Abstract
Background: The death after liver transplantation (LT) was most commonly caused by HCC recurrence. Golgi protein 73 (GP73), a type II Golgi membrane protein, has been proved to be a better serum marker for HCC. Objective: This study aims to clarify the relationship between serum GP73 levels and tumor recurrence as well as survival of HCC patients after LT. Methods: Between November 2003 and July 2008, serum samples from 60 liver transplantation patients and 72 healthy individuals were collected. ELISA and microparticle enzyme immunoassay were used to measure serum GP73 and AFP levels. Patient survival was analyzed using log-rank test along with Kaplan-Meier method. Receiver operating characteristic (ROC) curve was utilized to analyze the diagnostic value of serum GP73 levels. Cox regression was utilized to analyze prognostic factors with multiple variables. Results: Serum GP73 concentrations in HCC patients were much higher than that in healthy controls (P<0.001). Patients with lower serum GP73 levels at LT-6Month had better overall survival and recurrence-free survival than those with higher serum GP73 levels. ROC analyzing results showed that higher serum GP73 levels at 6 month post-LT could significantly predict mortality (P=0.020) as well as HCC recurrence (P=0.001) after liver transplantation. Multivariate analysis revealed that serum GP73 levels at LT-6Month was an independent predictor of good prognosis (P=0.002). Conclusion: Serum GP73 levels could be used to predict tumor recurrence and survival in HCC sufferers after LT.
Keywords: GP73, prognosis, liver transplantation, hepatocellular carcinoma
Introduction
Chronic liver disease remains a major challenge to global public health which could cause approximately 800,000 deaths yearly all around the world [1,2]. Hepatic injury could develop into hepatocirrhosis, hepatocellular carcinoma (HCC) and eventually death. Liver transplantation (LT) has been considered as the only effective solution method for patients with end-stage liver disease [3,4]. Especially for HCC patients, LT could possibly offer the best chance for cure due to the fact that tumors could be cleared and the underlying chronic liver disease could be treated [5]. However, even under stringent selection criteria, the recurring rate of HCC in transplant recipients was 10%-20% and it is a major cause of post-LT death [6-8]. Therefore, there is an urgent need for identifying reliable biomarkers to predict post-LT HCC recurring. At present, alpha-fetoprotein (AFP) has been considered as the main tumor biomarker that is most widely used to manage HCC [9]. Although it has been reported that AFP could not be utilized effectively to screen HCC [10], the value of which for the prediction of post-LT outcome has been demonstrated in several reports [11,12].
Golgi protein 73 (GP73), also named as GOLM1 or GOLPH2, is a 73-kDa type II Golgi membrane protein expressed mostly in epithelial cells of many human tissues [13]. In normal livers, GP73 is consistently present in biliary epithelial cells [14]. Nevertheless, the expression of GP73 was notably increased in numerous liver diseases, especially in HCC cells, which could attributed to the process that GP73 migrates to the plasma membrane and diffusion into the circulation. Therefore, GP73 is considered to be a novel potential biomarker for HCC and several studies have reported that GP73 could be used as a serum marker for HCC [15-17]. However, it is unknown the association between serum GP73 levels and survival in post-LT HCC patients. The present study aimed to investigate the relationship between serum GP73 levels and tumor recurrence as well as survival in liver transplantation patients with HCC.
Materials and methods
Subjects
A total 60 patients with HCC treated with orthotopic liver transplantation between November 2003 and July 2008 in the Liver Transplantation Center, The General Hospital of Chinese People’s Armed Police Forces, were recruited as subjects for the present study. All of the livers for transplantation were obtained from brain death donors. The study was approved by the Ethical Committee of The General Hospital of Chinese People’s Armed Police Forces. Written informed consent was signed by the patients or their family members. The clinical information of patients including sex, age, and serum AFP levels before LT are summarized in Table 1. The follow-up of survival patients included serum AFP levels and computed tomography (CT) or ultrasonography examinations of the abdomen every 6 months. The blood samples were collected from the HCC recipientsat pre-liver transplantation (pre-LT), 3 months after liver transplantation (LT-3Month) and 6 months after liver transplantation (LT-6Month) respectively. 72 healthy individuals enrolled from the Department Physical Examination Center of The General Hospital of Chinese People’s Armed Police Forces were chosen as healthy controls during the same period. All subjects in this control group were documented to have normal liver biochemistry (serum specimens were negative for HBsAg, anti-HIV, anti-TP, and anti-HCV; AFP<10 ng/ml, CEA<4 ng/ml, PSA<4 ng/ml, ALT<40 IU/ml), with no history of liver disease, and no risk factors for viral hepatitis.
