Abstract
Background: Oral cancer overexpressed 1 (ORAOV1) which is a novel candidate oncogene is a useful biomarker of metastasis and prognosis in various cancers. CD133 which is a biomarker of cancer stem cells is overexpressed in many cancers and promotes cancer cells growth and metastasis. WW domain-containing oxidoreductase (WWOX) which is a suppressor gene of tumor can inhibit proliferation and promote apoptosis in various cancers. However, associations among ORAOV1, CD133, and WWOX and their clinicopathological significance in gastric adenocarcionma (GAC) are unclear. In this study, we analyzed associations among ORAOV1, CD133, and WWOX in GAC, and their respective associations with clinicopathological characteristics and survival in GAC. Method: Positive expression of ORAOV1, CD133, and WWOX in 236 whole GAC tissue samples were detected by immunohistochemistry staining. Patients’ clinical data were also collected. Results: Levels of ORAOV1 and CD133 were significantly higher, and levels of WWOX significantly lower, in GAC tissues than in normal gastric tissues. Levels of ORAOV1 and CD133 were positively associated with tumor grade, invasion of depth, lymph node metastasis (LNM), and tumor-node metastasis (TNM) stages, and inversely with patients overall survival time; levels of WWOX was negatively correlated with tumor grade, invasion of depth, LNM, and TNM stages, and the WWOX-positive subgroup had significantly longer overall survival time than did the WWOX-negative subgroup. In multivariate analysis, high expression of ORAOV1 and CD133, invasion of depth, and TNM stages, and low expression of WWOX were potential to be independent prognostic factors for overall survival time in patients with GAC. Conclusions: The expression of ORAOV1, CD133, and WWOX represent promising biomarkers for metastasis and prognosis, and potential therapeutic targets for GAC.
Keywords: Gastric adenocarcinoma, ORAOV1, CD133, WWOX, prognosis
Introduction
In 2012, stomach cancer was reportedly found in 951,600 newly diagnosed cases, caused about 723,100 deaths [1], making it the third most lethal cancer in the worldwide. In China, an estimated 679,100 new stomach cancer cases and 498,000 death occurred in 2015 [2], making it the second most lethal cancer. Gastric adenocarcinoma accounts for about 90% of all diagnosed stomach cancers. Because stomach cancer is usually asymptomatic in its early stages, majority of patients diagnosed with stomach cancer in China have advanced stages disease.
Oral cancer overexpressed 1 (ORAOV1), also named as Tumor Amplified and Overexpressed Sequence 1 (TAOS1), which was originally identified a candidate oncogene in oral squamous cell carcinoma [3]. This gene is located on human chromosome 11q13. Accumulating evidence has demonstrated that ORAOV1 is associated with the cell cycle, apoptosis, angiogenesis, and tumorigenesis [4-6]. Overexpression of ORAOV1 was able to promote tumor cell proliferation, invasion, and metastasis and should be considered as a useful biomarker of metastasis and prognosis [4-9].
Cancer stem cells, which are a subpopulation of cancer cells, are considered as an important role in the initiation, progression, metastasis, and recurrence of cancers, because of their ability of self-renewal, therapy-resistance and high tumorigenicity [10]. CD133, also named as prominin-1, is considered as a biomarker of cancer stem cells in various human cancers [11-16]. CD133 is a 120 kDa five transmembrane domain cell surface glycoprotein that is encoded by the PROM1 gene. CD133 can promote cancer progression by their ability to self-renewal [17], induce promotion of angiogenesis and vasculogenic mimicry, and inhibit cancer cells apoptosis [18,19]. Therefore, CD133 is also considered as a useful biomarker for metastasis and prognosis in various cancers.
WW domain-containing oxidoreductase (WWOX) which was originally identified as a tumor suppressor in breast cancer was located human chromosome 16q23.3-24.1 [20]. WWOX which has 2 N-terminal WW domains and a high homology domain of the short chain dehydrogenase and/or reductase family encodes a 414-amino acid protein [20,21]. WWOX can inhibit tumor initiation, proliferation, and progression [22-24]. It is a common event in almost all cancer types to find WWOX loss which includes hemizygous deletions in majority cases and homozygous deletions [25,26]. Down-regulation of WWOX is associated with tumor progression, metastasis, and occurrence [27,28].
Overall, studies of ORAOV1, CD133, and WWOX in association with tumor metastasis and prognosis showed that these biomarkers affect tumor progression; however, the associations among ORAOV1, CD133, and WWOX in GAC have not yet been widely reported. In this study, we investigated the hypothesis that these biomarkers are mutual associated and correlated with metastasis and prognosis in GAC.
