Abstract
Dysregulation of microRNAs (miRNAs) are found in various human cancers, but the roles of miR-641 in lung cancer are still unclear. Our purpose is to explore miR-641 effects on the cellular behavior of A549 cells and the related molecular mechanisms. RT-qPCR assay was conducted to examine the expression of miR-641 in lung cancer and normal lung cell lines. A549 cells were transfected with miR-641 mimic and inhibitor, programmed cell death 4 (PDCD4) targeted siRNA and corresponding controls. Then, cell viability, migration, invasion and apoptosis were analyzed by Cell Counting Kit-8 (CCK-8), Transwell and flow cytometry assays. The expression of apoptosis-related factors and epithelial mesenchymal transition (EMT)-related factors were detected by western blot and RT-qPCR. A target gene of miR-641 was validated by dual-luciferase assay. Besides, the main factors expressions of JAK/STAT and PI3K/AKT signal pathways were measured by western blot. Results showed that miR-641 was low expressed in A549, H1650 and H1299 cells compared to WI-38 and HEL-1 cells. MiR-641 overexpression inhibited cell viability, migration, invasion but promoted apoptosis and apoptosis-related factors levels. Moreover, miR-641 overexpression inhibited TGF-β1-induced EMT in A549 cells. Additionally, PDCD4 was a direct target of miR-641 and PDCD4 silencing notably induced apoptosis, and relieved miR-641 suppressing promoted cell viability, migration and invasion. Finally, PDCD4 silencing blocked miR-641 suppression-induced activations of JAK/STAT and PI3K/AKT signal pathways. In conclusion, miR-641 inhibited cell proliferation and metastasis but promoted apoptosis in lung cancer cells by targeting PDCD4 and blocking JAK/STAT and PI3K/AKT signal pathways.
Keywords: microRNA-641, lung cancer, proliferation, metastasis, apoptosis, PDCD4
Introduction
Lung cancer is a malignant tumor which characterized by uncontrolled cell growth in tissues of the lung [1]. According to their unique clinic-pathological features, lung cancer is divided into two types: small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC) [2,3]. Among them, NSCLC is the most common subtype accounting for 85-90% of all cases [4]. If not treated promptly, lung cancer tumor cells will spread to other tissues through the cancer metastasis and which will serious threat to human health. Although the current study has confirmed that adjuvant chemotherapy can improve the survival rate of lung cancer, however, only 5-15% of treatment patients finally benefit [5,6]. Therefore, it is urgent needed to develop a new therapeutic method and strategy for treatment of lung cancer.
Recently, many studies concentrated on exploration of cancer molecular mechanisms by investigating miRNAs functions in various human cancers [7]. Increasing evidences suggested that the expression of miRNAs were great difference between in normal tissues and tumor tissues [8]. In addition, miRNAs could regulate diverse cellular functions including proliferation, differentiation, metastasis and apoptosis [9,10]. In lung cancer, abnormal expression of miRNAs including miR-34a, miR-7 and miR-25 have been reported to involve in regulation of cell proliferation, migration and apoptosis [11-13]. To our best knowledge, the functions of miR-641 have only been found in ovarian cancer and hepatocellular cancer (HCC) [14,15]. Whether miR-641 involved in the regulation of lung cancer has not been proved.
Programmed cell death 4 (PDCD4) is a novel tumor suppressor gene which inhibits tumor progression, invasion and apoptosis [16]. Previous study showed that miR-183 could inhibit apoptosis by repressing the PDCD4 expression in HCC [17]. Ying et al. demonstrated that miR-208a-3p promoted cell growth but suppressed apoptosis by targeting PDCD4 in gastric cancer [18]. However, there is no report about a possible correlation between PDCD4 and miR-641 in lung cancer.
In our study, we addressed these points by investigating the effect of miR-641 on lung cancer. The expression of miR-641 was examined in lung cancer and normal lung cell lines. Cell viability, migration, invasion, apoptosis as well as the expression levels of apoptosis-related factors and epithelial mesenchymal transition (EMT)-related factors were analyzed in A549 cells after transfection with miR-641 mimic, inhibitor and their corresponding controls. Moreover, a target of miR-641 was predicated and their interaction was confirmed by luciferase reporter assay. Furthermore, the important factors of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT) signal pathways were detected to explore the potential mechanisms. This study will provide new molecular therapeutic targets for human lung cancer.
Materials and methods
Cell culture and treatment
Human lung cancer cell lines A549, H1650 and H1299 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The WI-38 and HEL-1 human diploid cell lines from normal embryonic (3 month gestation) lung tissues were also purchased from ATCC. These cells were grown in Roswell Park Memorial Institute (RPMI)-1640 (Gibco, Grand Island, NY) medium supplemented with 1 × antibiotic-antimycotic mixture (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT) and maintained at 37°C in an atmosphere of 5% CO2 and 95% air. And 10 ng/ml of transforming growth factor-beta 1 (TGF-β1, Pepro Tech, Rocky Hill, NJ, USA) was used to induce EMT.
Cell transfection
MiR-641 mimic, miR-641 inhibitor, their corresponding controls (mimic control and inhibitor control) and PDCD4 target siRNA were synthesized by GenePharma Co. (Shanghai, China). A549 cell line was used for cellular plasmid transfection. In brief, A549 cells were cultured in 6-well plate at a density of 2 × 105 cells per well. Subsequently, cell transfections were conducted using Lipofectamine 3000 reagent (Invitrogen) following the manufacturer’s protocol.
Cell viability assay
Cells were seeded in 96-well plate with 5000 cells/well, and cell viability was assessed by a Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Gaithersburg, MD). Briefly, A549 cells were transfected with miR-641 mimic, miR-641 inhibitor and their corresponding controls. After 48 h transfection, the 10 μl CCK-8 solution was added to the culture medium, and the cultures were incubated for 1 h at 37°C in humidified 5% CO2 and 95% air. The absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA).
