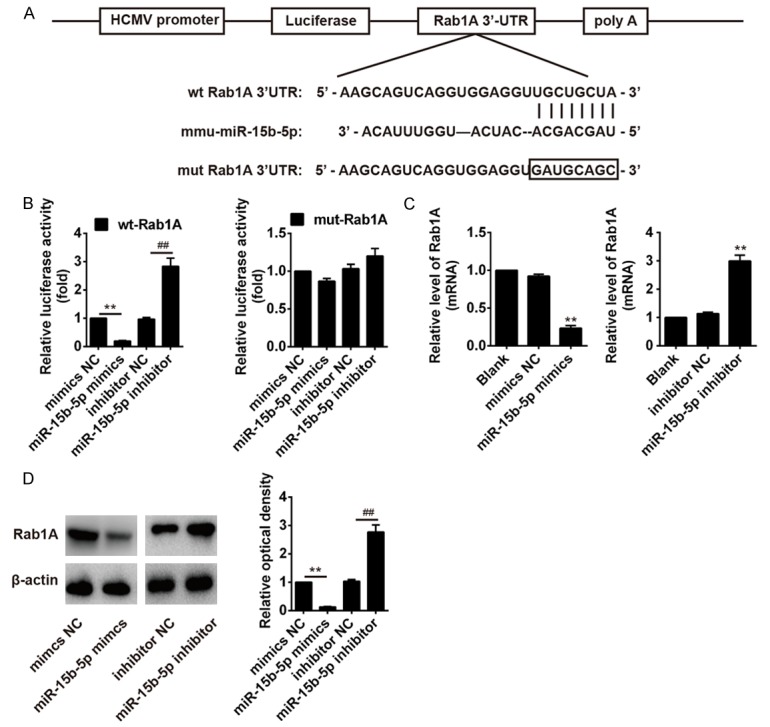
miR-15b-5p inhibits Rab1A expression by directly targeting its 3’-UTR. A. Scheme of the potential binding sites of miR-15b-5p in the Rab1A-3’-UTR. B. The relative luciferase activity of Rab1A wild type or mutant 3’-UTR in HT22 cells after transfection with the miR-15b-5p mimic/inhibitor or corresponding NC. C and D. The HT22 cells were transfected with miR-15b-5p mimic/inhibitor or corresponding NC, andqRT-PCR and Western blot analysis were used to measure the Rab1A mRNA and protein levels, respectively. β-actin was used as an internal control. The data represent the mean ± SEM of three replications. **P < 0.01 vs mimic NC, ##P < 0.01 vs inhibitor NC.
Statistical analysis
All statistical analysis was performed using SPSS 14.0 software (Chicago, IL). Numerical data presented as the mean ± standard deviation. The difference between means was analysed with Student’s t test. Probability value of < 0.05 was considered significant and < 0.01 was considered very significant.
Results
Sev promotes HT22 cells apoptosis
To investigate whether Sev can induce apoptosis, the cells were exposed to Sev (0%, 4.1% or 8%) for 6 h and CCK-8 assay was used to measure the cell viability. Our results showed that cell viability was significantly decreased after exposure to 4.1% or 8% Sev compared with control (P < 0.01; Figure 1A). Consistent with the CCK-8 assay, flow cytometry also demonstrated that the apoptotic cells were markedly increased after treatment with different concentration of Sev (P < 0.01; Figure 1B). To further examine this apoptotic mechanism, the cleaved-caspase-3 level was investigated in HT22 cells after treatment with different concentration of Sev (4.1% or 8%) using Western blot analysis. The results indicated that the cleaved-caspase-3 protein was significantly upregulated in cells after Sev treatment compared with control (P < 0.01; Figure 1C). Moreover, the caspase-3 activity was significantly increased after Sev treatment compared with control (P < 0.01; Figure 1D). These results suggested that Sev induces apoptosis in HT22 cells after treatment with Sev.
Figure 1.
Sevoflurane (Sev) induced apoptosisin HT22 cells. A. The Cell Counting Kit-8 (CCK-8) assay used to measure cell viability in HT22 cells exposed to 0%, 4.1% or 8% Sev for 6 h. B. The apoptotic cells were detected using flow cytometry analysis after treatment with 0%, 4.1% or 8% Sev for 6 h. C. After exposure to Sev, the cleaved-caspase-3 and total-cleaved-caspase-3 protein levels were measured using Western blot analysis. β-actin was used as an internal control. D. The colorimetric activity assay kit was used to measure caspase-3 activity following treatment with 0%, 4.1% or 8% Sev for 6 h. The data represent the mean ± SEM of three replications. **P < 0.01 vs control.
