Abstract
We describe a rare intraosseous myoepithelioma, arising in a rib. The patient was a 14-year-old female. The tumor, composed of epitheliod cells and plump spindle cells, was immunopositive for AE1/AE3, S-100 protein, HHF-35, desmin, smooth muscle actin (SMA), CD10, and c-kit, indicating a diagnosis of myoepithelioma. The tumor had invaded surrounding soft tissues, indicating a locally aggressive tumor. Positive staining for CD10 supported the diagnosis of myoepithelioma. To our knowledge, c-kit immunoreactivity has not been reported in intraosseous myoepithelioma. This form of myoepithelioma, arising in a rib and showing c-kit positivity, is very rare.
Keywords: Intraosseous myoepithelioma, rib, CD10, c-kit
Introduction
Myoepithelioma is a tumor which can arise in a salivary gland, soft tissue, skin, viscera and, rarely, bone [1]. Bone is a rare site for the malignant form of this tumor. The rib is a very rare site of intraosseous myoepithelioma. C-kit expression has been demonstrated in myoepithelial carcinoma arising in a salivary gland, but there are no reports describing c-kit expression in bone myoepithelioma [2]. CD10 expression in myoepithelioma was recently reported [3,4]. However, the clinical significance of CD10 expression in bone myoepithelioma remains unknown. Herein, we present a case with myoepithelioma arising in a rib, then invading the surrounding soft tissues, showing positive staining for c-kit (although the area of positive staining accounted for less than 5% of total tumor cells) and CD10. This is the first case report, to our knowledge, describing a myoepithelioma of bone origin expressing c-kit. The expression of CD10 supports the diagnosis of myoepithelial origin.
Case report
Clinical study
The patient was a 14-year-old female. She complained of left chest pain, and visited a hospital. She was then referred to our hospital. Swelling at the left 7th rib was detected on radiographic images. Magnetic resonance imaging revealed a tumor with an iso-intense signal on T1-weighted images and a high-intensity signal on T2-weighted images; the lesion was osteolytic. Three months later, an open biopsy was performed, yielding a diagnosis of spindle cell tumor. Two months after the open biopsy, the left 7th rib was resected. Postoperatively, she complained of mild pain and vomiting, but symptoms then diminished. Neither recurrence nor tumor metastasis was detected during the 1 year and 7 months, to date, since the operation.
Pathological study
Grossly, the specimen obtained by open biopsy consisted of small bone tumor fragments. The specimen obtained by resection of the rib was a 5 cm × 5 cm × 3 cm bone tumor and showed decalcification. On gross inspection, it was white, homogeneous, solid and firm (Figure 3A).
Figure 3.
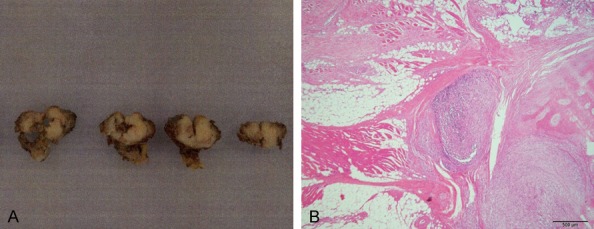
Specimen obtained by resection. A: Gross (bar is 2 cm), B: Invasive part of the tumor (HE) (40 ×) (bar is 500 μm).
Microscopically, the tumor showed a solid growth pattern. The tumor was composed of epithelioid cells and spindle cells arranged in interlacing fascicles. The spindle cells were plump. (Figure 1A-C). Examination of the resected specimen confirmed that the tumor had invaded the surrounding soft tissue (Figure 3B).
Figure 1.
