Abstract
It has been documented that secreted frizzled-related protein 1 (SFRP1) is epigenetically silenced in laryngeal carcinoma. However, the function of SFRP1 in laryngeal carcinoma remains elusive. In this study, we performed gain-of-function studies to determine the roles of SFRP1 in laryngeal carcinoma growth, tumorigenesis, and cisplatin resistance. Laryngeal carcinoma cell lines were treated with 5-aza-2’-deoxycytidine (5-aza-dC) and examined for SFRP1 expression. The effects of overexpression of SFRP1 on cell proliferation, colony formation, apoptosis, tumorigenesis, and cisplatin sensitivity were assessed. It was found that 5-aza-dC exposure significantly induced the expression of SFRP1 in both Hep-2 and SNU899 laryngeal carcinoma cells. Ectopic expression of SFRP1 significantly decreased cell proliferation and colony formation in vitro and retarded xenograft tumor growth in vivo. SFRP1-overexpressing Hep-2 cells displayed a higher percentage of apoptosis and enhancement of caspase-3 cleavage, which was coupled with loss of Δψm and increased release of cytochrome c from the mitochondria to the cytosol. Moreover, SFRP1 overexpression sensitized laryngeal carcinoma cells to cisplatin and decreased intracellular pH values. Mechanistically, SFRP1 inhibited the expression of Na+/H+ exchanger 1 (NHE1) and overexpression of NHE1 reversed the suppressive activity of SFRP1 on laryngeal carcinoma cells. In conclusion, we demonstrate that SFRP1 induces mitochondrial apoptosis and increases cisplatin sensitivity in laryngeal carcinoma cells via downregulation of NHE1. Delivery of SFRP1 may offer therapeutic benefits in the treatment of laryngeal carcinoma.
Keywords: Apoptosis, chemoresistance, growth, laryngeal carcinoma, pH homeostasis
Introduction
Laryngeal carcinoma is one of the most common malignancies of the head and neck [1]. Platinum-based chemotherapy is an important treatment option for advanced laryngeal carcinoma [2,3]. Early recurrence and development of drug resistance are responsible for poor prognosis of patients with laryngeal carcinoma [4,5]. Understanding the mechanisms for laryngeal carcinoma progression is of significance in developing effective therapies for this malignancy.
Drug-induced apoptosis is frequently accompanied by intracellular acidification [6]. It has been suggested that acidosis can trigger apoptosis through cytochrome c-mediated caspase-3 activation [7]. The Na+/H+ exchanger (NHE) family members play a critical in intracellular pH homeostasis [8]. Aberrant expression of NHE1 has been linked to aggressive phenotype in many types of cancers [9,10]. It has been reported that upregulation of NHE1 induces resistance to cytotoxic temozolomide therapy in glioblastoma cells [11]. Therefore, NHE1 represents a potential target for anticancer treatment.
Secreted frizzled-related protein 1 (SFRP1) is a modulator of the Wnt signaling pathway, which forms an inhibitory complex with frizzled receptors [12,13]. SFRP1 downregulation due to epigenetic modification is associated with poor prognosis in gastric cancer [12]. Similarly, frequent hypermethylation of SFRP1 is detected in laryngeal carcinoma tissues [14], suggesting its implication in tumor progression. However, the role of SFRP1 in laryngeal carcinoma is still unclear.
In this study, we performed gain-of-function studies to uncover the roles of SFRP1 in laryngeal carcinoma growth, survival, tumorigenesis, and chemoresistance. The associated molecular mechanism(s) is checked.
Materials and methods
Cell culture and treatment
Human laryngeal carcinoma cell lines (Hep-2, SNU899, TU212, and SNU46) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified incubator with 5% CO2. For demethylation treatment, cells were incubated with 5-aza-2’-deoxycytidine (5-aza-dC; Sigma-Aldrich) at a final concentration of 1 μM for 48 h. Dimethyl sulfoxide (DMSO; Sigma-Aldrich) was used as a vehicle control.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription was performed using a PrimeScript One-Step RT-PCR Kit (TaKaRa, Dalian, China). Real-time PCR was conducted using the following primers [15]: forward, 5’-CTACTGGCCCGAGATGCTTA-3’ and reverse, 5’-GCTGGCACAGAGATGTTCAA-3’ for SFRP1; and forward, 5’-TGGTCACCAGGGCTGCTT-3’ and reverse, 5’-AGCTTCCCGTTCTCAGCCTT-3’ for GAPDH. Cycling conditions were as follows: 5 min at 95°C and 40 cycles of 95°C for 15 sec, 60°C for 20 sec and 72°C for 30 sec. The relative mRNA level of SFRP1 was determined after normalization to that of GAPDH.