Sev triggers ER stress-induced apoptosis signaling pathway in HT22 cells
Previous study reported that Sev could trigger ER stress and may result in neuronal cell apoptosis [8]. To explore the effect of Sev-induced ER stress on HT22 cells, the expression of the ER stress markers (GRP78 and CHOP) and apoptosis-related proteins (cleaved-caspase-12 and cleaved-PARP) were evaluated after treatment with 4.1% Sev for 6 h. As shown in Figure 2, Western blot analysis revealed that ER stress-induced apoptosis signaling pathway-related proteins (GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP) were dramatically upregulated in HT22 cells treated with 4.1% Sev compared with control (P < 0.01; Figure 2A-C). These data suggested that Sev may prompt HT22 cells apoptosis through triggering ER stress-induced apoptosis signaling pathway.
Figure 2.
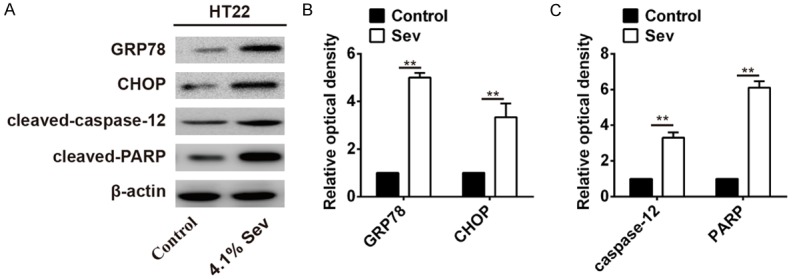
Effects of Sev on ER stress-induced apoptosis signaling pathway-related genes in HT22 cells. A. After exposure to 4.1% Sev, the Western blot analysis was performed to measure the ER stress-induced apoptosis signaling pathway-related genes (GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP) in HT22 cells. β-actin was used as an internal control. B. Densitometric analysis of GRP78 and CHOP levels. C. Densitometric analysis of cleaved-caspase-12 and cleaved-PARP levels. The data represent the mean ± SEM of three replications. **P < 0.01 vs control.
Sev induces miRNAs aberrant expression in HT22 cells
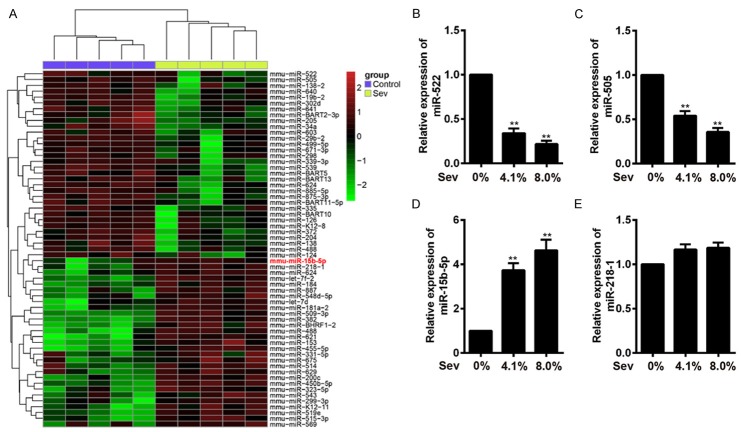
Mounting evidence demonstrated that miRNAs played key roles in neuro development and are likely to be important mediators of plasticity [18-20], and miRNA dysfunction has been implicated in neurodegenerative diseases [27]. Recent study showed that miR-21 exerted an anti-apoptotic function in human glioblastomacells [28]. Therefore, we hypothesized that Sev induces the HT22 cell apoptosis via modulating miRNAs expression. To confirm this notion, we performed microarray analysis to determine miRNA levels in HT22 cells after treatment with different concentration of Sev (4.1% or 8%). We observed that a large set of miRNAs expression were altered following Sev treatment compared with control (Figure 3A). To further verify these differential expressions of miRNAs, the 4 miRNAs (miR-15b-5p, miR-218-1, miR-522 and miR-505) which were the most significantly upregulated or downregulated, were selected and quantified by qRT-TPCR analysis. Consistent with the results in microarray analysis, miR-522 and miR-505 were significantly downregulated, miR-15b-5p was significantly upregulated compared with control (P < 0.01; Figure 3B-D), but miR-218-1 has no alteration compared with control (Figure 3E). These results indicated that Sev post-treatment could lead to miRNAs aberrant expression in HT22 cells.
