Abstract
Background: Exosomes which mainly function in intercellular communication and lncRNA HIF1A-AS1 which can be transferred by exosomes, have been shown to play a significant role in atherosclerosis. This study aims to explore the diagnostic value of exosomes and exosomal lncRNA HIF in atherosclerosis. Methods: Plasma sample from 35 patients with atherosclerosis and from 28 healthy adults were collected for further investigation. The exosomes were extracted from the plasma sample and then the concentration of exosomes was measured by flow cytometry. Then the exosomal RNA was isolated and exosomal lncRNA HIF1A-AS1 was quantified using real-time polymerase chain reaction (qRT-PCR). Finally, the concentration of exosomes and the expression level of exosomal lncRNA HIF1A-AS1 were analyzed using Spearman’s correlation test, and receiver operating characteristic (ROC) curves were used for evaluation of diagnostic accuracy of exosomes and exosomal lncRNA HIF1A-AS1. Results: The concentration of exosomes was significantly higher in patients with atherosclerosis than that in healthy people, the same as the expression level of exosomal lncRNA HIF1A-AS1. Furthermore, the result of Spearman’s correlation test indicated that there was a positive correlation between them. Last, we also showed that the area values under the ROC curves of exosomes and lncRNA HIF1A-AS1 were 0.856 and 0.823, respectively. Conclusion: Exosomes and exosomal lncRNA HIF1A-AS1 could act as potential biomarkers for atherosclerosis.
Keywords: Exosomal lncRNA, HIF1A-AS1, atherosclerosis, biomarker
Introduction
As the main underlying cause of cardiovascular disease (CVD), atherosclerosis is a chronic inflammatory condition of major blood vessels. Unstable atherosclerosis can lead to thrombus formation which causes serious consequences, including myocardial infarction and stroke [1,2]. Exosomes are extracellular nanovesicles of 30-100 nm in size which are released by many different cell types and can be found in most bodily fluids [3]. Extended evidences have shown that exosomes play an important part in atherosclerosis. Gao et al. [4] demonstrated that exosomes derived from mature dendritic cells could increase endothelial inflammation and atherosclerosis. Min Yin et al. [5] reported that exosomes can act as new pharmacological targets to treat atherosclerosis. However, the difference of exosomes expression between patients with atherosclerosis and healthy people is undecided.
Long noncoding RNA (lncRNA, >200 nucleotides) is an exclusive category of RNAs that do not code for functional polypetides, but has emerged as another important layer of gene regulation [6]. Jing Wang et al. [7] reported that LncRNA HIF 1 alpha-antisense RNA 1 (HIF1A-AS1) silencing could reduce palmitic acid-induced apoptosis and promote proliferation of human vascular endothelial cells (HUVECs), which indicated that lncRNA HIF1A-AS1 might play a vital role in the pathogenesis of cardiovascular disease via endothelial cells lesion. Wang et al. [8] also reported that lncRNA HIF1A-AS1 can regulate proliferation and apoptosis of vascular smooth muscle cells (VSMC). However, the expression level of circulating exosomal lncRNA HIF1A-AS1 in atherosclerosis is unknown.
Therefore, this study aims to investigate the diagnostic value of circulating exosomes and exosomal lncRNA HIF1A-AS1 as potential biomarkers for atherosclerosis.
Materials and methods
Study subjects
A total of 65 blood samples were collected from patients diagnosed with atherosclerosis and 68 blood samples from healthy people. All subjects are from Affiliated Hospital of Yangzhou University aged 45 and older. Subjects were excluded if they were diagnosed with acute coronary syndrome or with recent myocardial infarction or unstable angina, were known or suspected to suffer from infectious diseases, including human immunodeficiency virus or hepatitis B, or were diagnosed with malignant tumor, lung diseases, hepatic or hematologic disorders, or chronic renal failure requiring dialysis. All procedures to collect blood samples were performed according to the recommendations of Ethical Committee of Affiliated Hospital of Yangzhou University and all patients gave written informed consent.
Isolation and purification of exosomes from blood sample
The plasma was collected at 1500 g for 10 min and then underwent a centrifugation at 3000 g for 15 min to eliminate cell debris. Exosomes were extracted from the obtained supernatant according to the PureExo® Exosome Isolation Kit (for serum or plasma, Cat.#: P101). The final exosome pellet was resuspended in 100 ul PBS and stored at -80°C for subsequent studies.