Migration and invasion assay
For migration assay, cell migration was determined by using a modified two-chamber migration assay with an 8-mm size of pore (Greiner 662638). Briefly, cells suspension with 200 μl of serum-free medium were seeded on the upper compartment of 24-well Transwell culture chamber and 600 μl of complete medium was added to the lower compartment. After 24 h of incubation at 37°C, the migrated cells were fixed with 70% methanol (NIST, USA) for 30 min. Non-traversed cells were removed from the upper surface of the filter carefully with a cotton swab. Traversed cells on the lower side of the filter were stained with 0.1% crystal violet (Merck, Darmstadt, Germany) for 15-20 min and counted under microscope (Leica Microsystems, Wetzlar, Germany). The invasion assay of A549 cells were determined using 24-well Millicell Hanging Cell Culture inserts with 8 mm PET membranes (Millipore, Bedford, Massachusetts, USA). Invasion capacity detection was performed the same as migration assay.
Apoptosis assay
Analysis of cell apoptosis was performed using propidium iodide (PI) and fluorescein isothiocynate (FITC)-conjugated Annexin V staining (Beijing Biosea Biotechnology, Beijing, China). Briefly, suspended cells were washed twice in phosphate buffered saline (PBS), and then added 100 μl Binging Buffer and 10 μl FITC-Annexin V (20 μg/ml) and incubated for 30 min at room temperature in the dark. After this, 5 μl PI (50 μg/ml) was added to the above solution and incubated for other 5 min. Subsequently, flow cytometry analysis was done by using a FACS can (Beckman Coulter, Fullerton, CA, USA). The data were analyzed by using FlowJo software version 9 (Tree Star, Inc., Ashland, OR, USA).
Dual luciferase activity assay
The 3’untranslated region (3’UTR) target site was generated by PCR and the luciferase reporter constructs with the PDCD4 3’UTR carrying a putative miR-641-binding site into pMiR-report vector were amplified by PCR. Cells were co-transfected with the reporter construct, control vector and miR-641 mimic or corresponding controls using Lipofectamine 3000 (Invitrogen). Reporter assays were done using the dual-luciferase assay system (Promega, Fitchburg, WI, USA) following to the manufacturer’s information.
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from cells by using Trizol reagent (Life Technologies Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. First-strand complementary DNAs (cDNAs) were synthesized using 1 μg RNAs by PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). The One Step SYBR® PrimeScript® PLUS RT-RNA PCR Kit (TaKaRa) was used for the Real-Time PCR analysis to test the expression levels of miR-641 in cells. U6 was used as the endogenous control. Data were analyzed with the 2-ΔΔCt method.
Western blot
The protein used for western blot was extracted using radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitors (Roche, Basle, Switzerland). The proteins were quantified using the BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The western blot system was established using a Bio-Rad Bis-Tris Gel system according to the manufacturer’s instructions. Primary antibodies of B-cell lymphoma 2 (Bcl-2; ab32124), Bcl-2-associated X (Bax, ab32503), pro-caspase-3 (ab32150), cleaved caspase-3 (ab13585), pro-caspase-9 (ab32068), E-cadherin (ab1416), N-cadherin (ab18203), Vimentin (ab92547), Snail (ab53519), PDCD4 (ab51495), JAK (ab47435), phosphorylated (p)-JAK (ab138005), STAT1 (ab31369), p-STAT1 (ab30645), STAT2 (ab93445), p-STAT2 (ab53132), GAPDH (ab8245, Abcam, Cambridge, UK) and cleaved caspase-9 (#9501), Zinc finger E-box-binding homeobox 1 (ZEB1; #3396), AKT (#9272), p-AKT (#4060), PI3K (#4292) and p-PI3K (#13857, Cell signaling Technology) were prepared in 5% blocking buffer at a dilution of 1:1,000. Primary antibodies were incubated with the membrane at 4°C overnight, followed by wash and incubation with secondary antibody (1:5000, Abcam, USA) marked by horseradish peroxidase for 1 h at room temperature. After rinsing, the Polyvinylidene Difluoride (PVDF) membrane carried blots and antibodies were transferred into the Bio-Rad ChemiDoc™ XRS system, and then 200 μl Immobilon Western Chemiluminescent HRP Substrate (Millipore, MA, USA) was added to cover the membrane surface. The signals were captured and the intensity of the bands was quantified using Image Lab™ Software (Bio-Rad, Shanghai, China).
Statistical analysis
All experiments were repeated three times. The results of multiple experiments are presented as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS 19.0 for Windows statistical software (SPSS Inc., Chicago, IL). The P-values were calculated using a one-way analysis of variance (ANOVA). Statistical significance was declared at P < 0.05.
Results
MiR-641 was low expressed in lung cancer cells
In order to reveal the role of miR-641 in human lung cancer cells, we conducted RT-qPCR to evaluate the expression levels of miR-641 in the lung cancer cell lines (A549, H1650 and H1299) and normal embryonic lung tissue cell lines (WI-38 and HEL-1). As displayed in Figure 1, miR-641 expression levels in three lung cancer cell lines were remarkably lower than in their two normal groups (P < 0.05 or P < 0.01). Thus, the result illustrated that the expression of miR-641 might be involved in pathogenesis of lung cancer. Additionally, lung cancer cell line A549 was selected for further investigations.
Figure 1.