Western blot analysis
For preparation of whole-cell lysates, cells were lysed in ice-cold radioimmunoprecipitation lysis buffer (Sigma-Aldrich) containing protease and phosphatase inhibitors (Sigma-Aldrich). Mitochondrial and cytosolic proteins were extracted using the Mitochondria/Cytosol Fractionation Kit following the manufacturer’s instructions (ab65320; Abcam, Cambridge, UK). Protein concentration was quantified using a Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein samples (40 µg per lane) were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The primary antibodies are as follows: anti-SFRP1 (ab126613; Abcam; 1:300 dilution), anti-cleaved caspase-3 (#9661; Cell Signaling Technology, Beverly, MA, USA; 1:300 dilution), anti-cytochrome c (#4272; Cell Signaling Technology; 1:300 dilution), anti-NHE1 (sc-136239; Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:300 dilution), anti-HSP70 (ab2787; Abcam; 1:500 dilution), and anti-β-actin (A5316; Sigma-Aldrich; 1:2000 dilution). Horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology Inc.) was used to detect the primary antibodies. Protein signals were detected using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA, USA) and quantified by Quantity One software version 4.6.2 (Bio-Rad Laboratories, Inc., Richmond, CA, USA).
Plasmids and transfection
Human SFRP1 and NHE1 cDNA was obtained from Origene Technologies (Rockville, MD, USA) and inserted into pcDNA3.1(+) vector. Transfection of the plasmids into Hep-2 and SNU899 cells was achieved using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Forty-eight hours posttransfection, cells were tested for gene expression, viability, and apoptosis. In some experiments, Hep-2 cells were transfected with the SFRP1-expressing plasmid or vector 24 h before exposure to cisplatin (8 µM). For stable expression of SFRP1, cells were transfected with the SFRP1-expressing plasmid or empty vector and then selected for 2 weeks after the addition of 600 µg/ml G418 (Sigma-Aldrich).
MTT assay
Cells were plated in 96-well plates (5 × 103 cells/well) and cultured for 48 or 72 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution (Sigma-Aldrich; 0.5 mg/mL) was added to each well. After incubation for 4 h at 37°C, DMSO was added to the culture. Absorbance was recorded at 570 nm. For the cisplatin toxicity experiment, cells were exposed to different concentrations of cisplatin (0.5, 1, 2, 4, 6, 8, 10, 12, and 14 μM) for 48 h and cell viability was measured using the MTT assay. The 50% inhibitory concentration (IC50) value was determined based on the viability curve.
Colony formation assay
Cells were plated in a 6-well plate (600 cells/well) and cultured at 37°C for 10 days. Colonies were stained with 0.1% crystal violet and counted.
Apoptosis analysis
For apoptosis detection, the Annexin V-FITC Apoptosis Detection Kit (Merk Millipore, Billerica, MA, USA) was used. In brief, cells were washed and incubated with Annexin V-FITC and propidium iodide (PI) in the dark for 15 min. Stained cells were analyzed by flow cytometry (BD Biosciences, Franklin L, NJ, USA).
Animal experiments
Ten male BALB/c nude mice (4-week-old) were used in this experiment. Hep-2 cells stably transfected with the SFRP1-expressing plasmid or vector were inoculated subcutaneously into the hind limb of mice (2 × 106 cells/mouse). Tumor size was measured weekly for 5 weeks. Tumor sections were subjected to immunohistochemistry for Ki-67 using the anti-Ki-67 antibody (ab15580; Abcam; 1:500 dilution). All studies involving animals were approved by the Institutional Animal Care and Use Committee of Second Military Medical University (Shanghai, China).
