Abstract
Tumor infiltrating lymphocytes in a certain tumor microenvironment are associated with the prognosis of cancer patients. The function of CD4+ and CD8+ T cells in the microenvironment of pancreatic cancer remains largely unknown. This study aimed to investigate the prognostic value of both CD4+ and CD8+ TIL subsets and their combined role in pancreatic cancer. In this study, pancreatic cancer tissues and corresponding adjacent normal tissues were collected from 90 patients. The expression levels of CD4 and CD8+ T cells in pancreatic cancer tissues were detected by immunohistochemistry method. The results showed that CD4+ iTIL expression was significantly correlated with tumor stage. CD8+ iTILs were significantly correlated with lymphatic vessel invasion and tumor stage; CD8+ sTILs not only showed correlation with lymphatic vessel invasion and tumor stage, but also had correlation with pathologic differentiation; the survival time of high CD4 expression group was longer compared to the low CD4 expression group. CD4+ T cells were capable of killing tumor cells and prolonging the survival time of patients either directly or indirectly. According to Cox regression analysis, it was indicated that pathological differentiation, lymphatic vessel invasion, tumor stage, CD4+ and CD8+ TILs were the principle risk factors of pancreatic cancer prognosis. Especially multivariate analysis showed that pathological differentiation and the combination of CD4+ and CD8+ TILs expression were independent predictors of pancreatic cancer survival. Expression levels of CD4+ and CD8+ TILs in pancreatic cancer may provide promising and useful markers for prognosis of pancreatic cancer.
Keywords: Pancreatic cancer, tumor infiltrating lymphocytes, marker
Introduction
The immune system contains both the innate and adaptive immune systems. The innate immune system mediates tumor-promoting inflammation that contributes to development and immune escape of the tumor, which is recognized as a hallmark of cancer [1]. There are various innate cells, such as macrophages, mast cells and neutrophils, which contribute to tumor angiogenesis and tumor infiltration and lead to a poor prognosis [2]. On contrary, abundant infiltrating lymphocytes often indicate a favorable prognosis [3]. Accumulated evidence has demonstrated that abundant tumor infiltrating lymphocytes (TILs) in the certain tumor microenvironment are correlated with the prognosis of cancer, which plays an important role in tumor immune responses [4-6].
Generally, it is thought that CD8+ TILs are a favorable prognostic indicator of many types of cancer, including esophageal cancer, colorectal cancer, and non-small cell lung cancer [7,8]. CD4 T cells play a key role in regulating the immune response via sending signals to other types of immune cells [4]. CD4 T cells are significant in resisting cancer cells. Naïve CD4 T cells can be differentiated into four types, including helper T cells (Th1, Th2, Th17) and regulatory T cells (Tregs), which play important and various roles in tumor immune microenvironment, tumor immune evasion, immune homeostasis, and anti-tumor immunity [6,9,10]. Pancreatic cancer patients exhibited Th17/Treg balance disorders with higher Treg and lower Th17 cells, which affect cytokine IL-10, IL-23, INF-γ, TGF-β, and IL-17 expression changes mainly through regulating transcription factors such as RORα, RORγt, FoxP3 and CTLA-4 [11].
However, the function of CD4+ and CD8+ T cells in the microenvironment of pancreatic cancer remains largely unknown. In this study, expression levels of CD4 and CD8+ T cells in pancreatic cancer tissues were detected by immunohistochemistry method. This study aimed to investigate the prognostic value of both CD4+ and CD8+ TIL subsets and their combined role in pancreatic cancer.
Material and methods
Patients
Pancreatic cancer tissues and corresponding adjacent normal tissues were harvested from 90 cases (57 males and 33 females) in the Inner Mongolia Medical College Affiliated Hospital. Pancreatic cancer samples were completely removed and confirmed pathologically. The adjacent normal tissues were resected 3 cm from the pancreatic cancer tissues. Infor-med consent was obtained from all patients prior to surgery. Pancreatic cancer was diagnosed and staged according to the 7th edition Staging Manual of American Joint Committee on Cancer. The pathological types of pancreatic cancer consist of duct adenocarcinoma, mucinous adenocarcinoma, mucinous cystadenocarcinoma and adenosquamous carcinoma. Complete follow-up history was available for at least 5 years. Overall survival referred to the interval from operation to death. The protocol was approved by the Ethics Committee of Inner Mongolia Medical College Affiliated Hospital.