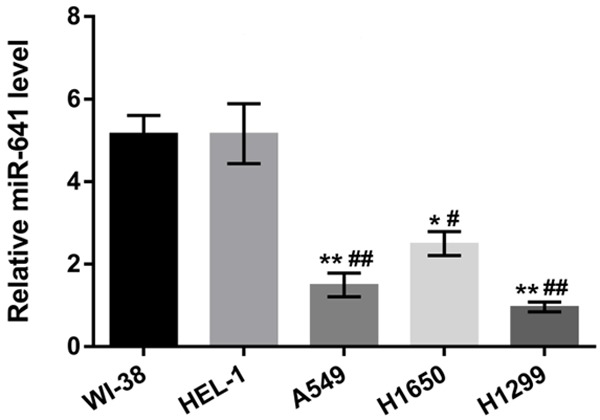
MiR-641 was low expressed in lung cancer cells. The expressions of miR-641 in human lung cancer cell lines (A549, H1650 and H1299) and normal embryonic lung tissue cell lines (WI-38 and HEL-1) were detected by RT-qPCR. MiR-641: microRNA-641; RT-qPCR: real-time quantitative PCR; *, P < 0.05, **, P < 0.01 when compared with WI-38 cell line; #, P < 0.05, ##, P < 0.01 when compared with HEL-1 cell line.
MiR-641 inhibited A549 cells viability, migration, invasion but promoted apoptosis
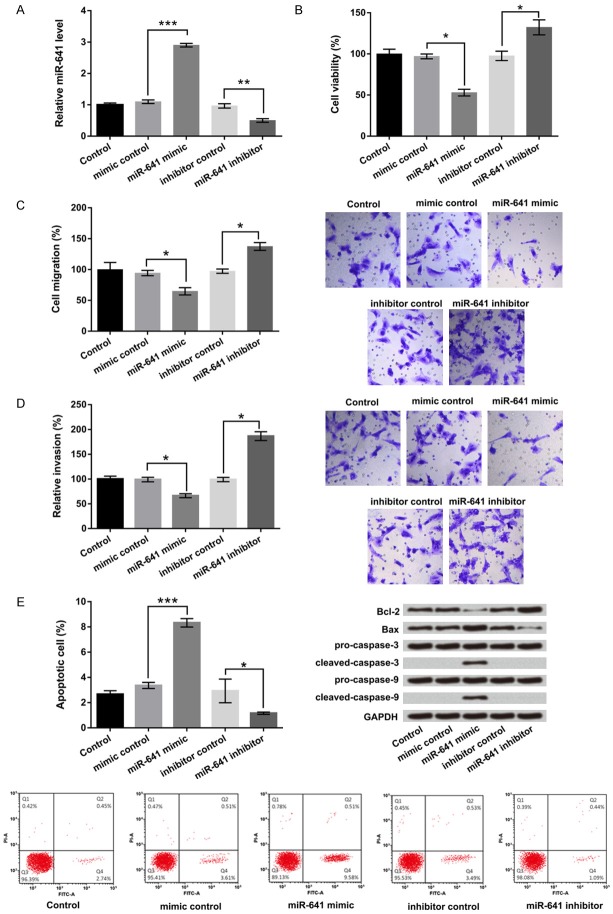
To explore the function of miR-641 in lung cancer cells growth and metastasis, the specific miR-641 mimic, miR-641 inhibitor and their corresponding controls were transfected into A549 cells. After transfection for 48 h, cells were collected for texting miR-641 expression level by RT-qPCR. Results showed that miR-641 mimic significantly promoted miR-641 expression in A549 cells, but miR-641 inhibitor suppressed miR-641 expression (P < 0.01 or P < 0.001, Figure 2A).
Figure 2.
MiR-641 inhibited A549 cells viability, migration, invasion but promoted apoptosis. A549 cells were transfected with miR-641 mimic, miR-641 inhibitor and their corresponding controls. A. Relative miR-641 expression. B. Cell viability. C. Migration. D. Invasion. E. Apoptosis and apoptosis-related genes expressions were respectively assessed by RT-qPCR, CCK-8, Transwell, flow cytometry and western blot. MiR-641: microRNA-641; RT-qPCR: real-time quantitative PCR; CCK-8: Cell Counting Kit-8; *, P < 0.05, **, P < 0.01, ***, P < 0.001.
The effects of miR-641 on cell proliferation, migration and invasion were detected by CCK-8 and Transwell assays in vitro. Results showed in Figure 2B-D, miR-641 overexpression obviously inhibited cell viability, migration and invasion compared with its control, while miR-641 suppression resulted in an opposite effect (P < 0.05). In addition, flow cytometry and western blot assay were used to detect cell apoptosis and their relative gene expressions. As showed in Figure 2E, cell apoptosis remarkably increased by miR-641 overexpression, but decreased by miR-641 suppression (P < 0.05 or P < 0.001). Western blot showed that miR-641 overexpression up-regulated Bax, cleaved caspase-3 and cleaved caspase-9 expressions, but down-regulated Bcl-2 expression. The protein levels of pro-caspase-3 and pro-caspase-9 were not significantly regulated by miR-641. Above all, these results suggested that miR-641 might be act as a tumor suppressor gene in lung cancer A549 cells which inhibited cell growth and metastasis.
MiR-641 inhibited EMT in lung cancer A549 cells
Our results from Transwell assay have proved that miR-641 signally inhibited cell migration and invasion. However, whether miR-641 also disrupted the EMT process is unclear. RT-qPCR and western blot results showed in Figure 3A and 3B, miR-641 overexpression increased the expression of the epithelial marker E-cadherin, but reduced those of mesenchymal markers of N-cadherin and Vimentin and transcription markers of ZEB1 and Snail (P < 0.05). Reverse results were obtained by miR-641 suppression. These results suggested that miR-641 would disrupt A549 cells metastasis via EMT process in lung cancer.
Figure 3.