Exosome characterization [9]
TEM
The extracted exosomes were identified by transmission electron microscopy (TEM). In brief, about 30 µL of extracted exosomes was fixed in 1% glutaraldehyde for 10 min, washed, and contrasted in 2% uranylacetate. TEM was used to observe the morphological features of the exosomes.
Western blot analysis
The exosome pellets were dissolved in the protein lysis buffer, and the protein content was determined using a BCA protein assay kit (Thermo Scientific, USA). The proteins were separated on an SDS-PAGE gel before transferring to a PVDF membrane. The membrane was incubated with TSG101 (ab83, Abcam Inc., Cambridge, MA, USA), CD9 (ab92726, Abcam Inc., Cambridge, MA, USA) and CD63 (ab59479, Abcam Inc., Cambridge, MA, USA) primary antibodies at 4°C overnight, followed by incubation with the corresponding secondary antibodies at room temperature for 1 h. The TSG101, CD9 and CD63 expressions were measured using Western blot analyses.
Measurement of exosomes using rapid micro flow cytometer analysis
Before the sample being run by the flow cytometer, the reference ApogeeMix beads were used to assess the performance of Apogee MFC, and to compare the size distribution of the exosomes. The PBS was run as a background control. The isolated exosomes were diluted by PBS to tenfold. Then all the samples were measured by the flow cytometer.
RNA extraction
RNeasy kit (QIAGEN, Valencia, CA, USA) was used to extract RNA from the isolated exosomes. Briefly, 20 μl exosome suspension was mixed with 700 μl QIAzol lysis buffer, and the mixture was processed according to the manufacturer’s standard protocol. The isolated RNA was eluted using 25 ul of RNase-free water.
LncRNA analysis through RT-qPCR
Reverse transcriptase quantitative real-time PCR (RT-qPCR) was performed using the high-throughput BioMark Real-Time PCR system (Fluidigm, South San Francisco, CA) according to the manufacturer’s protocol to determine the quality and abundance of lncRNA. lncRNA was quantified using NanoDrop spectrophotometer (Thermo Fisher Scientific). The PCR reaction included an initial “hot start” for 10 min, followed by 45 cycles of amplification. Each cycle consisted of a denaturation step at 95°C for 10 s, annealing starting at 60°C for 20 s and decreasing by 2°C every two cycles down to 55°C, and amplification at 72°C for 30 s. Relative expression was calculated according to comparative Quantification cycle (Cq) method. The levels of exosomal lncRNA HIF1A-AS1 were normalized using GAPDH, as recommended in other studies [10,11].
Statistical analysis
IBM SPSS Statistics version 18.0 was used for data analysis. Variables were evaluated using the Kolmogorov-Smirnov test to assess normality. Normally distributed data was expressed as the mean ± standard deviation (SD) or as numbers. Spearman’s correlation test was used to analyze correlations between exosomes and lncRNA HIF1A-AS1. Receiver operating characteristic (ROC) curves were used for evaluation of diagnostic accuracy of the concentration of exosomes and the expression of lncRNA HIF1A-AS1. Results were considered to be statistically significant when P<0.05.
Results
Clinical characteristics of the study subjects
In total, 133 subjects were finally recruited. The clinical characteristics of those subjects were presented in Table 1. Among them, there were several variables showing significant difference, including BMI categories, waist, total cholesterol, LDL, and triglycerides.
Table 1.
The clinical characteristics of the study subjects
| Variable | Patients with Atherosclerosis n=65 | Healthy people n=68 | P-Value |
|---|---|---|---|
| Gender (% Male) | 46.2 | 47.1 | >0.05 |
| Age (years) | 55.6±8.3 | 53.4±9.4 | >0.05 |
| BMI categories | 28.7±2.6 | 22.8±2.4 | <0.01 |
| Waist (cm) | 101±19 | 93±15 | <0.01 |
| Systolic BP (mm Hg) | 115±15 | 111±16 | >0.05 |
| Diastolic BP (mm Hg) | 78±9 | 76±10 | >0.05 |
| Diabete mellitus (%) | 5.6 | 4.7 | >0.05 |
| Total cholesterol (mmol/L) | 7.85±1.54 | 4.31±0.93 | <0.01 |
| HDL (mmol/L) | 1.30±0.56 | 1.31±0.15 | >0.05 |
| LDL (mmol/L) | 4.56±0.34 | 2.84±0.21 | <0.05 |
| Triglycerides (mmol/L) | 4.5±1.4 | 1.5±0.6 | <0.01 |
| Cholesterol medication (%) | 92.3 | 0 |
Identification of circulating exosomes
Under the observation of TEM, the exosomes were round-shaped with diameters of 50-120 nm, as shown in Figure 1A. We also detected the presence of exosomal markers TSG101, CD9 and CD63, as shown in Figure 1B.