Immunohistochemistry
Immunohitochemistry was performed on 4 μm thick paraffin-embedded sections. Briefly, the sections were deparaffinized and then hydrated. The sections were washed with PBS. Then the sections were immersed in 0.01 mol/L citrate buffer solution (pH 6.0), and placed in microwave for 10 minutes. Peroxidase was quenched by 3% H2O2 in phosphate-buffered saline (PBS) for 15 minutes. The sections were incubated at 4°C overnight with the primary antibody following PBS washing. After washed by PBS for three times, the sections were treated with the Envision detection system (EnVision™ Detection System, Peroxidase/DAB+, Rabbit/Mouse, Dako, Denmark). Finally, the sections were counterstained with hematoxylin (Hematoxylin, Sigma-Aldrich, Germany) to visualize the nuclei. Images were obtained with LEICA AMIL (LEICA, Germany). CD4 (clone L26 and CD8 (clone 1A5) antibodies from Ventana Medical (Tucson, Ariz, USA) were employed in this study.
Evaluation of staining
A couple of pathologists who were blinded to the scores of the other markers and the patient outcomes independently scored the samples. TILs were classified into two parts: iTILs and pTILs. The former was defined as TILs within intratumoral tissues, and the latter as TILs within peritumoral tissues (Figure 1). According to reference [12], the area of tumor and stromal components was determined in a 0.4×0.4 mm microscopic grid under 200× magnification, and the number of immunoreactive cells was counted in the specified area. The number of CD4+ cells and CD8+ cells was counted in the same region. For the definition of high and low TILs, the median TIL value as the cut-off was employed. The expression levels of the combined CD4 and CD8 were scored individually in the same tumor.
Figure 1.
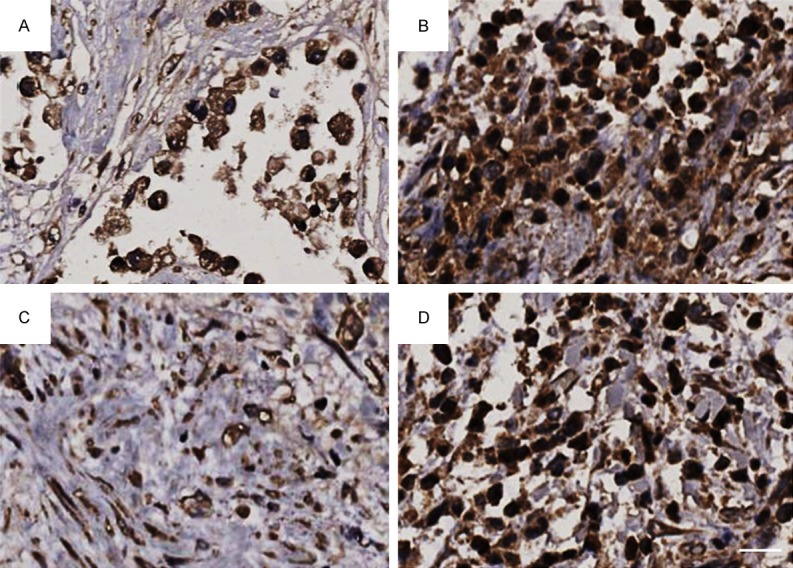
Immunohistochemical staining of TILs in pancreatic intratumoral cancer. A. Low CD4+ iTILs; B. High CD4+ iTILs; C. Low CD8+ iTILs; D. High CD8+ iTILs. Bar: 50 μm.
Statistics
The statistical package IBM SPSS version 18 was employed for data analysis. The Chi-square test was used to compare the data between different groups. Prognostic factors were examined by univariate and multivariate analyses. Kaplan-Meier method and the COX regression model were used to analyze survival and prognostic factors, respectively. P value < 0.05 was considered significant.
Results
As showed in Figures 1 and 2, CD4+ iTILs and CD8+ iTILs were stained in pancreatic intratumoral cancer and peritumoral cancer, respectively. The relationship between TILs and clinicopathological factors is summarized in Table 1. There was significant correlation between CD4+ iTILs and tumor stage (P = 0.005). However, there was no significant correlation between CD4+ pTILs and these clinical pathological factors (Table 2). CD8+ iTILs were significantly correlated with lymphatic vessel invasion and tumor stage (P = 0.010 and 0.005, respectively) (Table 3); CD8+ pTILs were not only correlated with lymphatic vessel invasion and tumor stage (P = 0.033 and P < 0.001, respectively), but also correlated with pathologic differentiation (P = 0.009) (Table 4).
