Abstract
Objective: This study aimed to investigate the human telomerase reverse transcriptase (hTERT) expression and telomere length in ameloblastoma, and expolre the role of hTERT in the invasiveness and recurrence of ameloblastoma. Methods: hTERT expression was detected by immunohistochemistry and Western blotting and telomere length by fluorescence in situ hybridization (FISH) in human ameloblastoma, normal mucosa, and oral squamous cell carcinoma (OSCC). Association between clincopathological characteristics and hTERT expression as well as telomere length in ameloblastoma was analyzed. Results: hTERT expression in ameloblastoma was higher than that in normal oral mucosa, and the highest in OSCC (P<0.05). The hTERT positive rate and expression increased with the recurrence and malignant transformation. hTERT expression was significantly different among different types of ameloblastoma. The telomere was the shortest in OSCC and the longest in normal oral mucosa. Conclusion: Ameloblastoma has shortened telomere, and is positive for hTERT expression which is related to the recurrence and malignancy of ameloblastoma. These findings indicate that telomerase is involved in the occurrence and development of ameloblastoma.
Keywords: Ameloblastoma, human telomerase reverse transcriptase, telomere
Introduction
A telomere is a region of repetitive nucleotide sequences ([TTAGGG]n) at each end of a chromatid, and has two main functions: (1) it maintains the integrity of chromosome structure, prevents the nuclease induced degradation of chromosomes, and prevent chromosomes from fusion with neighboring chromosomes; (2) it prevents the chromosome from shortening during replications (with loss of genetic information), which resolves the end replication problem [1]. A telomere will be shortened by 50-200 bp in each mitosis, and then the telomere will become dysfunctional with the mitosis. Under this condition, it can not protect the chromosome terminal, leading to the initiation of response to DNA damage, which may induce the senescence and apoptosis of cells. This can avoid carcinogenesis in cells with gene mutation. In cell division, the telomere is abnormally lengthened, leading to the unlimited cell proliferation, which is a cause of carcinogenesis. In some malignancies, the telomere is abnormally lengthened, which is mainly ascribed to the elevated telomerase activity in about 85% of cells and the alternative lengthening of telomeres (ALT) in remaining 15% of cells. In cell division, the excess lengthening of telomere may cause the acquisition of capability of unlimited proliferation. Telomerase can synthesize telomeres via reverse transcription with itself as a template to maintain the telomere length. As a rate limiting subunit in the telomerase catalyzing reaction, human telomerase reverse transcriptase (hTERT) plays a determinant role in the telomerase activity, and can regulate the telomerase activity at transcription level [2]. hTERT expression is up-regulated in germ cells, stem cells with differentiation potential, activated lymphocytes, some specific benign tumors, some precancerous lesions and all the malignancies, but normal tissues or some benign lesions have almost no hTERT expression [3].
Ameloblastoma is a common benign tumor of jaw, and accounts for about 1% of all oral tumors and 11%-18% of odontogenic tumors. The incidence of ameloblastoma follows the keratocystic odontogenic rumor (KCOT) and odontoma [4]. In China, ameloblastoma has an incidence of as high as 36.52%, following the KCOT, but its incidence is higher than that of odontoma [5]. Ameloblastoma is focally invasive, and shows local invasive growth. Some characteristics of ameloblastoma are similar to those of low-grade malignancies. In addition, ameloblastoma has a high post-operative recurrence rate and may become malignant or even metastasize [6]. Surgery is the main treatment for ameloblastoma, but extended excision is needed to acquire safe margin, which, however, brings difficulties to the post-operative repair and improvement of quality of life. Although extended excision has been performed, it still has a risk for recurrence [7,8]. There is evidence showing that the telomerase activity increases in ameloblastoma, which implies that telomerase is related to the special biological behaviors of ameloblastoma [9].
In present study, immunohistochemistry was employed to detect the hTERT expression in ameloblastoma, normal oral mucosa, and oral squamous cell carcinoma (OSCC), and the telomere length was measured by quantitative fluorescence in situ hybridization (Q-FISH), aiming to explore the role of hTERT in the occurrence and development of ameloblastoma, and analyze the change in telomere length in ameloblastoma.