Figure 3.
Sev induces miRNAs aberrant expression in HT22 cells. A. Differentially expressed miRNAs were identified in HT22 cells following treatment with 0%, 4.1% or 8% Sev for 6 h. The color code in the heat maps is linear, with green as the lowest and red as the highest. The miRNAs that were upregulated were shown in green to red, whereas the miRNAs that were downregulated were shown from red to green. B-E. The miR-522, miR-505, miR-15b-5p and miR-218-1 expression were quantified using qRT-PCR analysis, respectively. The data represent the mean ± SEM of three replications. **P < 0.01 vs control.
Knockdown of miR-15b-5p recuses Sev-induced apoptosis and ER stress
Recent study revealed that miR-15b-5p induces ER stress and apoptosis in human hepatocellular carcinoma (HCC) cells [29]. Therefore, we speculated whether Sev-induced miR-15b-5p aberrant expression also regulated apoptosis and ER stress in HT22 cell model. To verify this notion, the HT22 cells were exposed to 4.1% Sev for 6 h, then were transfected with miR-15b-5p inhibitor or inhibitor NC. Subsequently, the cell viability and apoptosis were measured using CCK-8 assay and flow cytometry analysis, respectively. Our results showed that Sev treatment remarkably decreased cell viability and increased apoptotic cell, but miR-15b-5p inhibitor dramatically reverse these effects compared with inhibitor NC (P < 0.01; Figure 4A and 4B). Moreover, Sev-induced caspase-3 activity enhancement was rescued by knockdown of miR-15b-5p in HT22 cells (Figure 4C). Additionally, we found that miR-15b-5p downregulation significantly repressed the ER stress-induced apoptosis signaling pathway-related genes (GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP) compared with Sev-treated cells (P < 0.01; Figure 4D). Taken together, these data suggested that the Sev-induced apoptosis and ER stress were recused by knockdown of miR-15b-5p in HT22 cells.
Figure 4.
Knockdown of miR-15b-5p rescues Sev-induced apoptosis and ER stress. The HT22 cells were exposed to 4.1% Sev for 6 h, and were transfected with miR-15b-5p inhibitor or inhibitor NC. A. Cell viability was measured using CCK-8 assay. *P < 0.05,**P < 0.01 vs control, ##P < 0.01 vs inhibitor NC. B. Flow cytometry analysis was performed to determine apoptotic cells. **P < 0.01 vs control, ##P < 0.01 vs inhibitor NC. C. The colorimetric activity assay kit was used to measure caspase-3 activity. *P < 0.05, **P < 0.01 vs control, ##P < 0.01 vs inhibitor NC. D. Western blot analysis was performed to detect the ER stress-induced apoptosis signaling pathway-related proteins (GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP). β-actin was used as an internal control. **P < 0.01 vs Sev. The data represent the mean ± SEM of three replications.
miR-15b-5p inhibits Rab1A expression by directly targeting its 3’-UTR
Previous study uncovered that Rab1A exerted as an important function in ER, inhibiting Rab1A function induced an ER stress signaling pathway referred to as the UPR [30]. It has been reported that miR-15b-5p induced ER stress in HCC cells via targeting and suppressing Rab1A [29]. To investigate whether miR-15b-5p also targets Rab1A in HT22 cells, we performed bioinformatic analysis to predicate the putative targets of miR-15b-5p, and found that Rab1A might be a target gene of miR-15b-5p and the target site located in the 3’-UTR (Figure 5A). To verify this bioinformatic predication, the wt or mut of Rab1A-3’-UTR was constructed and inserted into the pMIR-REPORT vector. Luciferase reporter assay showed that cotransfection of miR-15b-5p with wt of Rab1A-3’-UTR, but not with mut of Rab1A-3’-UTR, resulted in significant downregulation of luciferase activity than that in the transfection with control vector (P < 0.01; Figure 5B). To further identify this result, we performed qRT-PCR and western blot to detect the Rab1A mRNA and protein levels, respectively. We found that upregulation of miR-15b-5p dramatically suppressed the Rab1A mRNA and protein levels compared with NC, but knockdown of miR-15b-5p significantly increased the Rab1A mRNA and protein levels (P < 0.01; Figure 5C and 5D). These results suggested that Sev-induced miR-15b-5p upregulation resulted in ER stress through inhibiting Rab1A in HT22 cells.