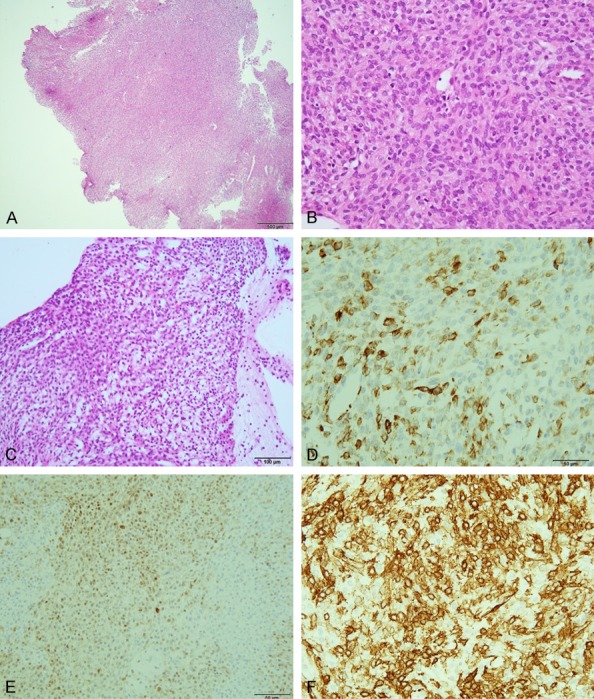
Specimen obtained by biopsy. (A) Hematoxylin and eosin (HE) (40 ×) (bar is 500 μm), (B) HE (400 ×) (bar is 50 μm), (C) HE (200 ×) (bar is 100 μm). The tumor was immunopositive for keratin (D), S-100 protein (E), α-smooth muscle actin (F) (400 ×). (D-F) Bar is 50 μm.
Immunohistochemical study
The tumor biopsy specimen was immunopositive for AE1/AE3, S-100 protein, HHF-35, Bcl-2, desmin, smooth muscle actin (SMA), and CD10 c-kit, while being negative for epithelial membrane antigen (EMA), CD31, CD34, calponin, 34βE12, p63, and glial fibrillary acidic protein (Figures 1D-F, 2A, 2B). In the resected specimen, staining for AE1/AE3, desmin and c-kit became negative, possibly due to decalcification. The other staining results were the same as those of the specimen obtained by biopsy.
Figure 2.
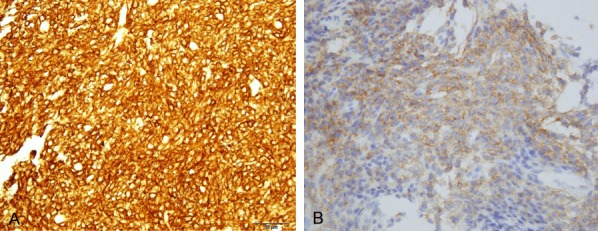
Specimen obtained by biopsy. The tumor was immunopositive for CD10 (A), c-kit (B). (A, B) (400 ×) (bar is 50 μm).
Discussion
In general, myoepithelioma is composed of epithelioid and spindle cells arranged in cords and nests, with a solid appearance [1]. Immunoreactivity for keratins and/or EMA, in conjunction with detection of S-100 protein or myogenic markers (calponin or SMA) is consistent with a diagnosis of myoepithelioma of soft tissue [5]. In this case, the tumor was composed of epithelioid cells and plump spindle cells, and immunohistochemical staining revealed the tumor to be positive for keratin, S-100 protein, SMA and CD10. We therefore diagnosed myoepithelioma.
The criteria for malignant myoepithelioma are controversial. However, nuclear atypia and mitosis are considered to indicate malignancy [5]. In the present case, tumor cells had hyperchromatic nuclei, but neither nuclear pleomorphism nor anisokaryosis was evident. No mitoses were detected. However, tumor cells had invaded the surrounding soft tissue. In salivary myoepithelioma, tumor invasion of surrounding tissue means malignancy. Hornick et al reported that in soft tissue, this criterion is not applicable, because many benign myoepitheliomas infiltrate adjacent tissues [5]. However, as to tumor arising in bone, invasion of the surrounding soft tissue indicates a locally aggressive tumor of low-grade malignancy. In the present case, tumor malignancy was unclear.