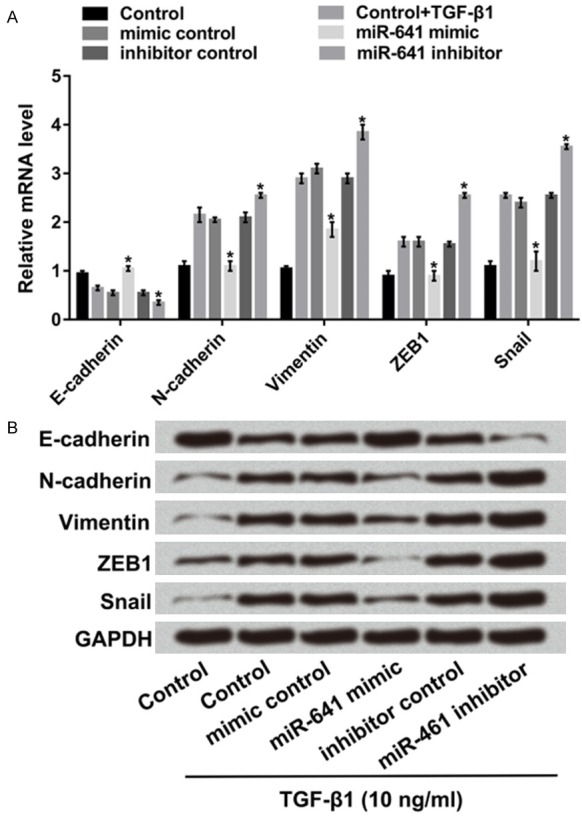
MiR-641 inhibited EMT process in A549 cells. A549 cells were transfected with miR-641 mimic, miR-641 inhibitor and their corresponding controls. After inducing by 10 ng/ml of TGF-β1, A. The mRNAs and B. The protein levels of E-cadherin, N-cadherin, Vimentin, ZEB1 and Snail were detected by RT-qPCR and western blot. MiR-641: microRNA-641; EMT: epithelial mesenchymal transition; TGF-β1: transforming growth factor-beta 1; ZEB1: Zinc finger E-box-binding homeobox 1; RT-qPCR: real-time quantitative PCR; *, P < 0.05.
PDCD4 was a direct target of miR-641
PDCD4 is one of the targets of some oncogene miRNAs which have been reported previously [19]. Whereas, if PDCD4 as a putative target of miR-641 still remains unclear. RT-qPCR and western blot assay were used to examine the PDCD4 expression level in miR-641 mimic, miR-641 inhibitor and their corresponding control transfected cells. Results demonstrated that the mRNA and protein expression levels of PDCD4 were significantly down-regulated in miR-641 overexpressing-cells, but were up-regulated in miR-641 suppressing-cells (P < 0.05 or P < 0.01 Figure 4A), which suggested that PDCD4 was negatively regulated by miR-641.
Figure 4.
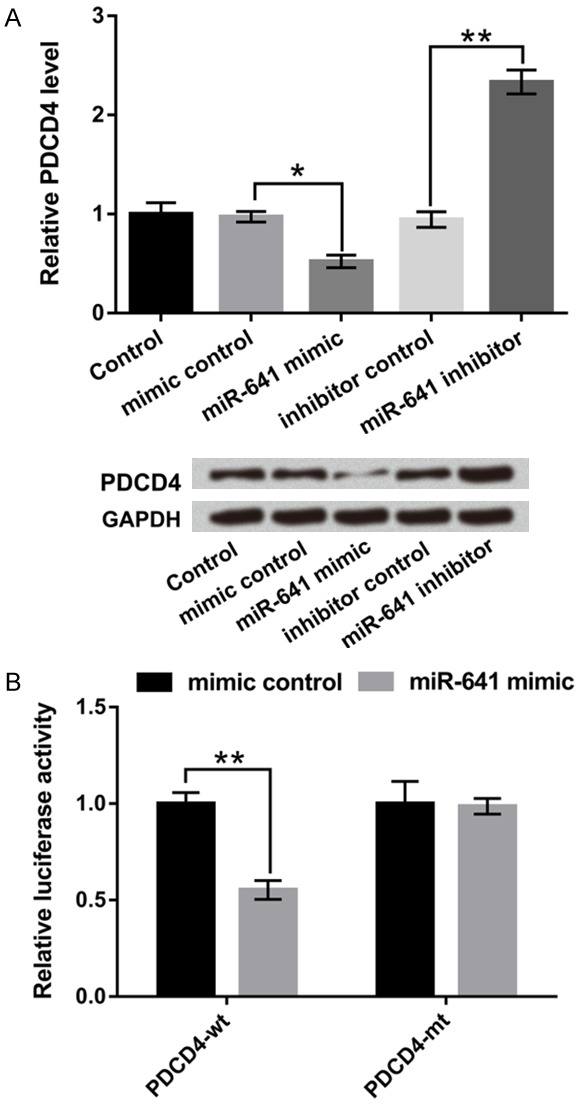
PDCD4 was a direct target of miR-641. A549 cells were transfected with miR-641 mimic, miR-641 inhibitor and their corresponding controls. A. The mRNA and protein levels of PDCD4 were determined by RT-qPCR and western blot. B. A target gene of miR-641 was analyzed by dual-luciferase assay. PDCD4: programmed cell death 4; miR-641: microRNA-641; RT-qPCR: real-time quantitative PCR; *, P < 0.05, **, P < 0.01.
Next, we verified PDCD4 as a target of miR-641 by performing dual luciferase report assay. A549 cells were co-transfected with miR-641 mimic and PDCD4 wild-type (PDCD4-wt) or PDCD4 mutant-type (PDCD4-mt) 3’UTR plasmids. The results in Figure 4B showed that co-transfected with miR-641 and PDCD4-wt notably reduced the luciferase active compared with their control groups (P < 0.01). But the luciferase activity revealed no significant difference in co-transfected with miR-641 and PDCD-mt cells. Above all, these results confirmed that PDCD4 might be a direct target of miR-641 and was negatively regulated by miR-641.