Patients and methods
Patients and tissue specimens
We collected specimens from all 236 patients (median age: 58.1 years; range: 28-78 years) who were treated for GAC at the Department of Pathology of the First Affiliated Hospital of Bengbu Medical College, from January 2010 to December 2011, along with 236 specimens of the corresponding adjacent normal tissues. Patients who had received preoperative anti-cancer therapy were excluded. All tissue samples were obtained with patients writing consent. The study was approved by the Bengbu Medical College’s ethics committee (BBMCEC) and performed in accordance with the guidelines of the Declaration of Helsinki. We collected the completely clinicopathological and follow-up data (at 6-months intervals by mail, e-mail, or phone) of patients. Overall survival (OS) time was calculated from the patients’ surgery date to his or her death date or December 2016 (mean OS: 48.6 months; range: 9-72 months). Tumor-node-metastasis stage was assessed according to the 7th edition of the American Joint Committee on Cancer (AJCC). Tumor was graded according to the World Health Organization (WHO) standards. Specific characteristics see Table 1.
Table 1.
Clinicopathological characteristics of patients with gastric adenocarcinoma
| Patients characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| <60 | 122 | 51.7 |
| ≥60 | 114 | 48.3 |
| Gender | ||
| Male | 159 | 67.4 |
| Female | 77 | 32.6 |
| Size (cm) | ||
| <4.0 | 48 | 20.3 |
| ≥4.0, <8.0 | 157 | 66.5 |
| ≥8.0 | 31 | 13.1 |
| Location | ||
| Antrum | 121 | 51.3 |
| Cardia | 79 | 33.5 |
| Pylorus | 36 | 15.3 |
| Type | ||
| Polypoid | 26 | 11.0 |
| Ulcerative | 158 | 66.9 |
| Infiltrative | 52 | 22.0 |
| Invasion of depth | ||
| Submucosa | 10 | 4.2 |
| Subserosa | 59 | 25.0 |
| Visceral peritoneum | 149 | 63.1 |
| Adjacent structure | 18 | 7.6 |
| Differentiation | ||
| Well | 29 | 12.3 |
| Moderate | 157 | 66.5 |
| Poor | 50 | 21.2 |
| LNM | ||
| N0 | 107 | 45.3 |
| N1 | 90 | 38.1 |
| N2 | 39 | 16.5 |
| TNM stage | ||
| I | 42 | 17.8 |
| II | 155 | 65.7 |
| III A | 39 | 16.5 |
N0: No regional lymph node metastasis; N1: the number of regional lymph node metastasis is no more than 2; N3: the number of regional lymph node metastasis is more than 2.
Immunohistochemistry
Immunohistochemistry was performed in accordance with the guideline of the ElivisionTM Plus detection kit instructions (Lab Vision, USA). Briefly, GAC- and the corresponding normal gastric mucosa tissues were fixed in 10% buffered formalin and embedded in paraffin. Continuous 4 µm thick sections were cut. All GAC and control sections were deparaffinized and dehydrated with xylene and graded ethanol, then washed for 10 min with PBS (pH 7.2). Endogenous peroxidase activity was quenched by incubation of sections in methanol containing 3% hydrogen peroxide for 10 min at room temperature; they were then placed in citrate buffer (pH 6.0) and heated to 95°C for antigen repair. After several washes with PBS, the sections were blocked with goat serum for 20 min at room temperature, then incubated with rabbit polyclonal antibody against human ORAOV1 (Abcam, Cambridge, MA, USA) or mouse monoclonal antibody against human CD133 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit polyclonal antibody against human WWOX (Abcam, Cambridge, MA, USA) for 1 h at 37°C. All sections were counterstained with hematoxylin, dehydrated, air-dried, and mounted. Negative controls were prepared by omitting primary antibodies from the staining procedure. ORAOV1 positive staining was mainly located in nucleus; CD133 positive staining was mainly located in membrane and cytoplasm; and WWOX positive staining was mainly located in the cytoplasm of cancer cells.
Evaluation of staining
Immunostaining results were interpreted semi-quantitatively by two independent pathologists who were blind to clinicopathological and follow-up data. Ten representative fields at high-power-field (HPF) from different areas of per GAC’s slide were analyzed to overcome any intratumoral heterogeneity of antibody expression. Immunostaining results were scored according to intensity (none staining mean 0; weak staining mean 1; moderate staining mean 2; strong staining mean 3) and extent (<11% positive cells = 1; 11-50% positive cells = 2; 51-75% positive cells = 3; >75% positive cells = 4). Then the scores for the intensity and extent were multiplied to yield final scores that ranged 0 from 12. Score ≥3 was considered positive. For sections that were positive for all three of ORAOV1, CD133, and WWOX, an average final score of each section was taken.