Figure 2.
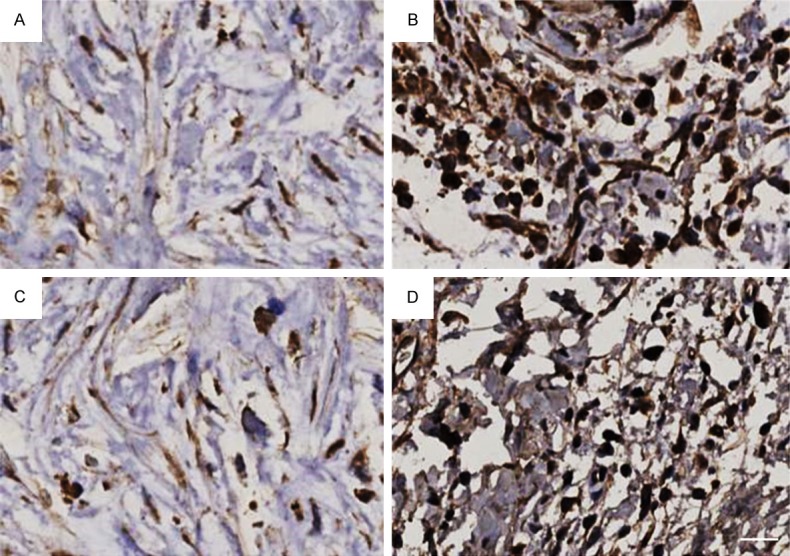
Immunohistochemical staining of TILs in peritumoral cancer. A. Low CD4+ iTILs; B. High CD4+ iTILs; C. Low CD8+ iTILs; D. High CD8+ iTILs. Bar: 50 μm.
Table 1.
Relationship between CD4+ iTILs and clinicopathological factors
| Clinical pathological factors | CD4+ iTILs | P value | ||
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Gender | Male | 30 | 27 | 0.351 |
| Female | 14 (31.8%) | 19 (41.3%) | ||
| Age | ≤ 60 years | 19 | 23 | 0.517 |
| > 60 years | 25 (58.8%) | 23 (50%) | ||
| Pathologic differentiation | High | 27 | 32 | 0.413 |
| Low | 17 (38.6%) | 14 (30.4%) | ||
| Tumor size | ≤ 4 cm | 24 | 27 | 0.691 |
| > 4 cm | 20 (45.5%) | 19 (41.3%) | ||
| Nerve invasion | Negative | 24 | 29 | 0.413 |
| Positive | 20 (45.5%) | 17 (37.0%) | ||
| Lymphatic vessel invasion | Negative | 27 | 30 | 0.705 |
| Positive | 17 (38.6%) | 16 (34.8%) | ||
| Stage (I + II vs II + IV) | I | 13 | 27 | 0.005 |
| II + IV | 31 (70.5%) | 19 (41.3%) | ||
Table 2.
Relationship between CD4+ pTILs and clinicopathological factors
| Clinical pathological factors | CD4+ pTILs | P value | ||
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Gender | Male | 23 | 34 | 0.929 |
| Female | 13 (36.1%) | 20 (37.0%) | ||
| Age | ≤ 60 years | 18 | 24 | 0.605 |
| > 60 years | 18 (50.0%) | 30 (55.6%) | ||
| Pathologic differentiation | High | 26 | 33 | 0.277 |
| Low | 10 (27.8%) | 21 (38.9%) | ||
| Tumor size | ≤ 4 cm | 22 | 29 | 0.487 |
| > 4 cm | 14 (38.9%) | 25 (46.3%) | ||
| Nerve invasion | Negative | 19 | 34 | 0.336 |
| Positive | 17 (47.2%) | 20 (37.0%) | ||
| Lymphatic vessel invasion | Negative | 23 | 34 | 0.929 |
| Positive | 13 (36.1%) | 20 (37.0%) | ||
| Stage (I vs II + IV) | I | 14 | 26 | 0.386 |
| II + IV | 22 (61.1%) | 28 (51.9%) | ||
Table 3.