Discussion
Previous studies have confirmed that volatile anesthetics, such as Sev, is very helpful for reduction of perioperative mortality [31,32]. But volatile anesthetics may also induce memory impairment by neurons lost in hippocampus through cells apoptosis [33]. However, the underlying molecular mechanisms are still elusive. In the present study, we demonstrated that neuronal model cells HT22 which exposure to Sev could induce apoptosis and ER stress. Meanwhile, our results confirmed that Sev could alter miRNAs expression in HT22 cells, and Sev-induced miR-15b-5p upregulation regulates apoptosis and ER stress through targeting Rab1A. It suggested that the Sev exposure may induce ER stress mediated apoptosis in HT22 cells via modulating miR-15b-5p/Rab1A signaling pathway.
The ER plays an important role in the synthesis, folding, and structural maturation of more than a third of all proteins made in the cell [34]. The folding of proteins was disturbed during physiological and pathological conditions in the ER, leading to ER stress [35]. Previous studies documented that ER stress-induced apoptosis was a vital pathological event in neurological disease processes and neuronal cell death [9,36,37]. It has been reported that glucose-regulated protein 78 (GRP78), C/EBP homologous protein (CHOP) and caspase-12 are key mediators of ER stress-induced apoptosis [38-40]. GRP78 plays a key role in the cell survival against ER stress, while CHOP acts as the principal executor of various pro-apoptotic events upon severe ER stress [41]. Prolonged ER stress results in transcriptional induction of CHOP, which modulates genes that participate in the apoptotic pathway [10,39]. Caspase-12 serves as a marker of ER stress-induced apoptosis, which is activated by ER stress, but apparently not by death receptor-mediated or mitochondrial-targeted apoptotic signals [40]. Recent study demonstrated that Sev anesthesia could induce apoptosis and ER stress in hippocampal neurons of aging rats [8]. In this study, our results demonstrated that Sev treatment significantly inhibited proliferation and prompted apoptosis in HT22 cells. Furthermore, we found that 4.1% Sev exposure could increase GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP protein levels in HT22 cells. It suggested that the Sev exposure may induce ER stress mediated apoptosis in the HT22 cells, by increasing the expression of GRP78, CHOP, cleaved-caspase-12 and cleaved-PARP.
miRNAs are important in the CNS as they affect various post-transcriptional regulation mechanisms, including cytoskeletal/neuronal growth, stress/death responses, signal transduction and synaptic plasticity [42-44]. Previous studies uncovered that Sev anesthesia could alter microRNAs expression in both animals and humans [21-24]. Moreover, recent study demonstrated that Sev induced apoptosis in H4 human neuroglioma cells [26]. Therefore, we speculated that Sev exposure induces ER stress-mediated apoptosis in the HT22 cells via mediating miRNAs expression. To verify this notion, we performed the microarray and RT-PCR analysis to identify miRNA levels in HT22 cells after Sev exposure. We found that a large set of miRNAs expression were altered following Sev treatment, and the miR-15b-5p was one of the miRNAs being most significantly upregulated. Additionally, it has been reported that miR-15b-5p is overexpression in HCC tissues and cells, induces ER stress and apoptosis [29]. Therefore, we further investigated the effects of miR-15b-5p downregulation on HT22 cells, we found that knockdown of miR-15b-5p aborted Sev-induced apoptosis and ER stress in HT22 cells. These results suggested that Sev exposure induces ER stress-mediated apoptosis in the HT22 cells through mediating miR-15b-5p expression. However, the possible molecular mechanism need further research to be understood deeply.
Rab proteins are a large family of small GTPases that function directly in intracellular vesicular trafficking [45]. Rab proteins acted as molecular switches, which localized on specific intracellular compartments where they participate in distinct steps in membrane trafficking pathways [46,47]. Rab proteins modulate membrane transport and impact cell signaling pathways via binding to effecter proteins. Rab1A, a member of the Rab family, which functions as a Golgi-resident Rab to control vesicle trafficking from the ER to the Golgi apparatus [48,49]. Inhibition of Rab1A function lead to the accumulation of protein within ER, which induces an ER stress signaling pathway referred to as the UPR [49]. Recently, it has been reported that miR-15b-5p induces ER stress and apoptosis in HCC, both in vitro and in vivo, via targeting Rab1A [29]. In the present study, our results also identified that Rab1A is a target gene of miR-15b-5p in HT22 cells, and upregulation of miR-15b-5p suppresses the Rab1A mRNA and protein levels. Taken together, these data indicated that Sev exposure induces ER stress mediated apoptosis in HT22 cells via regulating miR-15b-5p/Rab1A signaling pathway.