Statistical analysis
Correlations between clinicopathological parameters and ORAOV1, CD133, or WWOX were compared using Fisher’s exact test or Chi-square test. Correlations between ORAOV1, CD133, or WWOX were compared using Spearman’s coefficient test. Effects of ORAOV1, CD133, or WWOX on survival were determined by univariate and multivariate analyses. Independent prognostic factors were determined using the Cox regression model for multivariate analysis. The Kaplan-Meier method with log-rank test for univariate OS analysis was used to evaluate correlations between ORAOV1, CD133, or WWOX-positive results and clinicopathological parameters, using SPSS 19.0 software for Windows (Chicago, IL). P<0.05 was defined significant.
Results
Associations between ORAOV1, CD133, and WWOX expression and clinicopathological characteristics
To assess the contributions of ORAOV1, CD133, and WWOX to GAC, the results thereof were immunohistochemically evaluated for both GAC and corresponding normal gastric mucosa tissue specimens. These data were compared to patients’ clinicopathological characteristics. The positive rate of ORAOV1 results in the GAC specimens (58.5%, 138/236) was significantly higher than that in the corresponding normal gastric mucosa tissues (8.5%, 20/236; P<0.001; Figure 1A and 1B). The positive rate of ORAOV1 in GAC was positively related to tumor invasion, grade, LNM, and TNM stages, but not with patients’ age, gender, location, type, or size (Table 2).
Figure 1.
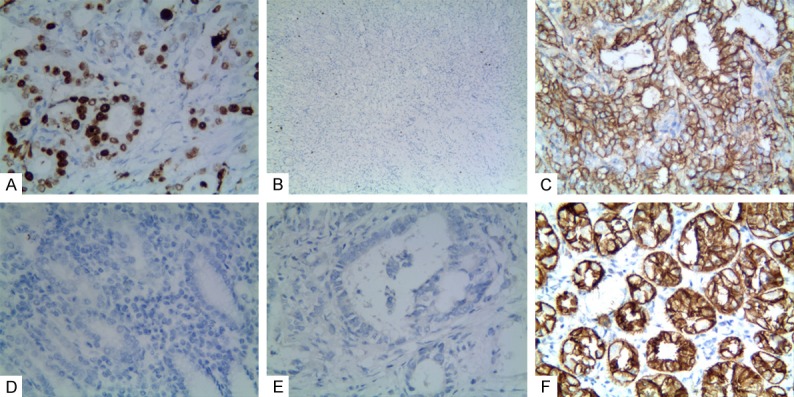
Positive staining of ORAOV1, or CD133, or WWOX in gastric adenocarcinoma or the control tissue. A: Positive staining of ORAOV1 in nuclei of GAC tissue (100 magnification); B: Negative staining of ORAOV1 in the control tissue (40 magnification); C: Positive staining of CD133 in the membrane and cytoplasm of cancer cells (400 magnification); D: Negative staining of CD133 in the control tissue (100 magnification); E: Negative staining of WWOX in the cancer cells (100 magnification); F: Positive staining of WWOX in the cytoplasm of the control tissue (400 magnification).
Table 2.
Associations between expression of ORAOV1, or CD133, or WWOX and clinicopathological characteristics of gastric adenocarcinoma (GAC)
| Variables | ORAOV1 | P | CD133 | P | WWOX | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| - | + | - | + | - | + | ||||
| Age | 0.536 | 0.899 | 0.786 | ||||||
| <60 years | 53 | 69 | 47 | 75 | 77 | 45 | |||
| ≥60 years | 45 | 69 | 43 | 71 | 70 | 44 | |||
| Gender | 0.161 | 0.069 | 0.783 | ||||||
| Male | 71 | 88 | 67 | 92 | 100 | 59 | |||
| Female | 27 | 50 | 23 | 54 | 47 | 30 | |||
| Size (cm) | 0.776 | 0.648 | 0.809 | ||||||
| <4.0 | 18 | 30 | 17 | 31 | 28 | 20 | |||
| ≥4.0, <8.0 | 66 | 91 | 63 | 94 | 99 | 58 | |||
| ≥8.0 | 14 | 17 | 10 | 21 | 20 | 11 | |||
| Location | 0.529 | 0.475 | 0.696 | ||||||
| Antrum | 46 | 75 | 44 | 77 | 74 | 47 | |||
| Cardia | 36 | 43 | 29 | 50 | 52 | 27 | |||
| Pylorus | 16 | 20 | 17 | 19 | 21 | 15 | |||
| Type | 0.613 | 0.655 | 0.184 | ||||||
| Polypoid | 13 | 13 | 12 | 14 | 18 | 8 | |||
| Ulcerative | 65 | 93 | 58 | 100 | 92 | 66 | |||