Materials and methods
General data
This study has been approved by the Ethics Committee of Affiliated Stomatological Hospital of China Medical University. A total of 89 patients who were hospitalized in the Affiliated Stomatological Hospital of China Medical University between 2005 and 2012 were recruited. They were pathologically diagnosed with ameloblastoma. In addition, 12 patients with OSCC and 10 healthy subjects were recruited as controls. Informed consent was obtained before study. There were 6 males and 4 females with a median age of 31 years (range: 18-47 years). Pathological assessment was done by two experienced pathologists according to the WHO classification criteria.
Immunohistochemistry for hTERT
Tissues were fixed in 10% formalin, embedded in paraffin and sectioned (3 μm in thickness). These sections were deparaffinized in xylene and then treated with a series of concentrations of ethanol. After incubation with 0.3% H2O2 at room temperature for 30 min, sections received antigen retrieval in citric acid buffer for 10 min. Following blocking in 10% BSA-PBST at room temperature for 1 h, sections were incubated with rabbit anti-human hTERT monoclonal antibody in 1% BSA-PBST (Abcam, 1:100) at 4°C over night. After incubation with biotinylated goat anti-rabbit IgG at room temperature for 2 h, sections were treated with Streptavidin-HRP at room temperature for 1 h. Visualization was done with DAB, followed by counterstaining with hematoxylin and mounting with neutral gum. hTERT positive cells had brown granules in the nucleus and/or cytoplasm. hTERT expression was evaluated by two experienced pathologists. The positive cells were counted on representative photos. The staining intensity (SI) was evaluated and scored as 0 (negative), 1 (weakly positive), 2 (moderately positive) and 3 (strong positive). The labeling indices (LI) was calculated as the proportion of positive cells in 300 counted cells: 0-10%, 0; 11-25%, 1; 26-50%, 2; >50%, 3. The labeling scores (LS) was calculated as the product of LI and SI: 0-1, negative (-); 2, weakly positive (+); 3-4, moderately positive (++); 5-6, strong positive (+++).
Fluorescence in situ hybridization (FISH) to detect the telomere length
In brief, the paraffin-embedded sections were deparaffinized and hydrated (100% ethanol I for 10 min; 100% ethanol II for 10 min; 95% ethanol for 5 min; 75% ethanol for 5 min). Then, sections were washed in PBS thrice, followed by antigen retrieval in citric acid buffer (0.1 M citric acid [9 ml], 0.1 M sodium citrate [41 ml], pH value: 6.0) for 10 min. After digestion in protease K (1:10) at 37°C for 10 min, sections were washed in PBS, and then dehydrated in a series of concentrations of ethanol. Sections were subsequently incubated with (CCCTAA)4-Cy3 (10 μl) in hybridization solution (1:9) at 95°C for 5 min, followed by hybridization in dark at room temperature for 2 h in a humidified box with 50% formamide. Then, sections were washed in 2×SSC at 42°C, followed by counterstaining with DAPI. After washing in methanol for 10 min, mounting was done with glycerin. Sections were observed under a fluorescence microscope (Olympus, CX61, Japan) and representative images were captured. Images were analyzed for the fluorescence intensity with Motic Image Advanced 3.2. Ten cells were randomly selected, and the sum of fluorescence intensity of each Cy-3 positive cells was calculated as the optical density (OD) of the telomeres and the intensity of DAPI as the OD of nuclei. The ratio of telomere OD to DAPI OD was calculated as the telomere length. The mean telomere length was calculated from 10 cells selected.
Statistical analysis
Statistical analysis was performed with SPSS version 13.0. Data from immunohistochemistry were analyzed with chi square test. A value of P<0.05 was considered statistically significant.