Table 1.
Relationship between GP73 expression and clinical information of 60 liver transplantation patients
| Characteristics | N | Patients with low -GP73 (≤150 ng/ml) | Patients with high -GP73 (>150 ng/ml) | P |
|---|---|---|---|---|
| Age | 0.200 | |||
| ≤55 years | 44 | 19 | 25 | |
| >55 years | 16 | 4 | 12 | |
| Gender | 1.000 | |||
| Male | 54 | 21 | 33 | |
| Female | 6 | 2 | 4 | |
| pre-LT serum AFP level | 0.727 | |||
| ≤400 (ng/ml) | 50 | 20 | 30 | |
| >400 (ng/ml) | 10 | 3 | 7 | |
| 3 month post-LT serum GP73 level | 0.561 | |||
| ≤150 (ng/ml) | 43 | 18 | 25 | |
| >150 (ng/ml) | 15 | 5 | 10 | |
| 6 month post-LT serum GP73 level | 0.506 | |||
| ≤150 (ng/ml) | 46 | 19 | 27 | |
| >150 (ng/ml) | 12 | 4 | 8 |
Laboratory testing
10 ml blood sample was drawn from each subject, spun, aliquoted, and serum stored at -80°C until testing. A commercial ELISA kit (Beijing Hotgen Biotech Co., Beijing, China) was utilized to detect serum GP73 levels quantitatively according to the manufacturer’s instructions. All samples were tested at the same time using the same batch of reagents by laboratory technicians with board certificates in a blind manner. A Roche Cobas 6000 Immunoassay System with original kits was used to perform the microparticle enzyme immunoassay for the measurement of serum AFP levels.
Statistical analysis
Statistical analyses were performed by SPSS 17.0 (SPSS, Chicago, IL). The Mann-Whitney U-test was used to determine statistical differences in serum GP73 levels between HCC patients and normal subjects. The chi-square test or Fisher exact test was used to determine the relationship between serum GP73 levels and clinical parameters. Recurrence-free survival (RFS) was defined as the time from the date of liver transplant surgery to the date of tumor recurrence. The Overall survival (OS) time was calculated from the date of surgery to the date of death from any cause or to their last follow-up date. Recurrence-free survival and overall survival were summarized using Kaplan-Meier curves and survival differences were carried out with the log-rank test. Cox-regression analysis was performed to examine the hazard ratio of GP73 and clinical factors in predicting overall and recurrence-free survival of HCC recipients. Multivariate Cox-regression analysis was performed to compare the significant factors in univariate cox-regression analysis. For all the analyses, P values <0.05 were considered to be statistically significant.
Results
General characteristics
Between November 2003 and July 2008, 60 HCC patients were enrolled in this study, including 54 males and 6 females, with the average age of 49 (range from 30 to 78). The clinical information of 60 patients was shown in Table 1. Among these 60 patients, 56 (93.0%) have chronic Hepatitis B virus (HBV) infection and 10 (16.7%) had elevated serum AFP levels (>400 ng/ml). The median follow-up duration was 38 months (range: 5-63 months). At the end of follow-up, 11 of 60 patients had recurrent tumors and 14 were dead. Among 72 healthy controls, the mean age was 41.8 years (range, 20-89 years) and 51.9% were men.
Expression of GP73 in the clinical samples
The median serum GP73 levels in the healthy subjects was 61.0 ng/mL (range: 37.0-92.0), while the median value of GP73 in HCC patients before LT was 118.9 ng/mL (range: 63.5-229.2), which remarkably higher than that of normal subjects (P<0.001). With the aim of avoiding influence of extreme value, HCC patients were divided into two subgroups according to serum GP73 levels (<150 ng/ml, Low; ≥150 ng/ml, High). Then the relationship between serum GP73 concentrations and clinical parameters was analyzed. No statistically significant differences between GP73 expression and clinical parameters were found (Table 1).
Association of GP73 with overall and recurrence-free survival
The overall survival and recurrence-free survival rates were analyzed to evaluate the prognostic significance of serum GP73 levels. By Kaplan-Meier survival curve analysis with log-rank test, we identified that HCC patients with higher serum GP73 levels at LT-6Month had a significantly shorter overall survival (Figure 1A) and recurrence-free survival (Figure 1B). However, there are no significant associations between serum GP73 levels and survival of HCC patients when serum GP73 levels were tested at pre-LT and LT-3Month.