In conclusion, our findings indicated that Sev induces ER stress-mediated apoptosis through modulating miR-15b-5p/Rab1A signaling pathway in HT22 cells, suggesting miR-15b-5p inhibitor may effectively protect against Sev-induced neuronal apoptosis in clinical patients.
Disclosure of conflict of interest
None.
References
- 1.Ma R, Wang X, Peng P, Xiong J, Dong H, Wang L, Ding Z. α-Lipoic acid inhibits sevo fl uraneinduced neuronal apoptosis through PI3K/Akt signalling pathway. Cell Biochem Funct. 2016;34:42–47. doi: 10.1002/cbf.3163. [DOI] [PubMed] [Google Scholar]
- 2.Alkire MT, Gruver R, Miller J, McReynolds JR, Hahn EL, Cahill L. Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc Natl Acad Sci U S A. 2008;105:1722–1727. doi: 10.1073/pnas.0711651105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, Eriksson LI. Object memory in young and aged mice after sevoflurane anaesthesia. Neuroreport. 2009;20:1419–1423. doi: 10.1097/WNR.0b013e328330cd2b. [DOI] [PubMed] [Google Scholar]
- 4.Rohan D, Buggy DJ, Crowley S, Ling FK, Gallagher H, Regan C, Moriarty DC. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anesth. 2005;52:137–142. doi: 10.1007/BF03027718. [DOI] [PubMed] [Google Scholar]
- 5.Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol Behav. 2015;151:16–23. doi: 10.1016/j.physbeh.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS One. 2013;8:e57870. doi: 10.1371/journal.pone.0057870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41–41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shore GC, Papa FR, Oakes SA. Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang WA, Groenendyk J, Michalak M. Endoplasmic reticulum stress associated responses in cancer. Biochim Biophys Acta. 2014;1843:2143–2149. doi: 10.1016/j.bbamcr.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti A, Chen AW, Varner JD. A review of the mammalian unfolded protein response. Biotechnol Bioeng. 2011;108:2777–2793. doi: 10.1002/bit.23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 14.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 15.Soo KY, Atkin JD, Farg M, Walker AK, Horne MK, Nagley P. Bim links ER stress and apoptosis in cells expressing Mutant SOD1 associated with amyotrophic lateral sclerosis. PLoS One. 2012;7:e35413. doi: 10.1371/journal.pone.0035413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang DX, Zhang LM, Zhao XC, Sun W. Neuroprotective effects of erythropoietin against sevoflurane-induced neuronal apoptosis in primary rat cortical neurons involving the EPORErk1/2-Nrf2/Bach1 signal pathway. Biomed Pharmacother. 2017;87:332–341. doi: 10.1016/j.biopha.2016.12.115. [DOI] [PubMed] [Google Scholar]
- 17.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 19.Krichevsky AM. MicroRNA profiling: from dark matter to white matter, or identifying new players in neurobiology. ScientificWorldJournal. 2007;7:155–66. doi: 10.1100/tsw.2007.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bak M, Silahtaroglu A, Møller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa MD, Tanaka MD, Arai MD, Genda MD, Sakamoto MD. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology. 2012;117:1245–1252. doi: 10.1097/ALN.0b013e3182746676. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S, Ishikawa M, Arai M, Genda Y, Sakamoto A. Changes in microRNA expression in rat lungs caused by sevoflurane anesthesia: a TaqMan® low-density array study. Biomed Res. 2012;33:255–263. doi: 10.2220/biomedres.33.255. [DOI] [PubMed] [Google Scholar]
- 23.Goto G, Hori Y, Ishikawa M, Tanaka S, Sakamoto A. Changes in the gene expression levels of microRNAs in the rat hippocampus by sevoflurane and propofol anesthesia. Mol Med Rep. 2014;9:1715–1722. doi: 10.3892/mmr.2014.2038. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto S, Ishikawa M, Nagano M, Sakamoto A. Influence of neonatal sevoflurane exposure on nerve development-related microRNAs and behavior of rats. Biomed Res. 2015;36:347–355. doi: 10.2220/biomedres.36.347. [DOI] [PubMed] [Google Scholar]