Results
Clinical characteristics of ameloblastoma patients
The primary ameloblastoma was found in 51 patients (57.3%) and recurrent ameloblastoma in 33 (37.1%). The interval to recurrence ranged from 4 months to 16 years. Malignant ameloblastoma was found in 5 patients (5.6%). There were 38 males (42.7%) and 51 females (57.3%). The age of ameloblastoma patients ranged from 14 to 72 years old median: 39 years old). Most patients (27%) were 31 to 40 years old. Ameloblastoma was noted in the mandible in 80 patients (89.89%), the maxilla in 7 (7.87%) and the periphery in 2 (gums; 2.24%). According to the WHO classification criteria for odontogenic tumors, solid/multicystic ameloblastoma was noted in 78 patients (87.64%), unicystic ameloblastoma in 7 (7.86%), desmoplastic ameloblastoma in 2 (2.25%) and peripheral ameloblastoma in 2 (2.25%). In addition, in 78 cases of solid multicystic ameloblastoma, follicular ameloblastoma was found in 32 patients (41.03%), plexiform ameloblastoma in 22 (28.21%), acanthomatous ameloblastoma (8.97%), basal cell ameloblastoma (6.41%) and follicular/plexiform mixed ameloblastoma in 12 (15.38%).
Expression and localization of hTERT in ameloblastoma
In 89 cases of ameloblastoma, 66 were positive for hTERT expression. hTERT expression was mainly found in the peripheral columnar and cubic epithelial cells as well as stellate reticulum-like cells. In addition, hTERT expression was observed in both cytoplasm and nucleus (Figure 1).
Figure 1.
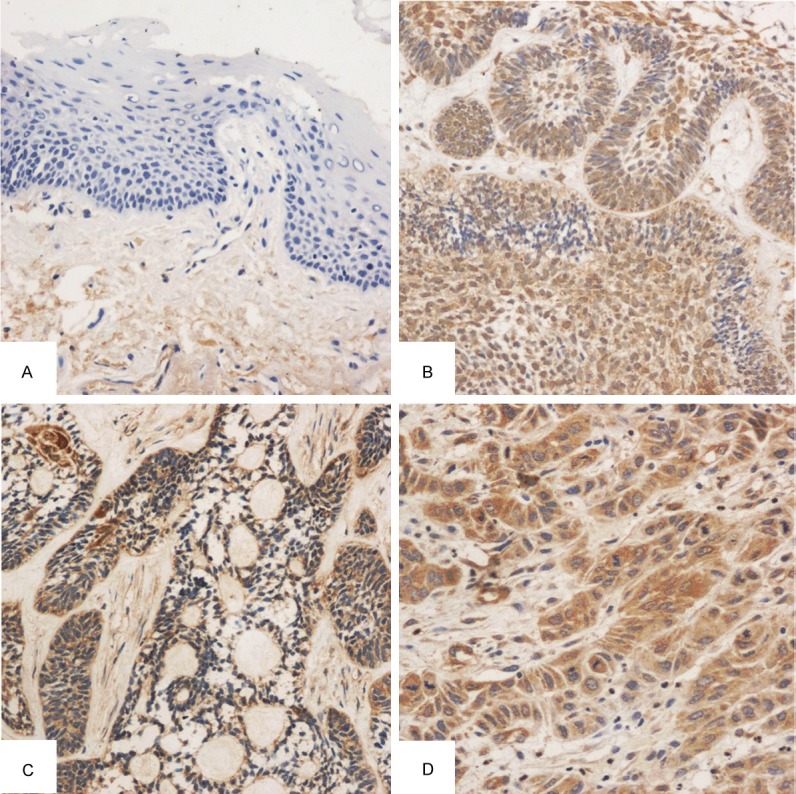
Immunohistochemistry for hTERT (A: NOM negative for hTERT, SP method, ×100; B: Epithelial bud-like hyperplasia in plexiform ameloblastoma and hTERT expression in the cytoplasm and some nuclei of peripheral columnar cells and stellate reticulum-like cells, SP method, ×100; C: hTERT expression in acanthomatous ameloblastoma and low expression in keratinized area, SP method, ×100; D: hTERT expression in the cytoplasm [strong positive] of moderately differentiated OSCC, SP method, ×200).