| Infiltrative | 20 | 32 | 20 | 32 | 37 | 15 | |||
| Invasion of depth | <0.001 | 0.003 | 0.022 | ||||||
| Submucosa | 7 | 3 | 6 | 4 | 6 | 1 | |||
| Subserosa | 34 | 25 | 32 | 27 | 32 | 27 | |||
| Visceral peritoneum | 57 | 92 | 49 | 100 | 92 | 57 | |||
| Adjacent structure | 0 | 18 | 3 | 15 | 17 | 1 | |||
| Grade | 0.020 | 0.025 | <0.001 | ||||||
| Well | 18 | 11 | 17 | 12 | 9 | 20 | |||
| Moderate | 65 | 92 | 59 | 98 | 101 | 56 | |||
| Poor | 15 | 35 | 14 | 36 | 37 | 13 | |||
| LNM | <0.001 | <0.001 | 0.002 | ||||||
| N0 | 49 | 58 | 55 | 52 | 64 | 43 | |||
| N1 | 44 | 46 | 34 | 56 | 49 | 41 | |||
| N2 | 5 | 34 | 1 | 38 | 34 | 5 | |||
| TNM stages | <0.001 | <0.001 | 0.007 | ||||||
| I | 26 | 16 | 26 | 16 | 25 | 17 | |||
| II | 67 | 88 | 63 | 92 | 89 | 66 | |||
| III A | 5 | 34 | 1 | 38 | 33 | 6 | |||
Similar to ORAOV1, CD133+ expression was significantly higher in GAC tissues (61.9%, 146/236) than that in the control tissues (7.6%, 18/236; P<0.001; Figure 1C and 1D). The positive rate of CD133 expression in GAC was related to tumor invasion, grade, LNM, TNM stages, but not to patients’ age, gender, location, type, or size (Table 2).
The positive rate of WWOX expression was significantly less in GAC tissues (37.7%, 89/236) than that in the control tissues (90.3%, 213/236; P<0.001; Figure 1E and 1F). The positive rate of WWOX expression was inversely associated with tumor invasion, grade, LNM, and TNM stages. No correlation was found between WWOX expression and patients’ age, gender, size, location, or type (Table 2).
Correlations among expression of ORAOV1, CD133, and WWOX in GAC
Spearman correlation coefficient analysis demonstrated that negative correlations between WWOX+ expression and that of ORAOV1 (r = -0.214, P = 0.001), or CD133 (r = -0.289, P<0.001). Expression of ORAOV1 was positive correlated with expression of CD133 (r = 0.454, P<0.001; Table 3).
Table 3.
Correlation among expression of ORAOV1, CD133, and WWOX in GAC
| Variable | ORAOV1 | r | P | WWOX | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | - | + | |||||
| ORAOV1 | -0.214 | 0.001* | ||||||
| - | 49 | 49 | ||||||
| + | 98 | 40 | ||||||
| CD133 | 0.454 | <0.001@ | -0.289 | <0.001* | ||||
| - | 63 | 27 | 40 | 50 | ||||
| + | 35 | 111 | 107 | 39 | ||||
Negative association;
Positive association.
Univariate and multivariate analyzes
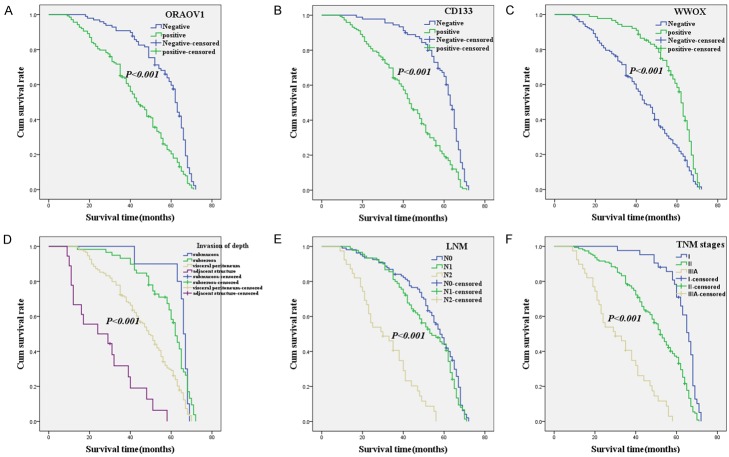
Follow-up data demonstrated that OS was significantly lower in GAC patients with ORAOV1+ specimens (42.6±16.8 months) compared with those with ORAOV1- (57.1±13.0 months; log-rank = 34.402, P<0.001; Figure 2A). Similarly, OS of CD133+ patients (42.2±16.6 months) was significantly lower than those of CD133- patients (59.0±11.4 months; log-rank = 46.895, P<0.001; Figure 2B). The OS of WWOX+ patients (57.3±11.9 months) was significantly longer than those who were WWOX- (43.3±17.3 months; log-rank = 24.276, P<0.001; Figure 2C). In univariate analysis, OS was significantly related to clinicopathological characteristics, including tumor invasion (log-rank = 71.549, P = 0.001, Figure 2D), LNM (log-rank = 82.580, P<0.001, Figure 2E), and TNM stages (log-rank = 93.666, P<0.001, Figure 2F; Table 4).