Only one case with intraosseous myoepithelioma involving a rib bone has been reported, to date [6]. Our case thus merits publication as intraosseous myoepithelioma of rib origin is very rare.
In this case, CD10 was positive. CD10 is positive in 67% of myoepitheliomas arising in soft tissue [7]. CD10 has recently come to be regarded as a marker of the myoepithelium [3,4]. Among previously reported intraosseous myoepithelioma cases, only one showed positive staining for CD10 [8]. Further investigation of CD10 is needed to determine the importance of CD10 in diagnosing myoepithelioma of bone.
This is the first report to document positive staining for c-kit in intraosseous myoepithelioma, though the area showing positive staining accounted for less than 5% of the specimen. Myoepithelioma of the salivary gland has been reported to occasionally express c-kit [2]. However, no other myoepithelioma sites have been reported to express c-kit. The capacity to express c-kit has, however, been noted at other myoepithelioma sites and imatinib mesilate, dasatinib hydrate or nilotinib hydrochloride hydrate might be effective for myoepithelioma of bone and other sites.
Conclusion
We experienced a rare case with myoepithelioma of rib origin. The tumor showed c-kit expression, documented for the first time in bone myoepithelioma, and CD10 expression supported the diagnosis of myoepithelioma.
Acknowledgements
We thank Dr. Tadashi Hasegawa Sapporo Medical University, School of Medicine, Department of Pathology, for aid in establishing the diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Kurzawa P, Kattapuram S, Hornicek FJ, Antonescu CR, Rosenburg AE, Nielsen GP. Primary myoepithelioma of bone. A report of 8 cases. Am J Surg Pathol. 2013;37:960–968. doi: 10.1097/PAS.0b013e3182858a0e. [DOI] [PubMed] [Google Scholar]
- 2.Jeng YM, Lin CY, Hsu HC. Expression of the c-kit protein is associated with certain subtypes of salivary gland carcinoma. Cancer Lett. 2000;154:107–111. doi: 10.1016/s0304-3835(00)00387-6. [DOI] [PubMed] [Google Scholar]
- 3.Leibel S, Moinfar F. Mammary NOS-type sarcoma with CD10 expression a rare entity with features of myoepithelial differentiation. Am J Surg Pathol. 2006;30:450–456. doi: 10.1097/00000478-200604000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kalof AN, Tam D, Beatty B, Cooper K. Immunostaining patterns of myoepithelial cells in breast lesions: a comparison of CD10 and smooth muscle myosin heavy chain. J Clin Pathol. 2004;57:625–629. doi: 10.1136/jcp.2003.013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornick JL, Fletcher CD. Myoepithelial tumor of soft tissue. A clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183–1196. doi: 10.1097/00000478-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Biradar P, Menon S, Ptil A, Karimundakal G, Jambhekar N. Primary myoepithelial carcinoma of rib bone: morphology, immunohistochemical evaluation and diagnostic dilemma in an unusual case. J Cancer Res Ther. 2015;11:647. doi: 10.4103/0973-1482.136035. [DOI] [PubMed] [Google Scholar]
- 7.Rekhi B, Sable M, Jambhekar NA. Histopatological, immunohistochemical and molecular spectrum of myoepithelial tumors of soft tissues. Virchows Arch. 2012;461:687–697. doi: 10.1007/s00428-012-1335-7. [DOI] [PubMed] [Google Scholar]
- 8.Rekhi B, Joshi S, Panchwagh Y, Gulia A, Borges A, Bajpai J, Jambhekar NA, Pant V, Mandholkar M, Byregowda S, Puri A. Clinicopathology features of five unusual cases of intraosseous myoepithelial carcinomas, mimicking conventional primary bone tumors, including EWSR1 rearrangement in one case. APMIS. 2016;124:278–290. doi: 10.1111/apm.12506. [DOI] [PubMed] [Google Scholar]