Relationship between CD8+ iTILs and clinicopathological factors
| Clinicopathological factors | CD8+ iTILs | P value | ||
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Gender | Male | 29 | 28 | 0.620 |
| Female | 15 (34.1%) | 18 (39.1%) | ||
| Age | ≤ 60 years | 21 | 21 | 0.844 |
| > 60 years | 23 (52.3%) | 25 (54.3%) | ||
| Pathologic differentiation | High | 27 | 32 | 0.413 |
| Low | 17 (38.6%) | 14 (30.4%) | ||
| Tumor size | ≤ 4 cm | 26 | 25 | 0.650 |
| > 4 cm | 18 (40.9%) | 21 (45.7%) | ||
| Nerve invasion | Negative | 24 | 29 | 0.413 |
| Positive | 20 (45.5%) | 17 (37.0%) | ||
| Lymphatic vessel invasion | Negative | 22 | 35 | 0.010 |
| Positive | 22 (50.0%) | 11 (23.9%) | ||
| Stage (I vs II + IV) | I | 13 | 27 | 0.005 |
| II + IV | 31 (70.5%) | 19 (41.3%) | ||
Table 4.
Relationship between CD8+ pTILs and clinicopathological factors
| Clinicopathological factors | CD8+ pTILs | P value | ||
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Gender | Male | 32 | 25 | 0.070 |
| Female | 12 (27.3%) | 21 (45.7%) | ||
| Age | ≤ 60 years | 20 | 22 | 0.822 |
| > 60 years | 24 (54.5%) | 24 (52.2%) | ||
| Pathologic differentiation | High | 23 | 36 | 0.009 |
| Low | 21 (41.7%) | 10 (21.7%) | ||
| Tumor size | ≤ 4 cm | 24 | 27 | 0.691 |
| > 4 cm | 20 (45.5%) | 19 (41.3%) | ||
| Nerve invasion | Negative | 24 | 29 | 0.413 |
| Positive | 20 (45.5%) | 17 (37.0%) | ||
| Lymphatic vessel invasion | Negative | 23 | 34 | 0.033 |
| Positive | 21 (47.7%) | 12 (26.1%) | ||
| Stage (I vs II + IV) | I | 11 | 29 | < 0.001 |
| II + IV | 33 (75.0%) | 17 (37.0%) | ||
The Kaplan-Meier survival curve was used to evaluate the overall survival of patients. The median survival of patients in high CD4+ TILs expression group (median, 27 months; 95% CI, 24.30-29.70 months) was significantly shorter than that in low CD4+ TILs expression group (median 17 months; 95% CI 12.14-21.86 months) (Figure 3). Similarly, the median survival time in high CD8 TILs expression group (median 28 months; 95% CI 22.62-33.39 months) was significantly longer than that in low CD8 TILs expression group (median 18 months; 95% CI 13.77-22.23 months) (P < 0.001) (Figure 4). The median survival time in the combination of CD4 high CD8 high TILs expression group (median 28 months; 95% CI 23.41-32.60 months) was shorter than that in the combination of low CD4 low CD8 low TILs expression group (median 15 months; 95% CI 11.08-18.92 months) (P < 0.05) (Figure 5).
Figure 3.
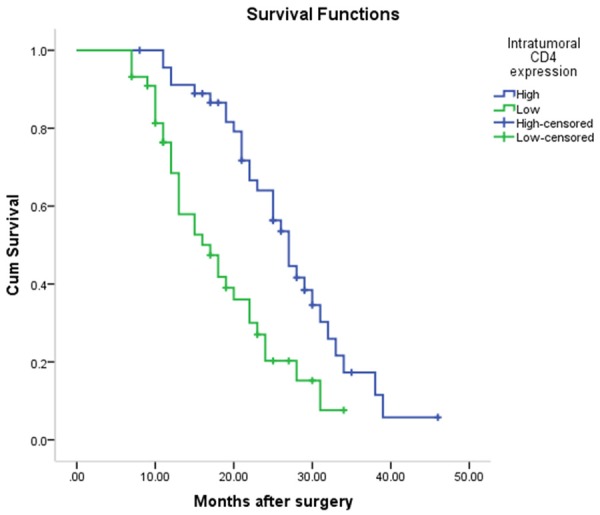
The median survival time in high CD4+ TILs expression group (median 27 months; 95% CI 24.299-29.701 months) was significantly shorter than that in low CD4+ TILs expression group (median 17 months; 95% CI 12.138-21.862 months) (P = 0.001).