Figure 2.
Kaplan-Meier analysis of the survival rate of patients with gastric adenocarcinoma. The y-axis represents the percentage of patients; the x-axis, their survival in months. (A) Overall survival of all patients in relation to ORAOV1 (log-rank = 34.402, P<0.001); (B) Overall survival of all patients in relation to CD133 expression (log-rank = 46.895, P<0.001); (C) Overall survival of all patients in relation to WWOX expression (log-rank = 24.276, P<0.001); In (A-C) analyses, the green line represents patients with positive ORAOV1, or CD133, or WWOX and the blue line representing the negative ORAOV1, or CD133, or WWOX group. In (D) analyses, the blue line represents patients with tumor invasion: submucosa group, the green line represents patients invasion: subserosa group, the brown line represents patients’ invasion: visceral peritoneum group; the purple line represents patients invasion: adjacent structure group. In (E) analyses, the blue line represents patients with N0 group, the green line represents patients with N1 group, the brown line represents patients with N2 group. In (F) analyses, the blue line represents patient with I stage group, the green line represents patients with II stage group, the brown line represents patients with III A stage group.
Table 4.
Results of univariate analyses of overall survival (OS) time
| Variable | n | Mean OS (months) | Log-rank | P value |
|---|---|---|---|---|
| ORAOV1 | 34.402 | <0.001 | ||
| Negative | 98 | 57.1±13.0 | ||
| Positive | 138 | 42.6±16.8 | ||
| CD133 | 46.895 | <0.001 | ||
| Negative | 90 | 59.0±11.4 | ||
| Positive | 146 | 42.2±16.6 | ||
| WWOX | 24.276 | <0.001 | ||
| Negative | 147 | 43.3±17.3 | ||
| Positive | 89 | 57.3±11.9 | ||
| Gender | 0.108 | 0.742 | ||
| Male | 159 | 49.6±16.2 | ||
| Female | 77 | 46.5±18.3 | ||
| Ages (years) | 3.650 | 0.056 | ||
| <60 | 122 | 47.9±16.3 | ||
| ≥60 | 114 | 49.4±17.6 | ||
| Type | 0.441 | 0.802 | ||
| Polypoid | 26 | 48.1±14.3 | ||
| Ulcerative | 158 | 48.9±17.1 | ||
| Infiltrative | 52 | 48.0±17.9 | ||
| Location | 1.084 | 0.582 | ||
| Antrum | 121 | 48.2±16.7 | ||
| Cardia | 79 | 47.9±17.9 | ||
| Pylorus | 36 | 51.5±15.4 | ||
| Size (cm) | 1.573 | 0.455 | ||
| D<4.0 | 48 | 46.9±18.8 | ||
| 4.0≤D<8.0 | 157 | 48.8±16.2 | ||
| 8.0≤D | 31 | 50.0±17.7 | ||
| Invasion of depth | 71.549 | <0.001 | ||
| Submucosa | 10 | 64.1±8.0 | ||
| Subserosa | 59 | 57.8±12.2 | ||
| Visceral peritoneum | 149 | 46.5±15.8 | ||
| Adjacent structure | 18 | 26.7±15.6 | ||
| Tumor grade | 0.546 | 0.761 | ||
| Well | 29 | 50.3±17.5 | ||
| Moderate | 157 | 48.5±17.1 | ||
| Poor | 50 | 48.0±16.1 | ||
| LNM | 82.580 | <0.001 | ||
| N0 | 107 | 53.6±15.2 | ||
| N1 | 90 | 30.3±15.0 | ||
| N2 | 39 | 30.8±13.7 | ||
| TNM stage | 93.666 | <0.001 | ||
| I | 42 | 62.6±8.5 | ||
| II | 155 | 49.2±15.4 | ||
| III A | 39 | 31.2±14.3 |
Multivariate analysis indicated that ORAOV1+, CD133+, and WWOX+ samples, tumor invasion, as well as TNM stages, were independent prognostic factors for GAC (Table 5).
Table 5.