MiR-641 inhibited A549 cells viability, migration, invasion but promoted apoptosis by down-regulation of PDCD4
To define whether PDCD4 was participated in miR-641-mediated biological behavior, PDCD4 target siRNA was transfected into A549 cells and used to inhibit PDCD4 expression. Western blot result showed in Figure 5A, co-transfected with miR-641 inhibitor and PDCD4 silence significantly suppressed PDCD4 expression. Cellular function results showed that co-transfected with miR-641 inhibitor and PDCD4 silence markedly depressed cell viability, migration and invasion compared with miR-641 inhibitor group (P < 0.05 or P < 0.01, Figure 5B-D). Nevertheless, cell apoptosis was strikingly enhanced by miR-641 inhibitor together with PDCD4 silence (P < 0.001, Figure 5E).
Figure 5.
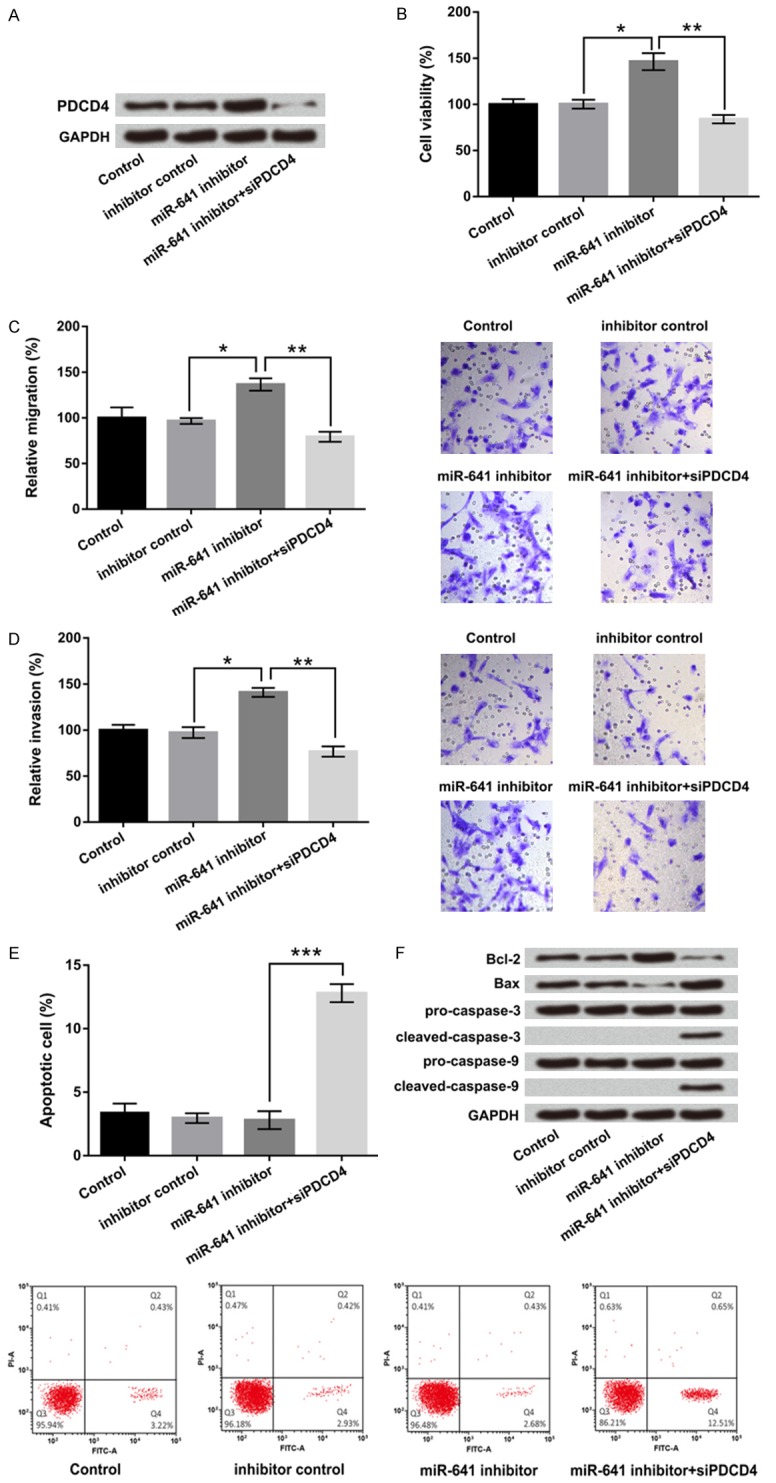
MiR-641 inhibited cell viability, migration, invasion but promoted apoptosis by down-regulation of PDCD4. A549 cells were co-transfected with miR-641 inhibitor and PDCD4 silence, then A. The protein level of PDCD4 was examined by western blot; and B. Cell viability. C. Migration. D. Invasion. E. Apoptosis and F. Apoptosis-related genes expressions were analyzed by CCK-8, Transwell, flow cytometry and western blot. MiR-641: microRNA-641; PDCD4: programmed cell death 4; CCK-8: Cell Counting Kit-8; *, P < 0.05, **, P < 0.01, ***, P < 0.001.
In addition, the expressions of apoptosis-related factors were further confirmed the effect of miR-641 and PDCD4 on apoptosis. The results demonstrated that co-transfected with miR-641 inhibitor and PDCD4 silence up-regulated pro-apoptosis gene of Bax expression, while down-regulated anti-apoptosis gene of Bcl-2 expression. The protein level of cleaved caspase-3 and cleaved caspase-9 were obviously up-regulated by miR-641 suppression together with PDCD4 silence. No obvious change in pro-caspase-3 and pro-caspase-9 were found (Figure 5F). In sum, the data uncovered that miR-641 could inhibit cell viability, migration, invasion but promoted apoptosis by down-regulation of PDCD4.