Positive rate of hTERT was 91.7% in OSCC cells and 74.2% in ameloblastoma cells. In NOM, being weakly positive for hTERT was noted in only 2 patients. The positive rate was significantly different among groups (Table 1). According to the clinically biological behaviors, the hTERT positive rate and expression increased with the recurrence and malignant transformation: 68.6% (35/51) of primary ameloblastoma and 78.8% (26/33) of recurrent ameloblastoma were positive for hTERT, but 100% (5/5) of malignant ameloblastoma was positive for hTERT, showing significant difference among groups (P<0.01; Table 2). hTERT expression was significantly different among different types of ameloblastoma (P<0.05; Table 3).
Table 1.
hTERT expression in ameloblastoma, NOM and OSCC
| n | - | + | ++ | +++ | Positive rate (%) | |
|---|---|---|---|---|---|---|
| NOM | 10 | 8 | 2 | 0 | 0 | 20.0 |
| Ameloblastomas | 89 | 23 | 36 | 22 | 8 | 74.2 |
| OSCC | 12 | 1 | 4 | 2 | 5 | 91.7 |
Table 2.
hTERT expression in primary, recurrent and malignant ameloblastoma
| Group | n | - | + | ++ | +++ | Positive rate (%) |
|---|---|---|---|---|---|---|
| Primary | 51 | 16 | 18 | 15 | 2 | 68.6 |
| Recurrent | 33 | 7 | 18 | 5 | 3 | 78.8 |
| Malignant | 5 | 0 | 0 | 2 | 4 | 100 |
Table 3.
Correlation between hTERT expression and pathological type of ameloblastoma
| Pathological type | n | - | + | ++ | +++ | Positive rate (%) |
|---|---|---|---|---|---|---|
| Plexiform | 22 | 7 | 9 | 4 | 2 | 68.2 |
| Follicular | 32 | 7 | 13 | 9 | 3 | 78.1 |
| Mixed | 12 | 2 | 5 | 3 | 2 | 83.3 |
| Acanthomatous | 7 | 1 | 3 | 3 | 0 | 85.7 |
| Basal cells | 5 | 1 | 1 | 2 | 1 | 80 |
| Unicystic | 7 | 4 | 2 | 1 | 0 | 42.9 |
Note: desmoplastic ameloblastoma in 2 patients and peripheral ameloblastoma in 2 patients were not included in this table.
Telomere length
FISH showed the mean telomere length was 0.1260 in 89 cases of ameloblastoma, 0.0487 in 12 cases of OSCC and 0.2105 in 10 cases of NOM, showing significant difference among groups (F=8.965, P<0.0001) (Figure 2 and Table 4).
Figure 2.

Telomere Cy-3 (A: NOM; B: Ameloblastoma; C: OSCC; ×400).
Table 4.
Telomere length in ameloblastoma, OSCC and NOM
| Group | n | Telomere length |
|---|---|---|
| NOM | 10 | 0.0487±0.0295 |
| Ameloblastoma | 89 | 0.1260±0.0886 |
| OSCC | 12 | 0.2105±0.1334 |
Correlation between telomere length and hTERT expression in ameloblastoma
The telomere length showed an increased tendency with the increase in hTERT expression although significant difference was not observed (F=1.984, P=0.144>0.05) (Table 5).
Table 5.
Correlation between telomere length and hTERT expression in ameloblastoma
| Group | n | Telomere length |
|---|---|---|
| hTERT (-) | 23 | 0.0965±0.0747 |
| hTERT (+) | 36 | 0.1364±0.0749 |
| hTERT (++) | 22 | 0.1401±0.1227 |
| hTERT (+++) | 8 | 0.1487±0.0893 |
In addition, patients were divided into 7 groups according to age. Results showed the telomere length in ameloblastoma had no relationship with the age at diagnosis (F=0.756, P=0.606>0.05). In addition, there was no marked correlation between telomere length and histological type in ameloblastoma (F=1.454, P=0.233>0.05).