Measurement of Δψm
Mitochondrial membrane potential (Δψm) was detected using the JC-1 Mitochondrial Membrane Potential Detection Kit (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer’s instructions. In brief, cells were stained with JC-1 at 37°C for 20 min and analyzed by flow cytometry. Loss of Δψm is reflected by the decrease in red to green fluorescence.
Intracellular pH measurement
Measurement of intracellular pH was done as described previously [16]. In brief, cells were loaded for 30 min with the pH-sensitive probe BCECF-AM (1 μM; Calbiochem, San Diego, CA, USA). The cells were rinsed with Ringer’s buffer (140 mM NaCl, 5 mM glucose, 5 mM potassium gluconate, 1 mM calcium gluconate, 1 mM MgSO4, 2.5 mM NaH2PO4, 25 mM NaHCO3, 10 mM HEPES, pH 7.4). The excitation wavelength is 490 nm, and the emission wavelength is 530 nm.
Statistical analysis
The results are expressed as the mean ± standard deviation. Comparisons between groups were performed by the Student’s t test or one-way analysis of variance followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Reexpression of SFRP1 suppresses cell proliferation and tumorigenesis
It has been reported that SFRP1 is epigenetically silenced in human laryngeal carcinoma specimens [14]. To confirm the aberrant methylation of SFRP1 in laryngeal carcinoma cells, we treated a panel of laryngeal carcinoma cell lines using 1 μM of 5-aza-dC for 48 h. qRT-PCR analysis showed that 5-aza-dC treatment markedly raised the abundance of SFRP1 mRNA in laryngeal carcinoma cell lines (Figure 1A). 5-aza-dC-treated Hep-2 and SNU899 cells had 4.2- and 6.9-fold higher levels of SFRP1 transcripts than corresponding controls, respectively. To clarify the biological function of SFRP1, we overexpressed SFRP1 in Hep-2 and SNU899 cells (Figure 1B).
Figure 1.
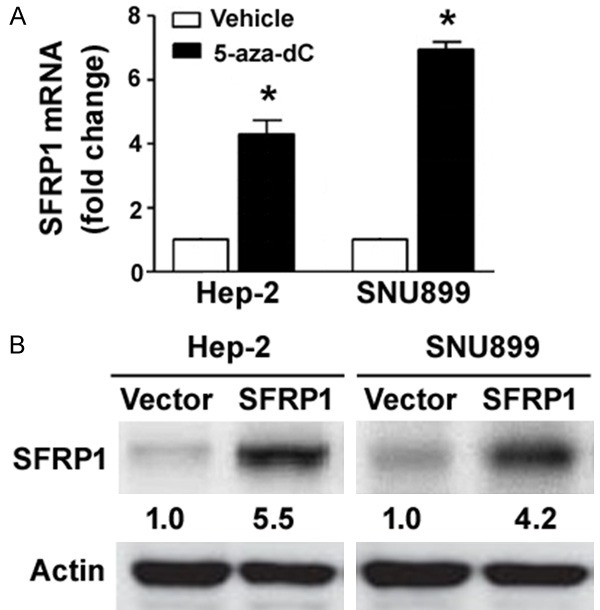
SFRP1 is epigenetically silenced in laryngeal carcinoma cell lines. A. qRT-PCR analysis of SFRP1 mRNA levels in laryngeal carcinoma cell lines treated with 1 μM of 5-aza-dC or DMSO (vehicle control) for 48 h. *P < 0.05 vs. vehicle-treated cells. B. Western blot analysis confirmed the overexpression of SFRP1 in Hep-2 and SNU899 cells transfected with SFRP1-expressing plasmid. Numbers below the Western blots indicate fold-change in SFRP1 protein levels.
MTT assay showed that ectopic expression of SFRP1 in both Hep-2 and SNU899 cells led to a significant reduction of proliferation, compared to vector-transfected control cells (Figure 2A). Colony formation assay further demonstrated that SFRP1-overexpressing Hep-2 and SNU899 cells gave rise to less colonies than control cells 14 days after low-density plating (Figure 2B). To determine the role of SFRP1 in tumorigenesis, we inoculated Hep-2 cells stably expressing SFRP1 and control cells into nude mice and monitored tumor development for 5 weeks. As shown in Figure 2C, reexpression of SFRP1 significantly retarded the growth of Hep-2 xenograft tumors, compared to control counterparts (P < 0.05). Ki-67 immunohistochemistry confirmed that SFRP1 overexpression was associated with a lower percentage of Ki-67-positive tumor cells (Figure 2D).