MiR-641 blocked JAK/STAT and PI3K/AKT signal pathways by regulation of PDCD4
Our finding showed that miR-641 dramatically suppressed cell proliferation, migration, invasion as well as promoted apoptosis in A549 cells. JAK/STAT and PI3K/AKT signal pathways have been found to be involved in regulating cellular processes including proliferation, metastasis and apoptosis [20]. However, the signal mechanisms responsible for the effect of miR-641 on lung cancer A549 cells remain to be elucidated. In JAK/STAT signal pathway, the result showed that expression of p-JAK, p-STAT1 and p-STAT2 were down-regulated by miR-641 overexpression, and up-regulated by miR-641 suppression. MiR-641 suppression together with PDCD4 silence abolished the promoting effect of miR-641 suppression on these three factors. JAK, STAT1 and STAT2 have no significant changes in different groups (Figure 6A). Similar results were found in PI3K/AKT signal pathway, miR-641 overexpression down-regulated p-PI3K, p-AKT and AKT, but miR-641 suppression up-regulated these three factors. Moreover, miR-641 suppression plus PDCD4 silence could weaken miR-641 suppression-induced up-regulations of these three factors. No obvious changes in PI3K expression were found in miR-641 and PDCD4 modified cells (Figure 6B). In a word, miR-641 blocked JAK/STAT and PI3K/AKT signal pathways by regulation of PDCD4.
Figure 6.
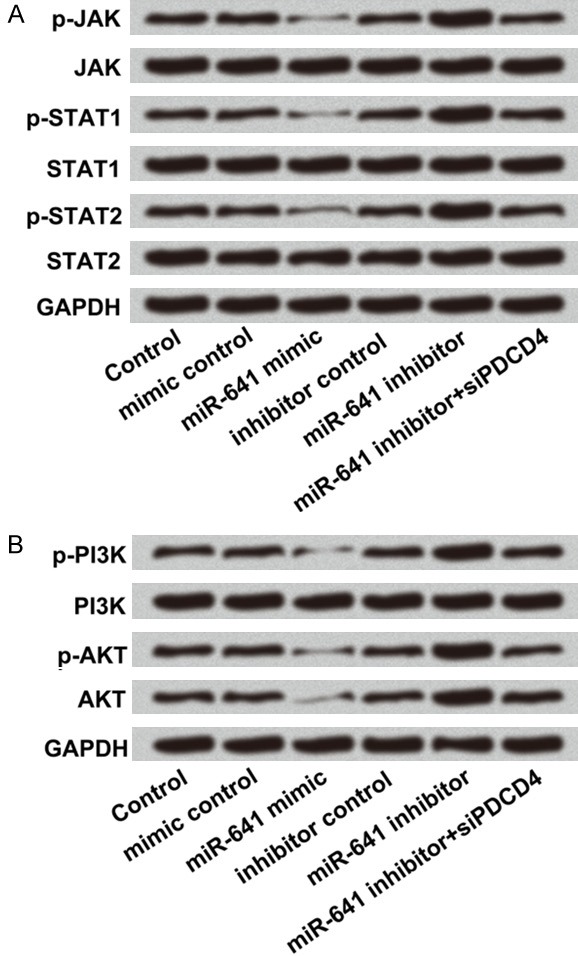
MiR-641 blocked JAK/STAT and PI3K/AKT signal pathways by down-regulation of PDCD4. A549 cells were transfected with miR-641 mimic, miR-641 inhibitor, co-transfected with miR-641 inhibitor and PDCD4 silence and their corresponding controls. A. The protein levels of JAK/STAT signal pathway factors and B. The protein levels of PI3K/AKT pathway factors were detected by western blot. MiR-641: microRNA-641; PDCD4: programmed cell death 4; JAK: Janus kinase; STAT: signal transducer and activator of transcription; PI3K: phosphatidylinositol 3 kinase; AKT: protein kinase B.
Discussion
In this study, we explored the function and mechanism of miR-641 in lung cancer A549 cell. We found that miR-641 was low expressed in lung cancer cell lines and overexpression of miR-641 inhibited cell viability, migration and invasion but promoted apoptosis. In addition, PDCD4 as a direct target of miR-641 was conformed and PDCD4 was negatively regulated by miR-641. Besides, PDCD4 could alleviate the suppressive effects of miR-641 on cells proliferation, metastasis and apoptosis. Further in vitro investigations displayed that miR-641 could block JAK/STAT and PI3K/AKT signal pathways in lung cancer A549 cells.
Mounting evidence indicated the importance of miRNAs in the progression of different kind of human tumors [21]. Accordingly, multiple miRNAs have been implicated in key biomarkers and involved in various biological processes in lung cancer [22,23]. A recent study confirmed that miR-494-3p improved cell growth and metastasis in lung cancer A549 cells [24]. In addition, the novel miR-9501 inhibited cell proliferation, migration and activated apoptosis in lung cancer [25]. So far, no beneficial studies were focused on the functions of miR-641 in lung cancer. In our study, we first confirmed that miR-641 was low expressed in lung cancer cell lines and inhibited cell viability, migration, invasion, EMT as well as promoted apoptosis in lung cancer. Similar with these results, Li et al. reported that miR-641 promoted cell proliferation, migration, invasion, EMT and cell growth in ovarian cancer [26].