Discussion
Early diagnosis before the invasion and metastasis is crucial for the prognosis and good therapeutic efficacy. Studies have shown that telomere and telomerase play important roles in the occurrence and development of malignancies, and determination of difference in the telomerase activity between malignancy and normal tissues is important. Generally, the telomerase expression in malignancies is higher than that in normal tissues, which renders telomerase a reliable biomarker in the early diagnosis of malignancies. A large number of studies confirmed that the telomerase activity increases in head and neck malignancies, including OSCC [10-12]. In oral precancerous lesions such as oral leukoplakia and oral submucosal fibrosis, the hTERT expression also increases [12-14]. Currently, only a few studies reported the hTERT expression in ameloblastoma [15]. Human ameloblastoma is a benign tumor derived from odontogenic epithelium, but shows focally invasive growth, susceptibility to recurrence and rapid proliferation of tumor cells, which are malignant characteristics [16].
Currently, some techniques can be employed to detect the activity and expression of telomerase. In situ hybridization (ISH) is able to specifically detect nucleic acid sequence [17]. ISH was employed to confirm the hTERT mRNA expression in ameloblastoma, and its expression increases with the recurrence and malignant transformation [18,19]. In this study, immunohistochemistry was employed to detect hTERT protein expression in ameloblastoma and results showed human ameloblastoma was positive for hTERT. This may be related to the activate proliferation of ameloblastoma cells which leads to the invasiveness and recurrence. In this study, results showed several NOMs were also positive for hTERT (weakly or moderately positive). This implies that oral mucosal epithelium has rapid turnover and basal cells may compensate the shedding mucosal cells by rapid division and differentiation. Some investigators speculated that oral mucosal epithelium with rapid proliferation had resident stem cells with the potential of persistent proliferation and hTERT is expressed in these stem cells [12,20]. Thus, in normal oral mucosal epithelium, there is low telomerase activity or low hTERT expression. In the present study, histological type had no relationship with hTERT expression. Krishna et al found MDM2 (a invasiveness related factor) expression in desmoplastic ameloblastoma was lower than in solid/polycystic and plexiform ameloblastoma and they speculated that the transformed granulocytes in follicular ameloblastoma had the most potent invasiveness and was susceptible to recurrence [21], but desmoplastic ameloblastoma possessed weak invasiveness. Kumamoto et al [22] found that the expression of Caspase3 and Fas in desmoplastic ameloblastoma was higher than that in solid/polycystic ameloblastoma and also confirmed that different types of ameloblastoma showed distinct capabilities of invasiveness and proliferation. In this study, desmoplastic ameloblastoma was found in only 2 patients, which might bias out results.
A telomere will be shortened by 50-200 kb after each normal cell division, and cell apoptosis will be initiated when the telomere is significantly shortened. In cancer cells, there are mechanisms for the elongation of telomeres which protect cells against senescence and death. Thus, telomere length has been regarded an important biomarker in the development of malignancies. Currently, there is still controversy on the change in telomere length in malignancies. Some studies on precancerous lesions and carcinoma in situ showed the telomere in early phase of carcinogenesis is shorter than in normal tissues [23,24]. Thus, it is assumed that the telomere is aberrantly shortened which may cause the chromosomal instability; subsequently, multiple genes are involved in the carcinogenesis, the telomerase is activated and the telomere is elongated.
The present study for the first time investigated the telomere length in ameloblastoma, and these results showed the telomere length in ameloblastoma was shorter than in normal oral mucosa, which was accompanied by telomerase activation. This may be related to the invasive growth of ameloblastoma. In studies, the correlations of telomere length with age, smoking and other factors are not evaluated; other benign odontogenic tumors may serve as controls, which will be a direction in future studies on ameloblastoma. In head and neck tumors and precancerous lesions, the telomerase activity increases [25,26], but the telomere is shortened in head and neck tumors, which is related to the tumor grade [27]. There is evidence showing that the telomere length in the tumor surrounding normal tissues is related to the prognosis [28]. In future studies, it is necessary to detect the telomere length in tissues and blood, and further evaluate its relationship with the biological characteristics of ameloblastoma.
According to the findings, we speculate that telomere length and telomerase provide references for the differentiation and proliferation of ameloblastoma cells as well as the outcome of ameloblastoma.