Figure 1.
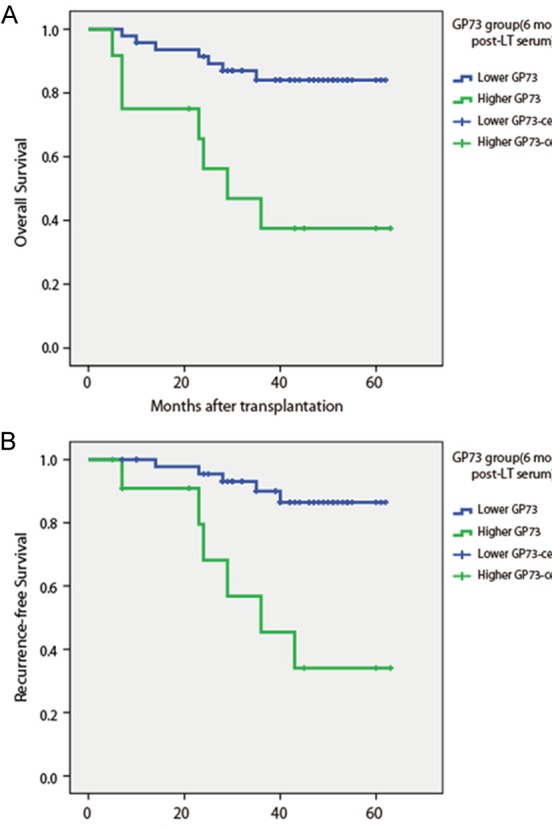
Overall survival (A) and recurrence-free survival (B) in 60 post-LT HCC patients according to serum GP73 levels at 6 month post-LT.
Then, we further examined the prediction value of GP73 (High group versus Low group) for mortality and tumor recurrence by ROC analysis. Results showed that higher serum GP73 levels at 6 month post-LT could significantly predict mortality [AUC =0.708 (95% CI: 0.540-0.875); Sensitivity =64.3%; Specificity =60.5%; P=0.020, Figure 2A] and HCC recurrence [AUC =0.822 (95% CI: 0.694-0.950); Sensitivity =72.7%; Specificity =81.2%; P=0.001, Figure 2B] after liver transplantation, while pre-LT serum GP73 levels and 3 month post-LT serum GP73 levels could not reach statistical significance. Pre-LT serum AFP levels could also predict HCC recurrence [AUC =0.672 (95% CI: 0.480-0.863); Sensitivity =63.6%; Specificity =73.5%; P=0.037, Figure 2C], but could not predict mortality of HCC recipients after liver transplantation (P=0.268).
Figure 2.
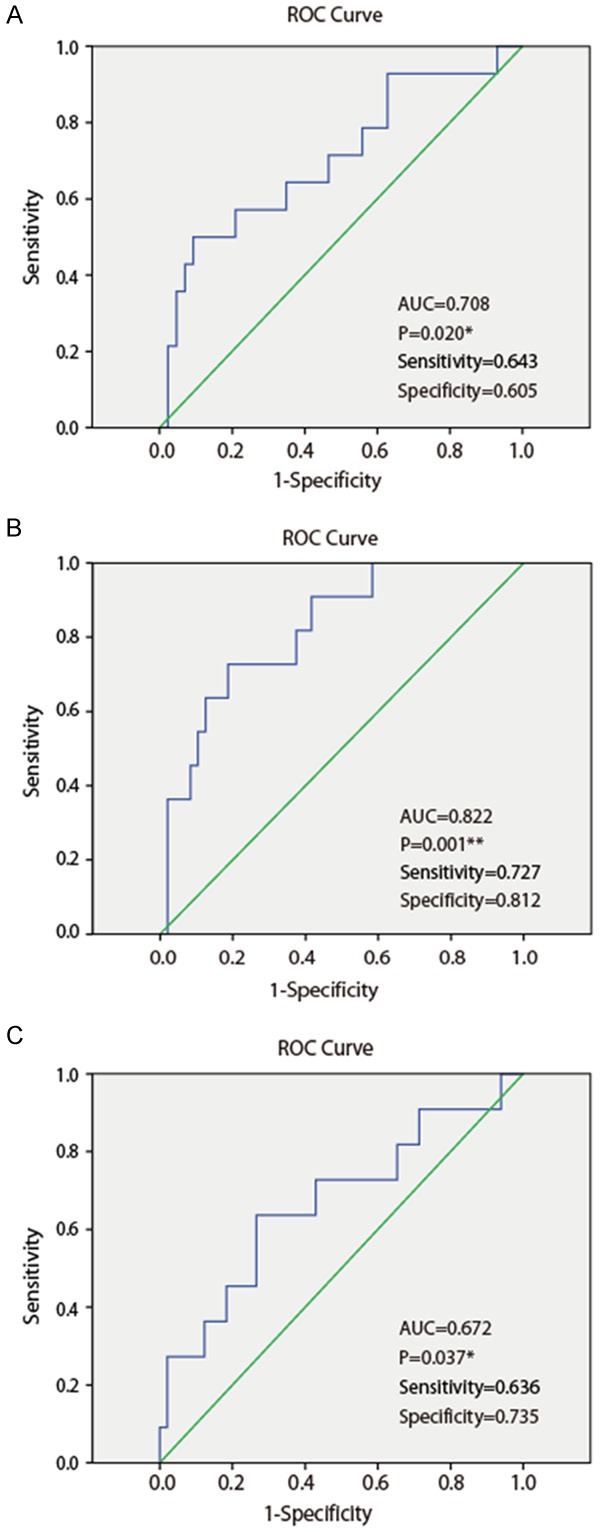
Receiver operating characteristic (ROC) curve of GP73 levels at LT-6Month (A, B) and AFP (C) to predict mortality (A) and HCC recurrence (B, C) in patients undergoing liver transplantation. AUC, area under curve; *, P<0.05; **, P<0.01.
Prognostic factors of patients with HCC undergoing LT
To further determine whether serum GP73 level was an independent prognostic factor, univariate and multivariate analyses were performed. Univariate Cox regression analysis revealed that high level of serum GP73 at LT-6Month was significantly associated with poor overall survival and recurrence-free survival (P=0.002) of HCC recipients after liver transplantation (Tables 2 and 3). The pre-LT serum AFP levels were also significant prognostic factor for overall survival (P=0.025) and recurrence-free survival (P=0.036). Significant prognostic factors in univariate analysis were then analyzed by multivariate analysis by using the Cox proportional hazards model [18]. The results showed that serum GP73 levels at LT-6Month was the only