Figure 1.
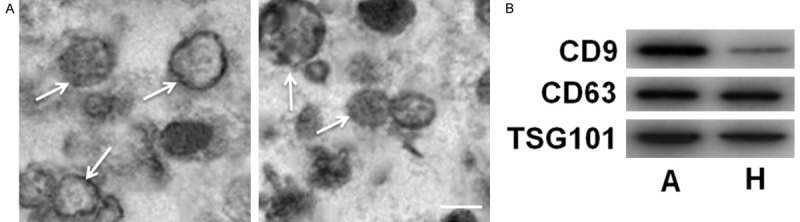
The identification of circulating exosomes. A: The observation of exosomes under electron microscope. B: The detection of exosomal markers CD9, CD63 and TSG101 by Western blot assay. Bar: 100 nm.
Expression of exosomes in plasma
The concentration of exosomes was measured by the following formula: the concentration of exosomes = the total concentration of particles-the concentration of particles in PBS (count/µl).
As shown in Figure 2, we found that the concentration of exosomes was significantly higher in patients with atherosclerosis than in controls.
Figure 2.
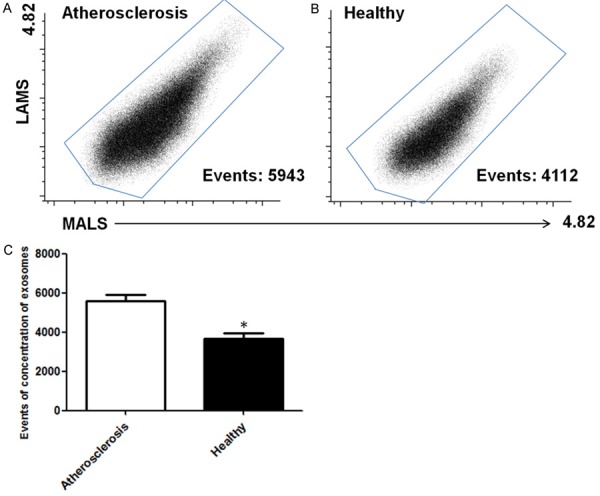
Expression of exosomes in plasma. A and B: The results of flow cytometry in atherosclerosis and healthy group. C: The statistics analysis of the results. *stands for P<0.05.
Expression of exosomal lncRNA HIF1A-AS1 in plasma
As shown in Figure 3, the result of RT-qPCR revealed that there was a significant difference between the expression of lncRNA HIF1A-AS1 between patients with atherosclerosis and healthy subjects.
Figure 3.
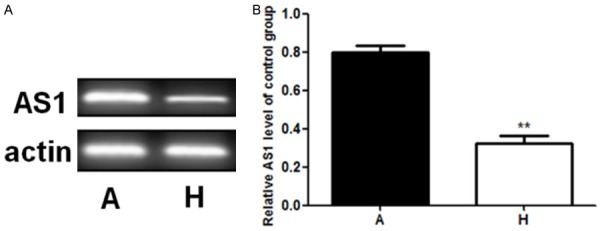
Expression of exosomal lncRNA HIF1A-AS1 in plasma. A. The result of RT-qPCR of AS1. B. Relative AS1 level of control group. **stands for P<0.01.
Correlations between circulating exosomes and exosomal lncRNA HIF1A-AS1 in atherosclerosis
As shown in Figure 4, in order to evaluate the possible relation between circulating exosomes and exosomal lncRNA HIF1A-AS1 in atherosclerosis, we analyzed if there was a correlation between them. It was revealed that the level of expression of exosomal lncRNA HIF1A-AS1 was significantly correlated with circulating exosomes.
Figure 4.