Figure 2.
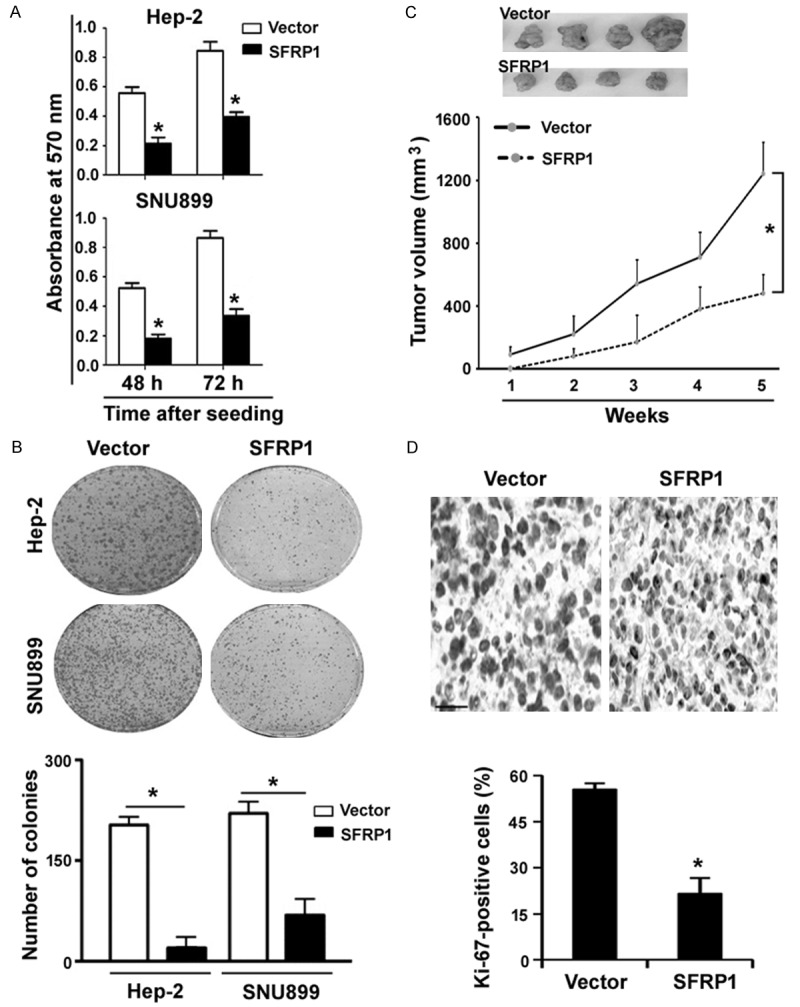
Overexpression of SFRP1 suppresses cell proliferation and tumorigenesis. A. MTT assay was performed to assess the viability of both Hep-2 and SNU899 cells transfected with SFRP1-expressing plasmid or vector after culturing for 48 or 72 h. B. Colony formation assay. Hep-2 and SNU899 cells stably expressing SFRP1 or vector were seeded at a low density and cultured for 10 days to allow to form colonies. C. Tumorigenic studies in nude mice. Hep-2 cells stably expressing SFRP1 and control cells were inoculated into nude mice, and tumor development was monitored for 5 weeks. Reexpression of SFRP1 significantly inhibited the growth of Hep-2 xenograft tumors. D. Tumor sections were subjected to Ki-67 immunohistochemistry. Representative images of Ki-67 staining are shown top panels. Scale bar = 50 μm. *P < 0.05 vs. the vector group.
Enforced expression of SFRP1 promotes apoptosis via the mitochondrial pathway
To understand the mechanism by which SFRP1 restrains laryngeal carcinoma growth, we examined the impact of reexpression of SFRP1 on apoptosis of Hep-2 cells. As determined by annexin-V and PI double staining, SFRP1-overexpressing Hep-2 cells had a higher apoptotic rate than control cells (25.5 ± 3.2% vs. 4.7 ± 1.5%, P < 0.05; Figure 3A). To confirm the induction of apoptosis by SFRP1 overexpression, we measured the level of cleaved caspase-3, an active form of caspase-3, by Western blot analysis. The results showed that there was a 4.4-fold increase in the amount of cleaved caspase-3 in SFRP1-overexpressing Hep-2 cells, compared to control cells (Figure 3B).