significant variable in predicting both overall survival (HR=5.137, P=0.002) (Table 2) and recurrence-free survival (HR=6.711, P=0.002) (Table 3) of HCC recipients after liver transplantation. Therefore, the above results indicated that serum GP73 levels at LT-6Month was an independent predictor for both recurrence-free survival and overall survival of HCC recipients undergoing LT.
Table 2.
Univariate and multivariate Cox regression analyses of overall survival in 60 HCC patients
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (>55 years) | 0.774 | 0.216~2.777 | 0.694 | |||
| Gender (Female) | 0.819 | 0.107~6.265 | 0.848 | |||
| AFP >400 (ng/ml) | 0.284 | 0.095~0.851 | 0.025 | |||
| pre-LT serum GP73 level >150 (ng/ml) | 0.842 | 0.292~2.430 | 0.751 | |||
| 3 month post-LT serum GP73 Level >150 (ng/ml) | 1.316 | 0.412~4.206 | 0.643 | |||
| 6 month post-LT serum GP73 Level >150 (ng/ml) | 5.137 | 1.794~14.711 | 0.002 | 5.137 | 1.794~14.711 | 0.002 |
Table 3.
Univariate and multivariate Cox regression analyses of recurrence-free survival in 60 HCC patients
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (>55 years) | 1.756 | 0.512~6.020 | 0.370 | |||
| Gender (Female) | 0.044 | 0.000~655.120 | 0.524 | |||
| AFP >400 (ng/ml) | 0.259 | 0.076~0.887 | 0.036 | |||
| pre-LT serum GP73 level >150 (ng/ml) | 3.084 | 0.665~14.293 | 0.150 | |||
| 3 month post-LT serum GP73 level >150 (ng/ml) | 1.887 | 0.550~6.473 | 0.313 | |||
| 6 month post-LT serum GP73 level >150 (ng/ml) | 6.711 | 2.044~22.039 | 0.002 | 6.711 | 2.044~22.039 | 0.002 |
Discussion
Several previous reports have indicated that some factors were closely related to poor prognosis in HCC patients who have underwent LT, such as pre-LT serum AFP levels [19,20], histologic grade, and vascular invasion [21]. In this study, we demonstrated that GP73 was associated with tumor recurrence and survival in HCC sufferers LT. We therein identified, for the first time, that serum GP73 levels at 6 month post-LT could be utilized as a potential prognostic marker to predict HCC recurrence and survival after LT.