Figure 4.
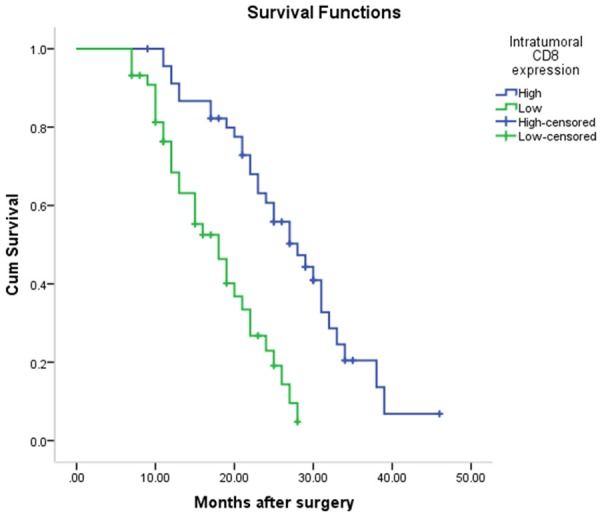
The median survival time in high CD8 TILs expression group (median 28 months; 95% CI 22.615-33.385 months) was significantly longer than that in low CD8 TILs expression group (median 18 months; 95% CI 13.770-22.230 months) (P < 0.001).
Figure 5.
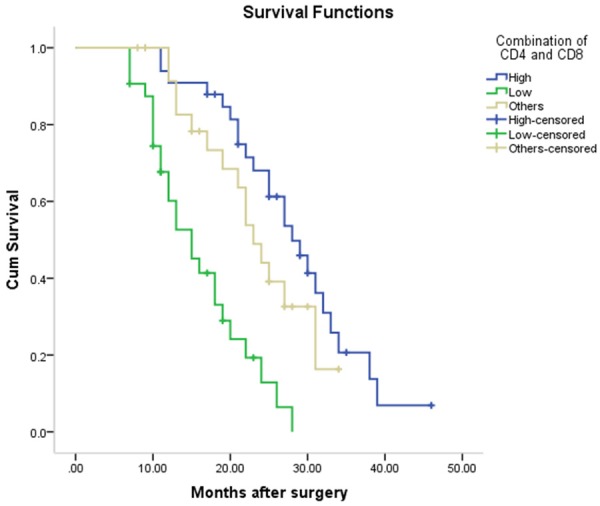
The median survival time in the combination of CD4 high CD8 high TILs expression group (median 28 months; 95% CI 23.407-32.593 months) was shorter than that in the combination of low CD4 low CD8 low TILs expression group (median 15 months; 95% CI 11.084-18.916 months) (P < 0.001).
The results of Cox regression analysis are shown in Tables 5 and 6. Univariate analysis demonstrated that the main prognostic factors of pancreatic cancer are pathological differentiation, lymphatic vessel invasion, tumor stage, CD4+ and CD8+ TILs in pancreatic cancer tissues (Table 5). Moreover, multivariate analysis clearly showed that pathological differentiation, and CD4+ and CD8+ TILs expression were independent prognostic factors for overall survival in pancreatic cancer (Table 6). The age, sex and tumor size were not correlated with the prognosis of pancreatic cancer.
Table 5.