Figure 3.
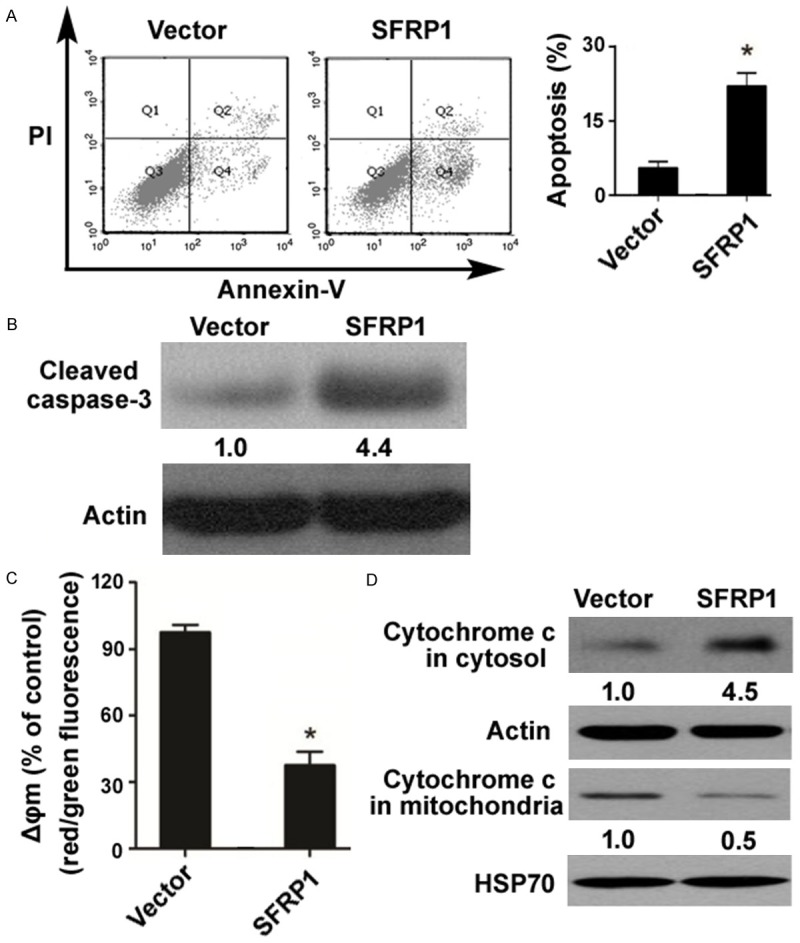
Enforced expression of SFRP1 promotes apoptosis via the mitochondrial pathway. A. Analysis of apoptosis in Hep-2 cells transfected with SFRP1-expressing plasmid or vector by flow cytometry after annexin-V/PI staining. B. Western blot analysis of cleaved caspase-3. Numbers below the Western blots indicate fold-change in cleaved caspase-3 levels. C. Measurement of Δψm changes using the JC-1 assay. Loss of Δψm is reflected by the decrease in red to green fluorescence. D. Western blot analysis of cytochrome c in the mitochondrial and cytosolic fractions. SFRP1 overexpression promoted the release of cytochrome c from the mitochondria to the cytosol. HSP70 and β-actin was used as a loading control for mitochondrial and cytosolic extracts, respectively. *P < 0.05 vs. the vector group.
Next, we asked whether SFRP1-mediated apoptosis involves activation of the mitochondrial pathway. Measurement of Δψm changes using the JC-1 assay revealed that SFRP1 overexpression led to a significant decline in the Δψm (Figure 3C). We also detected the release of cytochrome c from the mitochondria to the cytosol using Western blot analysis. The results showed that the level of cytochrome c in the mitochondrial fraction was 2-fold lower in SFRP1-overexpressing Hep-2 cells than in control cells (Figure 3D). In contrast, there was a 4.5-fold increase in the level of cytochrome c in the cytosolic fraction after SFRP1 overexpression (Figure 3D). These findings suggest that SFRP1 overexpression triggers mitochondrial dysfunction in laryngeal carcinoma cells.