GP73 is a type II Golgi-localized integral membrane protein that is normally expressed in epithelial cells of many human tissues [16]. Clinical studies demonstrated that serum GP73 increased in patients with liver disease, especially HCC, and GP73 was a better serum biomarker than AFP for early detection of HCC [22-24]. However, there are no reports about the relationship between serum GP73 levels and HCC patients who underwent LT. Our results indicated that HCC patients with higher serum GP73 levels at LT-6Month exhibited higher tumor recurrence rate and lower survival. The 5-year overall survival and recurrence-free survival rates in post-LT HCC patients with high serum GP73 levels were 41.7% and 49.9%, respectively. However, the 5-year rates among patients with low serum GP73 levels were 80.9% and 85.1%, respectively. All these finding suggested that serum GP73 levels at LT-6Month is an independent predictor of both overall survival and recurrence-free survival of HCC patients undergoing LT. Thus, monitor the serum GP73 levels of liver transplantation recipients could be important for patients’ prognosis, especially for those with normal serum AFP levels.
For the predictive of HCC recurrence, the sensitivity and specificity of GP73 was 72.7% and 81.2%, while those of AFP was only 63.6% and 73.5%, respectively (Figure 2C). Thus, the performance of GP73 as determined by ROC was found to be better than AFP. These data suggested that GP73 may be more advantageous than AFP in predicting HCC recurrence. Pre-LT serum AFP could be used as a prognostic factor to predict tumor recurrence after LT. However, contrary to AFP, the liver transplantation patients have a good prognosis only when the serum GP73 levels were steady low at least 6 months post operation, pre-LT serum GP73 levels and serum GP73 level at LT-3Month could neither predict mortality nor HCC recurrence. Moreover, there are no statistically significant differences between pre-LT serum GP73 levels and serum GP73 levels at LT-6Month. Wang et al [17,25] showed that in a few HCC patients, the GP73 levels were not markedly lower a week after surgical resection, but became lower 1.5-2 years after surgery. But, AFP levels usually decrease substantially within a week postresection. These results demonstrate that serum GP73 levels change slower than serum AFP levels.
The reason by which the serum GP73 levels at LT-6Month were possibly related to the prognosis of liver transplantation is still unrevealed. Chen et al [26] showed that increased expression of GP73 promotes proliferation and migration of HCC cell lines and growth of xenograft tumors in mice. Suppressing GP73 either by small hairpin RNA or antibody results in decreased cell malignant transformation, delayed tumorigenesis of xenografts, prolonged survival of tumor-bearing mice, or reduced metastasis. Thus, enhanced GP73 expression contributes to the development of HCC and post-transplantation hepatic injury may be an important determinant for the survival of HCC recipients. Several studies have revealed that the downregulation of autophagy observed in cancer cells was associated with tumor progression, and autophagy was well documented as a tumor suppression mechanism [27-29], particularly in hepatocellular carcinoma [30,31]. Zhou et al [29] reported that GP73 may play an important role in the inhibitory regulation of autophagy. This finding suggests a new molecular mechanism for GP73-related hepatoma progression. To some extent, this may explain the role of GP73 in HCC metastasis and the prognosis of liver transplant patients. However, the concrete mechanism need to be further studied.
In conclusion, the present study demonstrated that serum GP73 levels at LT-6Month could be a useful preoperative predictor of tumor recurrence and survival following liver transplantation for HCC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31571424), Beijing Novo Program (Z131102000413062), grants from People’s Armed Police Forces (WZ2010016, WZ2011021).