Univariate Cox regression analysis of the effect of TILs on survival
| Variables | Patients (n) | Median survival, 95% CI (months) | Log-rank P |
|---|---|---|---|
| Gender | |||
| Male | 57 | 24 (19.465-28.535) | 0.625 |
| Female | 33 | 22 (20.179-23.821) | |
| Age (yeas) | |||
| ≤ 60 | 42 | 24 (19.990-28.010) | 0.591 |
| > 60 | 48 | 21 (16.935-25.065) | |
| Pathologic differentiation | |||
| High | 59 | 24 (20.545-27.455) | 0.016 |
| Low | 25 | 18 (11.503-24.497 | |
| Tumor size | |||
| ≤ 4 cm | 51 | 23 (15.533-27.467) | 0.733 |
| > 4 cm | 39 | 22 (18.202-25.798) | |
| Nerve invasion | |||
| Negative | 53 | 23 (20.104-25.896) | 0.858 |
| Positive | 37 | 21 (13.997-28.003) | |
| Lymphatic vessel invasion | |||
| Negative | 57 | 27 (20.330-33.670) | 0.011 |
| Positive | 33 | 20 (14.920-25.080) | |
| Stage | |||
| I | 40 | 29 (24.093-33.907) | < 0.001 |
| II + IV | 50 | 19 (14.527-23.473) | |
| CD4+ | |||
| Low | 44 | 17 (12.138-21.862) | 0.001 |
| High | 46 | 27 (24.299-29.701) | |
| CD8+ | |||
| Low | 44 | 18 (13.770-22.230) | |
| High | 46 | 28 (22.615-33.385) | < 0.001 |
| Combination of CD4+ and CD8+ | |||
| Both Low | 29 | 15 (11.084-18.916) | < 0.001 |
| Both High | 31 | 28 (23.407-32.595) | |
| Others | 30 | 23 (19.727-26.273) |
Table 6.
Multivariate Cox regression analysis of the effect of TILs on survival
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Pathologic differentiation | ||
| High | 0.405 (0.380-1.471) | 0.405 |
| Low | 1.000 | - |
| Lymphatic vessel invasion | ||
| Negative | 1.740 (1.034-2.927) | 0.037 |
| Positive | 1.000 | |
| Stage | ||
| I | 2.779 (1.631-4.736) | < 0.001 |
| II + IV | 1.000 | - |
| Combination of CD4+ and CD8+ | ||
| Both Low | 1.000 | - |
| Both High | 3.264 (1.678-6.348) | 0.001 |
| Others | 0.646 (0.329-1.629) | 0.205 |
Discussion
Cellular immunity is significant for the immune system, which plays a major role in killing cancer and preventing inflammation. T cells are the key in cellular immunity [13]. Increasing evidence suggests that tumor-specific tumor-infiltrating T cells (TILs) but not circulating T cells predict clinical outcomes of cancer patients [14,15]. A model including three intratumoral infiltrating immune markers (CD15+, CD206+ and CD117+) and a SMAD4 mutation can be used to predict recurrence and survival in patients after surgery for pancreatic ductal adenocarcinoma [16]. Prognostic significance of CD3, CD8 and CD20 positive lymphocytes and survival have a correction in pancreatic ductal adenocarcinoma [17]. The involvement of both CD4+ and CD8+ T cells is required for an effective anti-tumor immune response [18,19]. CD8+ T cells have long been deemed as the ideal option for adoptive cell therapy due to their ability to lyse tumor cells directly. Although CD8+ T cells are important in antitumor immunity, it has been shown that they cannot always protect the host for tumor relapse [20]. One reason is that CD8+ T cells-mediated recognition of tumors is impaired [21]. Although only few studies investigated the role of CD4+ iTILs in a variety of cancers as a prognostic factor, the role of CD4+ T cells in anti-tumor immunity has been well investigated in both animal models and cancer patients [22].
In our current study, it was found that CD4+ iTILs expression was significantly correlated with tumor stage. There was significant correlation between CD8+ iTILs and lymphatic vessel invasion and tumor stage; CD8+ sTILs were not only correlated with lymphatic vessel invasion and tumor stage, but also correlated with pathologic differentiation. The results showed that survival in high CD4 expression group was longer compared to the low group. It was suggested that CD4+ T cells can directly or indirectly kill tumor cells and prolong the survival of patients. Cox regression analysis demonstrated that pathological differentiation, lymphatic vessel invasion, tumor stage, CD4+ and CD8+ iTILs were the main risk factors for pancreatic cancer prognosis. Especially multivariate analysis showed that pathological differentiation, and the CD4+ and CD8+ iTILs expression were independent predictors of survival of pancreatic cancer patients. Tumor-infiltrating CD4(+)T(high)/CD8(+)T(high)/%Treg(low) and %M1(high)/M2(low) are independent prognosticators useful for evaluating the immune microenvironment of PDC [23].