Results of multivariate analyses of overall survival (OS) time
| Covariate | B | SE | P | HR | 95% CI |
|---|---|---|---|---|---|
| ORAOV1 | 0.518 | 0.159 | 0.001 | 1.679 | 1.229-2.294 |
| CD133 | 0.492 | 0.170 | 0.004 | 1.635 | 1.173-2.280 |
| WWOX | -0.661 | 0.156 | <0.001 | 0.517 | 0.381-0.701 |
| TNM stage | 0.558 | 0.279 | 0.045 | 1.748 | 1.012-3.020 |
| Invasion of depth | 0.345 | 0.176 | 0.049 | 1.412 | 1.001-1.992 |
Discussion
GAC is a highly heterogeneous disease. This heterogeneity can interfere with the reproducibility of biomarker evaluation. Therefore, prognostic value of candidate biomarker must be thoroughly evaluated to confirm their validity. In this study, we analyzed ORAOV1 protein expression in GAC and matched normal gastric mucosa tissues from 236 patients and compared to clinicopathological characteristics. We found that ORAOV1 expression was significantly higher in GAC tissues than that in the control tissues. Furthermore, it was positively correlated with tumor invasion, grade, LNM, and TNM stages. Our results are similar to the previous studies suggesting that ORAOV1 should be useful as a clinical biomarker for GAC [4,5,7,8,29].
CD133, a cell surface biomarker for human hematopoietic stem cells, is present in various human cancer tissues [10-19]. In GAC, it has been demonstrated that CD133 was associated with progression [30] and shown to predict a poor prognosis to anti-cancer therapy [31]. In this study, we found that CD133 expression was significantly correlated with tumor invasion, grade, LNM, and TNM stages. Moreover, Kaplan-Meier survival analysis showed that GAC patients with positive CD133 expression had significantly reduced survival compared with that of those negative for CD133. The above results indicated that overexpression of CD133 should be involved in the process of GAC cells invasion and metastasis and mean a worse prognosis. Our results are consistent with the previous studies, including those of gastric cancers and other cancers [11-13,15,16,30].
WWOX is widely regarded as a suppressor of tumor in various cancers [20-28]. WWOX can inhibit tumor cells growth, invasion, metastasis, and promote apoptosis [23,32-34]. Results in this study also demonstrated that WWOX expression was significantly less in GAC tissues than that in the control tissues, and its expression was inversely associated with tumor invasion, grade, LNM, and TNM stages. In addition, Kaplan-Meier survival indicated that GAC patients with WWOX+ specimens had significantly higher survival time than did WWOX- patients. These results indicated that reduced-regulation of WWOX should induce GAC progression and metastasis, which are consistent with other studies [23,32-34].
TNM stages provide therapeutic strategies for patients with GAC, but not give exhaustive information about GAC’s biological behavior. Therefore, it is urgent to find novel and effective candidate biomarker to predict GAC’s biological behavior, metastasis, and prognosis of patients. In this study, multivariate analysis indicated that positive expression of ORAOV1, CD133, and WWOX and tumor invasion, as well as TNM stages are independent prognostic factors for GAC patients. The above results suggested that the expression of ORAOV1, CD133, and WWOX should be considered as credible biomarkers for GAC, especially in predicting metastasis and prognosis.
Gastric adenocarcinoma (GAC) is the leading common type of stomach cancer. Abnormal CD133 expression may be involved in the process of tumorigenesis, invasion, metastasis, and recurrence of GAC by its involvement in CSCs [35]. Among other peculiarity, self-renewal and aptitude for multiple differentiation promote GAC cells rapid growth. Meanwhile, Overexpression of ORAOV1 also contributes to tumorigenesis by the promotion of cancer cells proliferation and invasion through activation of a series Cyclins [4-6]. It is also involved in angiogenesis to promote tumor invasion and metastasis [4]. Indeed, the niche where CSCs reside is mainly composed of microvessel or microlymphatic vessels. Therefore, these microvessels support CSCs that further promote cancer cells invasion and metastasis. As reported, WWOX can inhibit CSCs proliferation, promote tumor cells apoptosis, and also involve in the process of epithelial-mesenchymal transition (EMT) [34,36,37]. The down-regulation of WWOX loses inhibiting the activation of proliferation and further promotes tumor cells invasion and metastasis.
Conclusions
Our results indicate that ORAOV1, CD133, and WWOX affects GAC evolution; and the combined detection of ORAOV1, CD133, and WWOX are valuable factors of metastasis and prognosis in GAC’s patients.