Disclosure of conflict of interest
None.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brundtland GH. From the World Health Organization. Reducing risks to health, promoting healthy life. JAMA. 2002;288:1974. doi: 10.1001/jama.288.16.1974. [DOI] [PubMed] [Google Scholar]
- 3.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American association for the study of liver diseases and the American society of transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. EASL clinical practice guidelines: liver trans-plantation. J Hepatol. 2016;64:433–485. [Google Scholar]
- 5.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Yamashiki N, Sugawara Y, Tamura S, Tateishi R, Yoshida H, Kaneko J, Matsui Y, Togashi J, Akahane M, Makuuchi M, Omata M, Kokudo N. Postoperative surveillance with monthly serum tumor markers after living-donor liver transplantation for hepatocellular carcinoma. Hepatol Res. 2010;40:278–286. doi: 10.1111/j.1872-034X.2009.00591.x. [DOI] [PubMed] [Google Scholar]
- 8.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811–1819. doi: 10.3748/wjg.v19.i11.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717–725. doi: 10.1634/theoncologist.2009-0038. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 13.Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein up-regulated by viral infection. Gene. 2000;249:53–65. doi: 10.1016/S0378-1119(00)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Y, Chen W, Zhao Y, Chen L, Peng T. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Ann Clin Biochem. 2009;46:38–43. doi: 10.1258/acb.2008.008088. [DOI] [PubMed] [Google Scholar]
- 15.Riener MO, Stenner F, Liewen H, Soll C, Breitenstein S, Pestalozzi BC, Samaras P, Probst-Hensch N, Hellerbrand C, Müllhaupt B, Clavien PA, Bahra M, Neuhaus P, Wild P, Fritzsche F, Moch H, Jochum W, Kristiansen G. Golgi phosphoprotein 2 (GOLPH2) expression in liver tumors and its value as a serum marker in hepatocellular carcinomas. Hepatology. 2009;49:1602–1609. doi: 10.1002/hep.22843. [DOI] [PubMed] [Google Scholar]
- 16.Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874–881. [PMC free article] [PubMed] [Google Scholar]
- 17.Gao G, Dong F, Xu X, Hu A, Hu Y. Diagnostic value of serum Golgi protein 73 for HBV-related primary hepatic carcinoma. Int J Clin Exp Pathol. 2015;8:11379–11385. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HY, Han ZB, Fan JW, Xia J, Wu JY, Qiu GQ, Tang HM, Peng ZH. miR-203 expression predicts outcome after liver transplantation for hepatocellular carcinoma in cirrhotic liver. Med Oncol. 2012;29:1859–1865. doi: 10.1007/s12032-011-0031-9. [DOI] [PubMed] [Google Scholar]
- 19.Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129–137. doi: 10.1111/j.1600-6143.2009.02750.x. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 21.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carci-noma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 22.Block TM, Comunale MA, Lowman M, Steel LF, Romano PR, Fimmel C, Tennant BC, London WT, Evans AA, Blumberg BS, Dwek RA, Mattu TS, Mehta AS. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc Natl Acad Sci U S A. 2005;102:779–784. doi: 10.1073/pnas.0408928102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T, Yang Z, Cai J, Li H, Chen J, Zhong S, Mohanti SR, Lopez-Soler R, Millis JM, Huang J, Zhang H. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- 24.Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Long RE, Comunale MA, Junaidi O, Marrero J, Di Bisceglie AM, Block TM, Mehta AS. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:1914–1921. doi: 10.1158/1055-9965.EPI-08-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, Wang Y, Tao J, Shi Y, Gai X, Huang F, Ma Q, Zhou Z, Chen H, Zhang H, Liu Z, Sun Q, Peng H, Chen R, Jing Y, Yang H, Mao Y, Zhang H. mTORC1 up-regulates GP73 to promote proliferation and migration of hepatocellular carcinoma cells and growth of xenograft tumors in mice. Gastroenterology. 2015;149:741–752. doi: 10.1053/j.gastro.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955–963. doi: 10.1093/carcin/bgr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B. Akt-mediated regulation of autophagy and tumorigenesis through beclin 1 phosphorylation. Science. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou YY, Jiang JC, You J, Zhou LF. Effect of Golgi phosphoprotein 2 (GOLPH2/GP73) on autophagy in human hepatocellular carcinoma HepG2 cells. Tumour Biol. 2015;36:3399–3406. doi: 10.1007/s13277-014-2974-x. [DOI] [PubMed] [Google Scholar]
- 30.Ni HM, Williams JA, Yang H, Shi YH, Fan J, Ding WX. Targeting autophagy for the treatment of liver diseases. Pharmacol Res. 2012;66:463–474. doi: 10.1016/j.phrs.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, Shi GM, Wang XY, Ke AW, Wu B, Fan J. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]