Conclusion
The telomere is shortened in ameloblastoma and ameloblastoma is positive for hTERT. In addition, the telomere length and hTERT expression are related to the recurrence and malignant transformation. These findings indicate that telomerase is involved in the occurrence and development of ameloblastoma.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81072197 and 81470758).
Disclosure of conflict of interest
None.
References
- 1.Adams Martin A, Dionne I, Wellinger RJ, Holm C. The function of DNA polymerase alpha at telomeric G tails is important for telomere homeostasis. Mol Cell Biol. 2000;20:786–796. doi: 10.1128/mcb.20.3.786-796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene. 2002;21:643–649. doi: 10.1038/sj.onc.1205070. [DOI] [PubMed] [Google Scholar]
- 4.Jhamb T, Kramer JM. Molecular concepts in the pathogenesis of ameloblastoma: implications for therapeutics. Exp Mol Pathol. 2014;97:345–353. doi: 10.1016/j.yexmp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Luo HY, Li TJ. Odontogenic tumors: a study of 1309 cases in a Chinese population. Oral Oncol. 2009;45:706–711. doi: 10.1016/j.oraloncology.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Gilijamse M, Leemans CR, Winters HA, Schulten EA, van der Waal I. Metastasizing ameloblastoma. Int J Oral Maxillofac Surg. 2007;36:462–464. doi: 10.1016/j.ijom.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Antonoglou GN, Sandor GK. Recurrence rates of intraosseous ameloblastomas of the jaws: a systematic review of conservative versus aggressive treatment approaches and meta-analysis of non-randomized studies. J Craniomaxillofac Surg. 2015;43:149–157. doi: 10.1016/j.jcms.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Nkenke E, Agaimy A, von Wilmowsky C, Eitner S. Mandibular reconstruction using intraoral microvascular anastomosis following removal of an ameloblastoma. J Oral Maxillofac Surg. 2013;71:1983–1992. doi: 10.1016/j.joms.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Cai ZJ. Telomerase reverse transcriptase expression in ameloblastoma of long bone. J Clin Rehab Tissue Engineer Res. 2008;12:512–514. [Google Scholar]
- 10.Chen HH, Yu CH, Wang JT, Liu BY, Wang YP, Sun A, Tsai TC, Chiang CP. Expression of human telomerase reverse transcriptase (hTERT) protein is significantly associated with the progression, recurrence and prognosis of oral squamous cell carcinoma in Taiwan. Oral Oncol. 2007;43:122–129. doi: 10.1016/j.oraloncology.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Freier K, Pungs S, Flechtenmacher C, Bosch FX, Lichter P, Joos S, Hofele C. Frequent high telomerase reverse transcriptase expression in primary oral squamous cell carcinoma. J Oral Pathol Med. 2007;36:267–272. doi: 10.1111/j.1600-0714.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 12.Palani J, Lakshminarayanan V, Kannan R. Immunohistochemical detection of human telomerase reverse transcriptase in oral cancer and pre-cancer. Indian J Dent Res. 2011;22:362. doi: 10.4103/0970-9290.84281. [DOI] [PubMed] [Google Scholar]
- 13.Luzar B, Poljak M, Marin IJ, Eberlinc A, Klopcic U, Gale N. Human telomerase catalytic subunit gene re-expression is an early event in oral carcinogenesis. Histopathology. 2004;45:13–19. doi: 10.1111/j.1365-2559.2004.01892.x. [DOI] [PubMed] [Google Scholar]
- 14.Shimamoto H. [Telomerase activity in oral squamous cell carcinoma and leukoplakia] . Kokubyo Gakkai Zasshi. 2001;68:125–133. doi: 10.5357/koubyou.68.125. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto H, Kinouchi Y, Ooya K. Telomerase activity and telomerase reverse transciptase (TERT) expression in ameloblastomas. J Oral Pathol Med. 2001;30:231–236. doi: 10.1034/j.1600-0714.2001.300407.x. [DOI] [PubMed] [Google Scholar]