SFRP1 increases the sensitivity to cisplatin and promotes intracellular acidification
Next, we determined the role of SFRP1 in the regulation of cisplatin chemosensitivity. Overexpression of SFRP1 significantly inhibited the viability of Hep-2 and SNU899 cells after cisplatin treatment, relative to vector-transfected cells, decreasing the IC50 value by 3.2- and 8.7-fold, respectively (Figure 4A). We also examined the effect of SFRP1 overexpression on intracellular pH homeostasis in laryngeal carcinoma cells. Compared to vector-transfected Hep-2 cells, SFRP1-overexpressing cells displayed a significant decline in intracellular pH (6.68 ± 0.02 vs. 7.25 ± 0.03, P < 0.05; Figure 4B). Such reduction was enhanced in the presence of cisplatin (8 μM).
Figure 4.
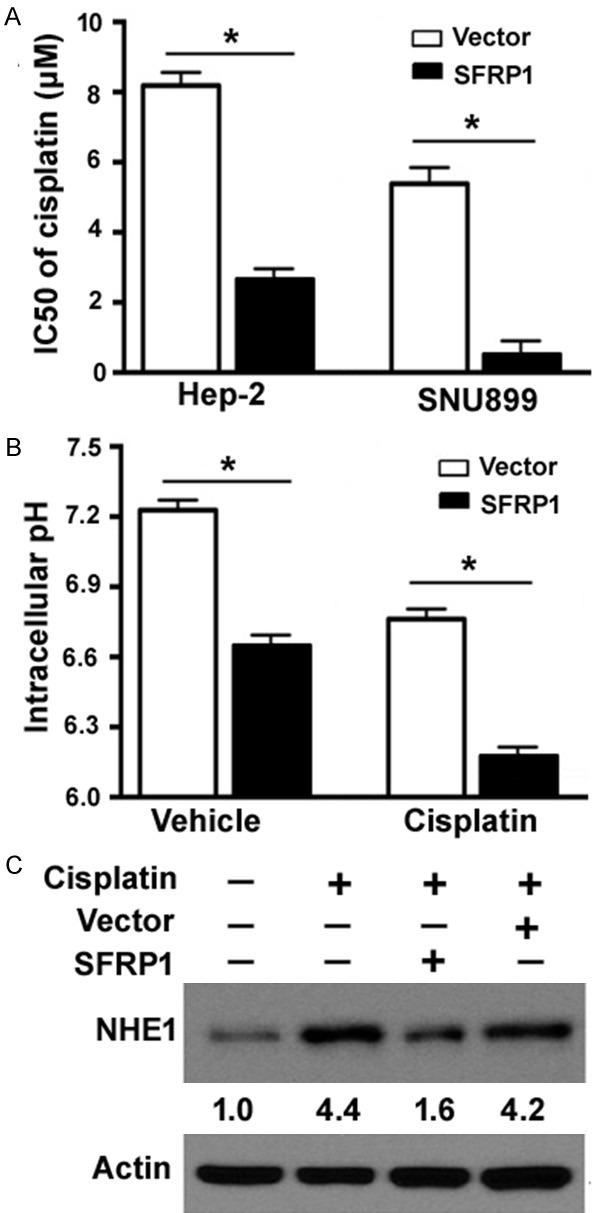
SFRP1 increases the sensitivity to cisplatin and promotes intracellular acidification. A. Hep-2 and SNU899 cells transfected with SFRP1-expressing plasmid or vector were treated with different concentrations of cisplatin (0.5, 1, 2, 4, 6, 8, 10, 12, and 14 μM) for 48 h, and cell viability was measured using the MTT assay. The 50% inhibitory concentration (IC50) value for cisplatin was determined based on the viability curve. *P < 0.05. B. Hep-2 cells transfected with SFRP1-expressing plasmid or vector were treated with or without cisplatin (8 μM) for 48 h and measured for intracellular pH values using the pH-sensitive probe BCECF-AM. *P < 0.05. C. Western blot analysis of NHE1 protein levels. Hep-2 cells were exposed to cisplatin (8 μM) for 48 h with or without transfection with SFRP1-expressing plasmid. Numbers below the Western blots indicate fold-change in NHE1 protein levels.