- 25.Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am J Physiol Cell Physiol. 2013;304:C1117. doi: 10.1152/ajpcell.00061.2013. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YF, Wang QX, Zhou HY, Chen G. Autophagy activation prevents sevoflurane-induced neurotoxicity in H4 human neuroglioma cells. Acta Pharmacol Sin. 2016;37:580–588. doi: 10.1038/aps.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nature reviews. Neuroscience. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Hou N, Wang X, Wang L, Chang Se, He K, Zhao Z, Zhao X, Song T, Huang C. miR-15b-5p induces endoplasmic reticulum stress and apoptosis in human hepatocellular carcinoma, both in vitro and in vivo, by suppressing Rab1A. Oncotarget. 2015;6:16227–16238. doi: 10.18632/oncotarget.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holstein SA, Hohl RJ. Isoprenoid biosynthetic pathway inhibition disrupts monoclonal protein secretion and induces the unfolded protein response pathway in multiple myeloma cells. Leuk Res. 2011;35:551–559. doi: 10.1016/j.leukres.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landoni G, Rodseth RN, Santini F, Ponschab M, Ruggeri L, Székely A, Pasero D, Augoustides JG, Del Sarto PA, Krzych LJ, Corcione A, Slullitel A, Cabrini L, Le Manach Y, Almeida RMS, Bignami E, Biondi-Zoccai G, Bove T, Caramelli F, Cariello C, Carpanese A, Clarizia L, Comis M, Conte M, Covello RD, De Santis V, Feltracco P, Giordano G, Pittarello D, Gottin L, Guarracino F, Morelli A, Musu M, Pala G, Pasin L, Pezzoli I, Paternoster G, Remedi R, Roasio A, Zucchetti M, Petrini F, Finco G, Ranieri M, Zangrillo A. Randomized evidence for reduction of perioperative mortality. J Cardiothorac Vasc Anesth. 2012;26:764–772. doi: 10.1053/j.jvca.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Bignami E, Biondi-Zoccai G, Landoni G, Fochi O, Testa V, Sheiban I, Giunta F, Zangrillo A. Volatile anesthetics reduce mortality in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:594–599. doi: 10.1053/j.jvca.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 34.Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, Schuldiner M. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schönthal AH. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Scientifica. 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23:239–252. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa S, Kitao Y, Hori O. Ischemia-induced neuronal cell death and stress response. Antioxid Redox Signal. 2007;9:573–587. doi: 10.1089/ars.2006.1516. [DOI] [PubMed] [Google Scholar]
- 38.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 39.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. NatCell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-[beta] . Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi H, Kuribayashi F, Mimuro H, Imajohohmi S. Biochemical and biophysical research communications lack of GCN5 remarkably enhances the resistance against prolonged endoplasmic reticulum stress-induced apoptosis through up-regulation of Bcl-2 gene expression. Biochem Biophys Res Commun. 2015;463:870–875. doi: 10.1016/j.bbrc.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K, Kim JH, Kwon OB, An K, Ryu J, Cho K, Suh YH, Kim HS. An activity-regulated microRNA, miR-188, controls dendritic plasticity and synaptic transmission by downregulating neuropilin-2. J Neurosci. 2012;32:5678–5687. doi: 10.1523/JNEUROSCI.6471-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001:2. doi: 10.1186/gb-2001-2-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucci C, Alifano P, Cogli L. The role of Rab proteins in neuronal cells and in the trafficking of neurotrophin receptors. Membranes. 2014;4:642–677. doi: 10.3390/membranes4040642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MT, Mishra A, Lambright DG. Structural mechanisms for regulation of membrane traffic by Rab GTPases. Traffic. 2009;10:1377–1389. doi: 10.1111/j.1600-0854.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allan BB, Moyer BD, Balch WE. Rab1 Recruitment of p115 into a cis-SNARE Complex: Programming Budding COPII Vesicles for Fusion. Science. 2000;289:444–8. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- 49.Ishida M, Ohbayashi N, Maruta Y, Ebata Y, Fukuda M. Functional involvement of Rab1A in microtubule-dependent anterograde melanosome transport in melanocytes. J Cell Sci. 2012;125:5177–87. doi: 10.1242/jcs.109314. [DOI] [PubMed] [Google Scholar]