- 16.Sathi GA, Inoue M, Harada H, Rodriguez AP, Tamamura R, Tsujigiwa H, Borkosky SS, Gunduz M, Nagatsuka H. Secreted frizzled related protein (sFRP)-2 inhibits bone formation and promotes cell proliferation in ameloblastoma. Oral Oncol. 2009;45:856–860. doi: 10.1016/j.oraloncology.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Mutirangura A, Supiyaphun P, Trirekapan S, Sriuranpong V, Sakuntabhai A, Yenrudi S, Voravud N. Telomerase activity in oral leukoplakia and head and neck squamous cell carcinoma. Cancer Res. 1996;56:3530–3533. [PubMed] [Google Scholar]
- 18.Zhong M, Li ZJ, Wang J, Zhang B, Hou L, Gong YB. [Expression of telomerase activity and c-myc and stimulatory protein 1 in human ameloblastoma] . Hua Xi Kou Qiang Yi Xue Za Zhi. 2004;22:499–502. [PubMed] [Google Scholar]
- 19.Zhong M, Zhang LH, Wang J, Zhang B, Hou L. [Expression of telomerase and p53 in ameloblastoma] . Shanghai Kou Qiang Yi Xue. 2003;12:127–131. [PubMed] [Google Scholar]
- 20.Fujimoto R, Kamata N, Taki M, Yokoyama K, Tomonari M, Nagayama M, Yasumoto S. Gene expression of telomerase related proteins in human normal oral and ectocervical epithelial cells. Oral Oncol. 2003;39:445–452. doi: 10.1016/s1368-8375(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 21.Krishna A, Kaveri H, Naveen Kumar RK, Kumaraswamy KL, Shylaja S, Murthy S. Overexpression of MDM2 protein in ameloblastomas as compared to adenomatoid odontogenic tumor. J Cancer Res Ther. 2012;8:232–237. doi: 10.4103/0973-1482.98976. [DOI] [PubMed] [Google Scholar]
- 22.Kumamoto H, Kimi K, Ooya K. Immunohistochemical analysis of apoptosis-related factors (Fas, Fas ligand, caspase-3 and singlestranded DNA) in ameloblastomas. J Oral Pathol Med. 2001;30:596–602. doi: 10.1034/j.1600-0714.2001.301004.x. [DOI] [PubMed] [Google Scholar]
- 23.Aida J, Kobayashi T, Saku T, Yamaguchi M, Shimomura N, Nakamura K, Ishikawa N, Maruyama S, Cheng J, Poon SS, Sawabe M, Arai T, Takubo K. Short telomeres in an oral precancerous lesion: Q-FISH analysis of leukoplakia. J Oral Pathol Med. 2012;41:372–378. doi: 10.1111/j.1600-0714.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 24.Mu Y, Zhang Q, Mei L, Liu X, Yang W, Yu J. Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol. 2012;29:893–898. doi: 10.1007/s12032-011-9866-3. [DOI] [PubMed] [Google Scholar]
- 25.Patel MM, Parekh LJ, Jha FP, Sainger RN, Patel JB, Patel DD, Shah PM, Patel PS. Clinical usefulness of telomerase activation and telomere length in head and neck cancer. Head Neck. 2002;24:1060–1067. doi: 10.1002/hed.10169. [DOI] [PubMed] [Google Scholar]
- 26.Raghunandan BN, Sanjai K, Kumaraswamy J, Papaiah L, Pandey B, Jyothi BM. Expression of human telomerase reverse transcriptase protein in oral epithelial dysplasia and oral squamous cell carcinoma: an immunohistochemical study. J Oral Maxillofac Pathol. 2016;20:96–101. doi: 10.4103/0973-029X.180953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 28.Boscolo-Rizzo P, Rampazzo E, Perissinotto E, Piano MA, Giunco S, Baboci L, Spinato G, Spinato R, Tirelli G, Da Mosto MC, Del Mistro A, De Rossi A. Telomere shortening in mucosa surrounding the tumor: biosensor of field cancerization and prognostic marker of mucosal failure in head and neck squamous cell carcinoma. Oral Oncol. 2015;51:500–507. doi: 10.1016/j.oraloncology.2015.02.100. [DOI] [PubMed] [Google Scholar]