Kaplan-Meier analysis showed that high CD4 and CD8 TILs expression is associated with better prognosis in pancreatic cancer. Our study also revealed that high CD4 and high CD8 iTILs and sTILs were correlated with good prognosis compared with low CD4 and low CD8 iTILs. The median survival in the high CD4 and high CD8 iTILs expression group was longer compared to low CD4 and low CD8 iTILs expression group. Our main finding was that the combination of CD4+ and CD8+ iTILs expression was a strong and independent prognostic factor in this group. Patients with low CD4+ and low CD8+ iTILs expression seemed to have poor prognosis. Our results indicated that CD4 and CD8 iILs may also play an important role in antitumor immune response and that the collaborative interaction between high CD8 and high CD4 iTILs was critical for cancer suppression. In pancreatic adenocarcinoma, the presence of CD4+ iTILs together with CD8+ iTILs serves as a good indicator of the patient’s outcome after surgical treatment [24].
Conclusions
Expression of CD4+ and CD8+ iTILs in pancreatic cancer may provide promising and useful markers for prognosis of pancreatic cancer. Determination of CD4 and CD8 iTILs expression in pancreatic cancer tissues may aid to understand the local immune response in tumor microenvironment, which provides a basis for postoperative antitumor immunity therapy.
Acknowledgements
The study was supported by the Natural Science Foundation of the Inner Mongolia (No. 2015BS (LH) 0801) and National Natural Science Foundation of China (81560384).
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Deng S, Hu B, Shen KP, Xu L. Inflammation, macrophage in cancer progression and chinese herbal treatment. J Basic Clin Pharm. 2012;3:269–272. doi: 10.4103/0976-0105.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hald SM, Bremnes RM, Al-Shibli K, Al-Saad S, Andersen S, Stenvold H, Busund LT, Donnem T. CD4/CD8 co-expression shows independent prognostic impact in resected non-small cell lung cancer patients treated with adjuvant radiotherapy. Lung Cancer. 2013;80:209–215. doi: 10.1016/j.lungcan.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 4.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uso M, Jantus-Lewintre E, Bremnes RM, Calabuig S, Blasco A, Pastor E, Borreda I, Molina-Pinelo S, Paz-Ares L, Guijarro R, Martorell M, Forteza J, Camps C, Sirera R. Analysis of the immune microenvironment in resected non-small cell lung cancer: the prognostic value of different T lymphocyte markers. Oncotarget. 2016;7:52849–52861. doi: 10.18632/oncotarget.10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016:4. doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 10.Cohen-Inbar O, Xu Z, Sheehan JP. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J Ther Ultrasound. 2016;4:2. doi: 10.1186/s40349-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Wang L, Mo Q, Dong Y, Wang G, Ji A. Changes of Th17/Treg cell and related cytokines in pancreatic cancer patients. Int J Clin Exp Pathol. 2015;8:5702. [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, Tan PH, Iqbal J. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016;156:237–247. doi: 10.1007/s10549-016-3743-x. [DOI] [PubMed] [Google Scholar]
- 13.Winter H, van den Engel NK, Ruttinger D, Schmidt J, Schiller M, Poehlein CH, Lohe F, Fox BA, Jauch KW, Hatz RA, Hu HM. Therapeutic T cells induce tumor-directed chemotaxis of innate immune cells through tumor-specific secretion of chemokines and stimulation of B16BL6 melanoma to secrete chemokines. J Transl Med. 2007;5:56. doi: 10.1186/1479-5876-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 15.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 16.Wang WQ, Liu L, Xu HX, Wu CT, Xiang JF, Xu J, Liu C, Long J, Ni QX, Yu XJ. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br J Surg. 2016;103:1189–1199. doi: 10.1002/bjs.10187. [DOI] [PubMed] [Google Scholar]
- 17.Tewari N, Zaitoun AM, Arora A, Madhusudan S, Ilyas M, Lobo DN. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer. 2013;13:1471–2407. doi: 10.1186/1471-2407-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 20.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opinion Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majchrzak K, Nelson MH, Bailey SR, Bowers JS, Yu XZ, Rubinstein MP, Himes RA, Paulos CM. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol Immunother. 2016;65:247–259. doi: 10.1007/s00262-016-1797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Ma C, Zhang Q, Ye J, Wang F, Zhang Y, Hunborg P, Varvares MA, Hoft DF, Hsueh EC, Peng G. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6:17462–17478. doi: 10.18632/oncotarget.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]