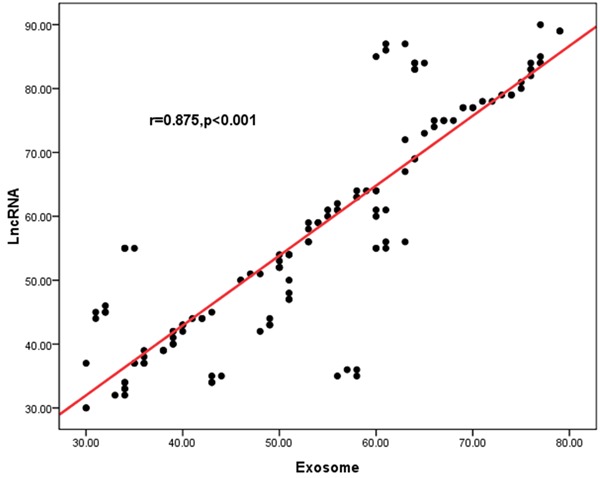
Possible relation between circulating exosomes and exosomal lncRNA HIF1A-AS1 in atherosclerosis. It was revealed that the level of expression of exosomal lncRNA HIF1A-AS1 was significantly correlated with circulating exosomes (r=0.875, P<0.001).
Circulating exosomes and exosomal lncRNA HIF1A-AS1 as potential biomarkers in atherosclerosis
As shown in Figure 5, to further investigate the efficiency of circulating exosomes and exosomal lncRNA HIF1A-AS1 as potential biomarkers of atherosclerosis, ROC curve analysis was performed between patients with atherosclerosis and healthy people. And it was demonstrated that the area value under the receiver operating characteristics curves of exosomes and exosomal lncRNA HIF1A-AS1 were 0. 861 and 0.823, respectively.
Figure 5.
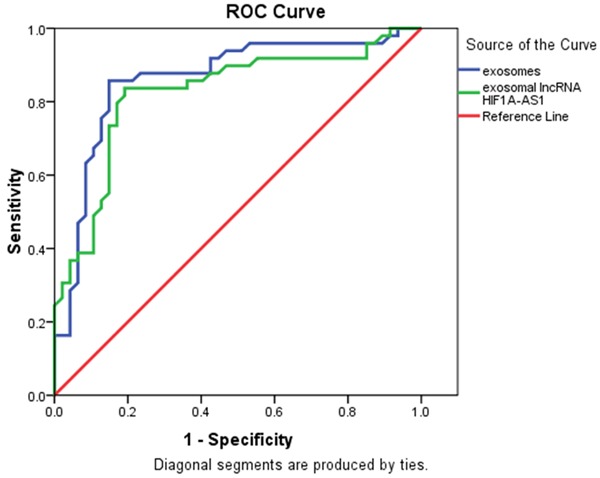
Circulating exosomes and exosomal lncRNA HIF1A-AS1 as potential biomarkers of atherosclerosis, ROC curve analysis was performed between patients with atherosclerosis and healthy people. The area value under the receiver operating characteristics curves of exosomes and exosomal lncRNA HIF1A-AS1 were 0. 861 and 0.823 respectively.
Discussion
In this study, for the first time we found that exosomes from plasma are significantly higher in atherosclerosis than in healthy people. It is well known that exosomes can be secreted by many different cell types. The activation of endothelial cells, migration of vascular smooth muscle cells, monocyte chemotaxis/adhesion are the main events in the processes of atherosclerosis [12]. Perrotta et al. [13] revealed that both lesional smooth muscle cells and endothelial cells are able to secrete exosomes in atherosclerosis, which suggested the possible existence of a new mechanism of intercellular communication in the plaque milieu. Since we found the concentration of plasma exosomes was higher in atherosclerosis, it is logically assumed that those mentioned cells secrete more exosomes in atherosclerosis than they are in normal condition and that the overexpression of exosomes might help with the progress of atherosclerosis. However, further research is needed to decide what mechanism behind this affects the development of atherosclerosis.
Furthermore, we demonstrated that the expression level of exosomal lncRNA HIF1A-AS1 was much higher in atherosclerosis. LncRNA HIF1A-AS1 was shown to regulate the apoptosis and proliferation of endothelial cells [7] and VSMCs [8,14,15] in atherosclerosis and thoracoabdominal aorta aneurysm which usually results from atherosclerosis. This indicated that the overexpression of lncRNA HIF1A-AS1 might relate to those cells. However, the relation between lncRNA HIF1A-AS1 and those cells needs to be further explained. For example, is lncRNA HIF1A-AS1 overexpressed by those cells and then transferred to intracellular environment? In order to answer this question, we performed Spearman’s correlation test between the concentration of circulating exosomes and exosomal lncRNA HIF1A-AS1. Interestingly, we found that there was a positive correlation. As is known to us, exosomes are extracellular vesicles and can contribute to cell-to-cell communication and modulate cellular activities in recipient cells by transfer of their contents [16]. Exosomes contain a variety of biologically active molecules, including proteins, mRNA, miRNA [17] and also lncRNA [16]. Therefore, it is possible that the overexpression of lncRNA HIF1A-AS1 was the result of overexpression of exosomes secreted by endothelial cells and vascular smooth muscle cells.