As an important tumor suppressor, PDCD4 influenced transcription and translation of multiple genes and modulates different signal transduction pathways [27]. Until now, PDCD4 has been verified as a direct target of miR-499-5p, miR-106a and miR-155 respectively in colorectal cancer, ovarian cancer and oral cancer cells [28-30]. In terms of lung cancer, Ning et al. demonstrated that PDCD4 was a target of miR-182 and responsible for the miR-182-induced resistance in lung cancer [31]. However, whether miR-641 was another potential PDCD4-targeting miRNA have not been defined. In the present study, we demonstrated that PDCD4 was a direct target of miR-641 and was negatively regulated by miR-641. Moreover, we found that PDCD4 silencing notably induced apoptosis, and relieved miR-641 suppressing promoted cell viability, migration and invasion. Therefore, our data provided comprehensive evidence that PDCD4 was a direct target of miR-641 and participated in regulating cell growth and metastasis in lung cancer.
PI3K/AKT and JAK/STAT signal pathways were pivotal signal transduction pathways which were stimulated by cytokines [32]. Accumulating evidences indicated that PI3K/AKT and JAK/STAT signal pathways were linked to the formation, metastasis and apoptosis of tumors [33]. As Li et al. displayed that miR-294 promoted cellular proliferation and motility through the PI3K/AKT and JAK/STAT pathways by up-regulation of NRAS in bladder cancer [20]. Xia et al. reported that miR-107 inhibited tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in lung cancer [34]. Our data showed that miR-641 blocked JAK/STAT and PI3K/AKT signal pathways by regulation of PDCD4, and further regulated cell growth and metastasis.
In conclusion, all our results demonstrated that miR-641 as a tumor suppressive gene inhibited cell proliferation, metastasis but promoted apoptosis by targeting PDCD4 and inactivating JAK/STAT and PI3K/AKT signal pathways in A549 cells. This study provides a novel therapeutic strategy focusing on miR-641 and PDCD4 for lung cancer treatment. But the in-depth molecular mechanisms underlying lung cancer require further research.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81372407).
Disclosure of conflict of interest
None.
References
- 1.Kabel A, Aburas SH. Lung cancer: a focus on the new lines of management. 2016 [Google Scholar]
- 2.Incoronato M, Urso L, Portela A, Laukkanen MO, Soini Y, Quintavalle C, Keller S, Esteller M, Condorelli G. Epigenetic regulation of miR-212 expression in lung cancer. PLoS One. 2011;6:e27722. doi: 10.1371/journal.pone.0027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma ZL, Hou PP, Li YL, Wang DT, Yuan TW, Wei JL, Zhao BT, Lou JT, Zhao XT, Jin Y. MicroRNA-34a inhibits the proliferation and promotes the apoptosis of non-small cell lung cancer H1299 cell line by targeting TGFβR2. Tumor Biol. 2015;36:2481–90. doi: 10.1007/s13277-014-2861-5. [DOI] [PubMed] [Google Scholar]
- 4.Nigro E, Imperlini E, Scudiero O, Monaco ML, Polito R, Mazzarella G, Orrù S, Bianco A, Daniele A. Differentially expressed and activated proteins associated with non small cell lung cancer tissues. Respir Res. 2015;16:74. doi: 10.1186/s12931-015-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, Kim AW, Detterbeck FC, Blasberg JD, Boffa DJ. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–619. doi: 10.1001/jamaoncol.2016.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Sui A, Wang Z, Liu S, Yao R. Adenovirus-mediated TRAIL expression and downregulation of Bcl-2 expression suppresses non-small cell lung cancer growth in vitro and in vivo. Int J Mol Med. 2012;30:358–64. doi: 10.3892/ijmm.2012.998. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Kong X, Wang H, Huang G, Ye C, He Z. A novel microRNAs expression signature for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 2017;8:8775–8784. doi: 10.18632/oncotarget.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamfjord J, Stangeland AM, Hughes T, Skrede ML, Tveit KM, Ikdahl T, Kure EH. Differential expression of miRNAs in colorectal cancer: comparison of paired tumor tissue and adjacent normal mucosa using high-throughput sequencing. PLoS One. 2012;7:e34150. doi: 10.1371/journal.pone.0034150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh DK, Bose S, Kumar S. Role of microRNA in regulating cell signaling pathways, cell cycle, and apoptosis in non-small cell lung cancer. Curr Mol Med. 2016 [Epub ahead of print] [PubMed] [Google Scholar]
- 10.Xia W, Zhou J, Luo H, Liu Y, Peng C, Zheng W, Ma W. MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 2017;17:14. doi: 10.1186/s12935-017-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garofalo M, Jeon YJ, Nuovo GJ, Middleton J, Secchiero P, Joshi P, Alder H, Nazaryan N, Di Leva G, Romano G, Crawford M, Nana-Sinkam P, Croce CM. MiR-34a/c-dependent PDGFR-α/β downregulation inhibits tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer. PLoS One. 2013;8:e67581. doi: 10.1371/journal.pone.0067581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7:805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang J, Hang JB, Che JM, Li HC. miR-25 is up-regulated in non-small cell lung cancer and promotes cell proliferation and motility by targeting FBXW7. Int J Clin Exp Pathol. 2015;8:9147–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Yan L, Liu J, Zhao Y, Nie Y, Ma X, Kan Q, Zhang L. Suppression of miR-628-3p and miR-641 is involved in rifampin-mediated CYP3A4 induction in HepaRG cells. Pharmacogenomics. 2017;18:57–64. doi: 10.2217/pgs-2016-0088. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Richards E, Coppola M, Xu CX, Cheng JQ. Abstract 1904: MiR641 regulates EMT, ovarian cancer stem cell and angiogenesis by targeting p63/miR200 axis. 2016;76:1904–1904. [Google Scholar]