Acknowledgements
This work was supported by the Nature Science Foundation of Anhui Province (No. 1708085MH230) and the Nature Science Key Program of College and University of Anhui Province (No. KJ2017A224 and No. KJ2016A488) and Key projects of support program for outstanding young talents in Colleges and Universities of Anhui Province (No. gxyqZD2016160).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Gollin SM, Raja S, Godfrey TE. High-resolution mapping of the 11q13 amplicon and identification of a gene. TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci U S A. 2002;99:11369–74. doi: 10.1073/pnas.172285799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L, Zeng X, Yang H, Wang Z, Shen J, Bai J, Zhang Y, Gao F, Zhou M, Chen Q. Oral cancer overexpressed 1 (ORAOV1): a regulator for the cell growth and tumor angiogenesis in oral squamous cell carcinoma. Int J Cancer. 2008;123:1779–86. doi: 10.1002/ijc.23734. [DOI] [PubMed] [Google Scholar]
- 5.Jiang L, Zeng X, Wang Z, Ji N, Zhou Y, Liu X, Chen Q. Oral cancer overexpressed 1 (ORAOV1) regulates cell cycle and apoptosis in cervical cancer HeLa cells. Mol Cancer. 2010;9:20. doi: 10.1186/1476-4598-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Togashi Y, Arao T, Kato H, Matsumoto K, Terashima M, Hayashi H, de Velasco MA, Fujita Y, Kimura H, Yasuda T, Shiozaki H, Nishio N. Frequent amplification of ORAOV1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ROS production. Oncotarget. 2014;5:2962–73. doi: 10.18632/oncotarget.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu Y, Hibi K, Kodera Y, Akiyama S, Ito K, Nakao A. TAOS1, a novel marker for advanced esophageal squamous cell carcinoma. Anticancer Res. 2006;26:2029–32. [PubMed] [Google Scholar]
- 8.Xia J, Chen Q, Li B, Zeng X. Amplifications of TAOS1 and EMS1 genes in oral carcinogenesis: association with clinicopathological features. Oral Oncol. 2007;43:508–14. doi: 10.1016/j.oraloncology.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lázár V, Ecsedi S, Szöllosi AG, Tóth R, Vízkeleti L, Rákosy Z, Bégány A, Adány R, Balázs M. Characterization of candidate gene copy number alterations in the 11q13 region along with BRAF and NRAS mutations in human melanoma. Mod Pathol. 2009;22:1367–78. doi: 10.1038/modpathol.2009.109. [DOI] [PubMed] [Google Scholar]
- 10.Singh SR. Gastric cancer stem cells: a novel therapeutic target. Cancer Lett. 2013;338:110–9. doi: 10.1016/j.canlet.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang C, Fan C, Wang C, Huang Q, Meng W, Yu Y, Yang L, Peng Z, Hu J, Li Y, Mo X, Zhou Z. CD133+CD54+CD44+ circulating tumor cells as a biomarker of treatment selection and liver metastasis in patients with colorectal cancer. Oncotarget. 2016;7:77389–403. doi: 10.18632/oncotarget.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Wu M, Sun L, Li W, Fu W, Zhang X, Liu T. Clinicopathological and prognostic significance of cancer stem cell markers CD44 and CD133 in patients with gastric cancer. Medicine (Baltimore) 2016;95:e5163. doi: 10.1097/MD.0000000000005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi G, Li YD, Grahovac G, Rajaram V, Wadhwani N, Pundy T, Mania-Farnell B, James CD, Tomita T. Targeting CD133 improves chemotherapeutic efficacy of recurrent pediatric pilocytic astrocytoma following prolonged chemotherapy. Mol Cancer. 2017;16:21. doi: 10.1186/s12943-017-0593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argaw-Denboba A, Balestrieri E, Serafino A, Cipriani C, Bucci I, Sorrentino R, Sciamanna I, Gambacurta A, Sinibaldi-Vallebona P, Matteucci C. HERV-K activation is strictly required to sustain CD133+ melanoma cells with stemness features. J Exp Clin Cancer Res. 2017;36:20. doi: 10.1186/s13046-016-0485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu H, Qi XW, Yan GN, Zhang QB, Xu C, Bian XW. Is CD133 expression a prognostic biomarker of non-small-cell lung cancer? A systematic review and meta-analysis. PLoS One. 2014;9:e100168. doi: 10.1371/journal.pone.0100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sui YP, Jian XP, Ma LI, Xu GZ, Liao HW, Liu YP, Wen HC. Prognostic value of cancer stem cell marker CD133 expression in esophageal carcinoma: a meta-analysis. Mol Clin Oncol. 2016;4:77–82. doi: 10.3892/mco.2015.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–56. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Lai CY, Schwartz BE, Hsu MY. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72:5111–8. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adini A, Adini I, Ghosh K, Benny O, Pravda E, Hu R, Luyindula D, D’Amato RJ. The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis. 2013;16:405–16. doi: 10.1007/s10456-012-9323-8. [DOI] [PubMed] [Google Scholar]