Last and third, the diagnostic values of plasma exosomes and exosomal lncRNA HIF1A-AS1 were evaluated by ROC curve analysis. We demonstrated that the areas under the curve of plasma exosomes and exosomal lncRNA HIF1A-AS1 were 0.856 and 0.823, respectively. These evidence were valuable because they indicated that circulating exosomes and exosomal lncRNA HIF1A-AS1 could act as potential biomarkers for atherosclerosis.
In conclusion, we showed that the overexpression of circulating exosomes and exosomal lncRNA HIF1A-AS1 could result from the activation of endothelial cells and vascular smooth muscle cells VMSCs. And what’s more important is that they could serve as potential biomarkers for atherosclerosis.
Acknowledgements
This research is supported by The National Key Research and Development Program of China (No.2016YFE0126000) and National Natural Science Foundation of China (No.81570392).
Disclosure of conflict of interest
None.
References
- 1.Srikakulapu P, McNamara C. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol. 2017;312:H1060–H1067. doi: 10.1152/ajpheart.00859.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huber HJ, Holvoet P. Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr Opin Lipidol. 2015;26:412–419. doi: 10.1097/MOL.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation. 2015;131:2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappa B pathway. J Cell Mol Med. 2016;20:2318–2327. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin M, Loyer X, Boulanger CM. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol. 2015;763:90–103. doi: 10.1016/j.ejphar.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 6.Iwakiri J, Hamada M, Asai K. Bioinformatics tools for lncRNA research. Bba-Gene Regul Mech. 2016;1859:23–30. doi: 10.1016/j.bbagrm.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Chen L, Li H, Yang J, Gong Z, Wang B, Zhao X. Clopidogrel reduces apoptosis and promotes proliferation of human vascular endothelial cells induced by palmitic acid via suppression of the long non-coding RNA HIF1AAS1 in vitro. Mol Cell Biochem. 2015;404:203–210. doi: 10.1007/s11010-015-2379-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang SW, Zhang X, Yuan Y, Tan M, Zhang L, Xue X, Yan Y, Han L, Xu Z. BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long noncoding RNA HIF1A-AS1 in vitro. Eur J Cardiothorac Surg. 2015;47:439–446. doi: 10.1093/ejcts/ezu215. [DOI] [PubMed] [Google Scholar]
- 9.Jia HL, Liu CW, Zhang L, Xu WJ, Gao XJ, Bai J, Xu YF, Xu MG, Zhang G. Sets of serum exosomal microRNAs as candidate diagnostic biomarkers for Kawasaki disease. Sci Rep. 2017;7:44706. doi: 10.1038/srep44706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–85563. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isin M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel ÖB, Gezer U, Dalay N. Exosomal IncRNAp21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo HM, Hwang TL, Wu WB. A Phenanthrene derivative, 5,7-Dimethoxy-1,4-Phenanthrenequinone, inhibits cell adhesion molecule expression and migration in vascular endothelial and smooth muscle cells. Pharmacology. 2017;99:291–302. doi: 10.1159/000457802. [DOI] [PubMed] [Google Scholar]
- 13.Perrotta I, Aquila S. Exosomes in human atherosclerosis: an ultrastructural analysis study. Ultrastruct Pathol. 2016;40:101–106. doi: 10.3109/01913123.2016.1154912. [DOI] [PubMed] [Google Scholar]
- 14.Zhang QQ, Xu MY, Qu Y, Hu JJ, Li ZH, Zhang QD, Lu LG. TET3 mediates the activation of human hepatic stellate cells via modulating the expression of long non-coding RNA HIF1A-AS1. Int J Clin Exp Pathol. 2014;7:7744–7751. [PMC free article] [PubMed] [Google Scholar]
- 15.He Q, Tan J, Yu B, Shi W, Liang K. Long noncoding RNA HIF1A-AS1A reduces apoptosis of vascular smooth muscle cells: implications for the pathogenesis of thoracoabdominal aorta aneurysm. Pharmazie. 2015;70:310–315. [PubMed] [Google Scholar]
- 16.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127:1585–1594. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki E, Fujita D, Takahashi M, Oba S, Nishimatsu H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J Stem Cells. 2016;8:297–305. doi: 10.4252/wjsc.v8.i9.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