- 16.Kim YK, Kwon JT, Choi JY, Jiang HL, Arote R, Jere D, Je YH, Cho MH, Cho CS. Suppression of tumor growth in xenograft model mice by programmed cell death 4 gene delivery using folate-PEG-baculovirus. Cancer Gene Ther. 2010;17:751–760. doi: 10.1038/cgt.2010.28. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang J, Ma J, Hu XY, Li J, Hu MJ. MicroRNA-183 inhibits apoptosis and promotes proliferation and invasion of gastric cancer cells by targeting PDCD4. Int J Clin Exp Med. 2014;7:2519–2529. [PMC free article] [PubMed] [Google Scholar]
- 18.Yin K, Liu M, Zhang M, Wang F, Fen M, Liu Z, Yuan Y, Gao S, Yang L, Zhang W. miR-208a-3p suppresses cell apoptosis by targeting PDCD4 in gastric cancer. Oncotarget. 2016;7:67321. doi: 10.18632/oncotarget.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Uzair-ur-Rehman , Yu G, Liang H, Cheng R, Fei Y, Hong Y. miR-181b functions as an oncomiR in colorectal cancer by targeting PDCD4. Protein Cell. 2016;7:722–734. doi: 10.1007/s13238-016-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Shan Z, Liu C, Yang D, Wu J, Men C, Xu Y. microRNA-294 promotes cellular proliferation and motility through the PI3K/AKT and JAK-STAT pathways by upregulation of NRAS in bladder cancer. Biochemistry (Mosc) 2017;82:474–482. doi: 10.1134/S0006297917040095. [DOI] [PubMed] [Google Scholar]
- 21.Stiuso P, Potenza N, Lombardi A, Ferrandino I, Monaco A, Zappavigna S, Vanacore D, Mosca N, Castiello F, Porto S. MicroRNA-423-5p promotes autophagy in cancer cells and is increased in serum from hepatocarcinoma patients treated with sorafenib. Mol Ther Nucleic Acids. 2015;4:e233. doi: 10.1038/mtna.2015.8. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Zhan B, Luo P, Wang B. miRNA-375 regulates the cell survival and apoptosis of human non-small cell carcinoma by targeting HER2. Mol Med Rep. 2017;15:1387–1392. doi: 10.3892/mmr.2017.6112. [DOI] [PubMed] [Google Scholar]
- 23.Kontomanolis E, Mitrakas A, Giatromanolaki A, Kareli D, Panteliadou M, Pouliliou S, Koukourakis MI. A pilot study on plasma levels of micro-RNAs involved in angiogenesis and vascular maturation in patients with breast cancer. Med Oncol. 2017;34:20. doi: 10.1007/s12032-016-0881-2. [DOI] [PubMed] [Google Scholar]
- 24.Faversani A, Amatori S, Augello C, Colombo F, Porretti L, Fanelli M, Ferrero S, Palleschi A, Pelicci PG, Belloni E. miR-494-3p is a novel tumor driver of lung carcinogenesis. Oncotarget. 2017;8:7231–7247. doi: 10.18632/oncotarget.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi Y, Liang W, Sun C, Yang C, Feng Z, Li D. The novel miR-9501 inhibits cell proliferation, migration and activates apoptosis in non-small cell lung cancer. Med Oncol. 2016;33:124. doi: 10.1007/s12032-016-0837-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wei SY, Li JS, Zhang QF, Wang YX, Zhao SL, Yu J, Wang C, Qin Y, Wei QJ. Overexpression of heme oxygenase-1 prevents renal interstitial inflammation and fibrosis induced by unilateral ureter obstruction. PLoS One. 2016;11:e0147084. doi: 10.1371/journal.pone.0147084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Wang Y, Zang W, Wang H, Chu H, Li P, Li M, Zhang G, Zhao G. Downregulation of microRNA-182 inhibits cell growth and invasion by targeting programmed cell death 4 in human lung adenocarcinoma cells. Tumor Biol. 2014;35:39–46. doi: 10.1007/s13277-013-1004-8. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zhang Z, Sun L, Chai N, Tang S, Jin J, Hu H, Nie Y, Wang X, Wu K. MicroRNA-499-5p promotes cellular invasion and tumor metastasis in colorectal cancer by targeting FOXO4 and PDCD4. Carcinogenesis. 2011;32:1798–1805. doi: 10.1093/carcin/bgr213. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Xu H, Shen H, Li H. microRNA-106a modulates cisplatin sensitivity by targeting PDCD4 in human ovarian cancer cells. Oncol Lett. 2014;7:183–188. doi: 10.3892/ol.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zargar SA, Karunagaran D. Abstract 3070: MicroRNA-155 directly targets PDCD4 and activates BIC promoter through AP-1 dependent transcription to replenish miR-155 expression in SAS cells. Cancer Research. 2015;75:3070–3070. [Google Scholar]
- 31.Ning FL, Wang F, Li ML, Yu ZS, Hao YZ, Chen SS. MicroRNA-182 modulates chemosensitivity of human non-small cell lung cancer to cisplatin by targeting PDCD4. Diagn Pathol. 2014;9:143. doi: 10.1186/1746-1596-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jantaree P, Lirdprapamongkol K, Kaewsri W, Thongsornkleeb C, Choowongkomon K, Atjanasuppat K, Ruchirawat S, Svasti J. Homodimers of vanillin and apocynin decrease metastatic potential of human cancer cells by inhibiting the FAK/PI3K/Akt signaling pathway. J Agric Food Chem. 2017;65:2299–2306. doi: 10.1021/acs.jafc.6b05697. [DOI] [PubMed] [Google Scholar]
- 33.Bellido T, Manolagas SC. Printed in U. S.A. copyright © 1997 by the endocrine society activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes. International Journal of Mass Spectrometry & Ion Processes. 2013;159:137–152. [Google Scholar]
- 34.Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol. 2016;49:1325–1333. doi: 10.3892/ijo.2016.3628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]