- 20.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3‑24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–5. [PubMed] [Google Scholar]
- 21.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem. 2001;276:3361–70. doi: 10.1074/jbc.M007140200. [DOI] [PubMed] [Google Scholar]
- 22.Yan HC, Xu J, Fang LS, Qiu YY, Lin XM, Huang HX, Han QY. Ectopic expression of the WWOX gene suppresses stemness of human ovarian cancer stem cells. Oncol Lett. 2015;9:1614–20. doi: 10.3892/ol.2015.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Mare S, Salah Z, Ageilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem. 2009;108:737–45. doi: 10.1002/jcb.22298. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Liu J, Ren Y, Yang J, Liu P. Common chromosomal fragile site gene WWOX in metabolic disorders and tumors. Int J Biol Sci. 2014;10:142–8. doi: 10.7150/ijbs.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardenswartz A, Ageilan RI. WW domain-containing oxidoreductase’s role in myriad cancers: clinical significance and future implications. Exp Biol Med (Maywood) 2014;239:253–63. doi: 10.1177/1535370213519213. [DOI] [PubMed] [Google Scholar]
- 26.Baryła I, Styczeń-Binkowska E, Bednarek AK. Alteration of WWOX in human cancer, a clinical view. Exp Biol Med (Maywood) 2015;240:305–14. doi: 10.1177/1535370214561953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng HL, Liu YF, Su CW, Su SC, Chen MK, Yang SF, Lin CW. Functional genetic variant in the Kozak sequence of WW domaincontaining oxidoreductase (WWOX) gene is associated with oral cancer risk. Oncotarget. 2016;7:69384–96. doi: 10.18632/oncotarget.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroni P, Matteucci E, Bendinelli P, Desiderio MA. Functions and epigenetic regulation of Wwox in bone metastasis from breast carcinoma: comparison with primary tumors. Int J Mol Sci. 2017;18:75. doi: 10.3390/ijms18010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JU, Koo SH. ORAOV1 is a probable target within the 11q13.3 amplicon in lymph node metastasis from gastric adenocarcinoma. Int J Mol Med. 2012;29:81–7. doi: 10.3892/ijmm.2011.811. [DOI] [PubMed] [Google Scholar]
- 30.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC Cancer. 2010;10:218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Qian LJ, Su XY, He KF, Jin KT, Gu LH, Feng JG, Li GL, Zhou Q, Xu ZZ, Wang HH, Zhang J, Cao J, Teng LS. Establishment and characterization of GCSR1, a multi-drug resistant signet ring cell gastric cancer cell line. Int J Oncol. 2015;46:2479–87. doi: 10.3892/ijo.2015.2966. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Sun L, Mu Z, Huang Y, Fu C, Hu B. Ectopic WWOX expression inhibit growth of 5637 bladder cancer cell in vitro and in vivo. Cell Biochem Biophy. 2015;73:417–25. doi: 10.1007/s12013-015-0654-0. [DOI] [PubMed] [Google Scholar]
- 33.Zheng QW, Zhou YL, You QJ, Shou F, Pang QF, Chen JL. WWOX inhibits the invasion of lung cancer cells by downregulating RUNX2. Cancer Gene Ther. 2016;23:433–8. doi: 10.1038/cgt.2016.59. [DOI] [PubMed] [Google Scholar]
- 34.Yan H, Tong J, Lin X, Han Q, Huang H. Effect of the WWOX gene on the regulation of the cell cycle and apoptosis in human ovarian cancer stem cells. Mol Med Rep. 2015;12:1783–8. doi: 10.3892/mmr.2015.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Yu L, Zhu B, Wu S, Song W, Gong X, Wang D. Metastasis-associated in colon cancer-1 and aldehyde dehydrogenase 1 are metastatic and prognostic biomarker for non-small cell lung cancer. BMC Cancer. 2016;16:876. doi: 10.1186/s12885-016-2903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan H, Sun Y. Evaluation of the mechanism of epithelial-mesenchymal transition in human ovarian cancer stem cells transfected with a WW domain-containing oxidoreductase gene. Oncol Lett. 2014;8:426–30. doi: 10.3892/ol.2014.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Tan X, Ding Y, Mai B, Huang X, Hu G, Luo X. WWOX CNV-67048 functions as a risk factor for epithelial ovarian cancer in Chinese women by negatively interacting with oral contraceptive use. Biomed Res Int. 2016;2016:6594039. doi: 10.1155/2016/6594039. [DOI] [PMC free article] [PubMed] [Google Scholar]