Overexpression of NHE1 rescues the inhibitory effects of SFRP1
NHE1 plays an important role in the regulation of intracellular pH and prevents an acid overload response [17]. As determined by Western blot analysis, NHE1 was induced after cisplatin treatment (Figure 4C). Delivery of SFRP1 remarkably prevented the induction of NHE1 by cisplatin (8 μM).
To validate the involvement of NHE1 in the action of SFRP1, we co-expressed SFRP1 with NHE1 in Hep-2 cells (Figure 5A). As expected, enforced expression of NHE1 restored intracellular pH to baseline values in SFRP1-overexpressing cells (Figure 5B). Functional studies demonstrated that overexxpression of NHE1 abolished SFRP1-mediated growth suppression (Figure 5C) and apoptosis (Figure 5D) in Hep-2 cells. Taken together, SFRP1-mediated anticancer activity is causally linked to downregulation of NHE1.
Figure 5.
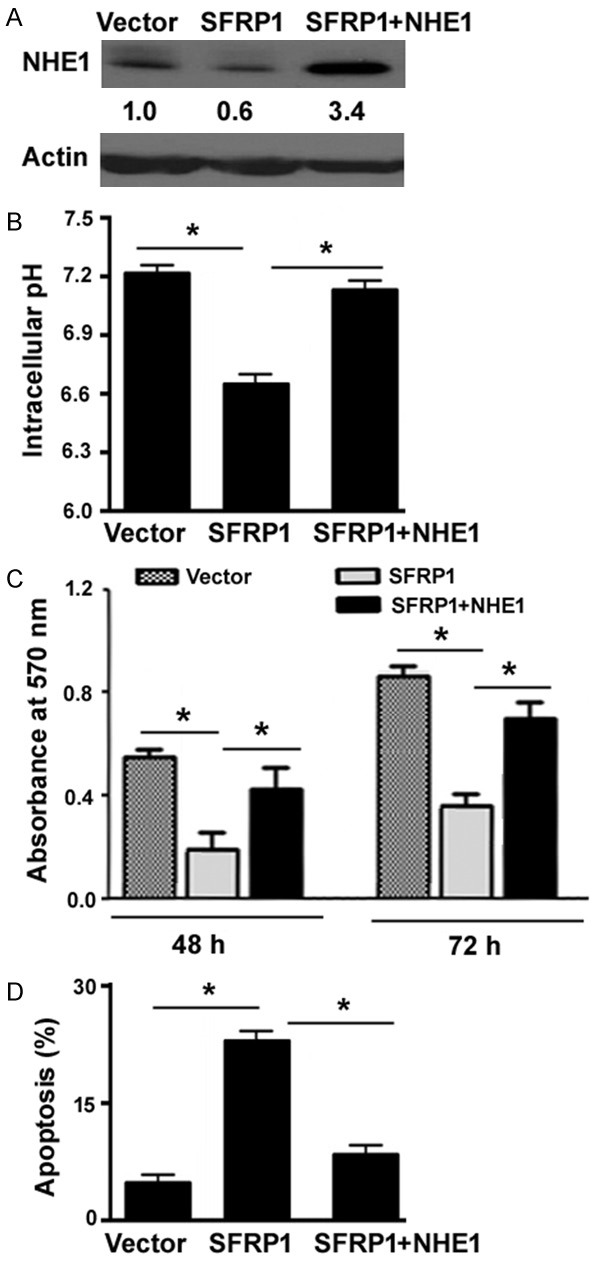
Overexpression of NHE1 rescues the inhibitory effects of SFRP1 on Hep-2 cells. A. Western blot analysis of NHE1 protein levels in Hep-2 cells transfected with the SFRP1-expressing plasmid or together with the NHE1-expressing plasmid. Numbers below the Western blots indicate fold-change in NHE1 protein levels. B. Measurement of intracellular pH values using the pH-sensitive probe BCECF-AM. C. Hep-2 cells transfected with indicated constructs were assessed for proliferation after culturing for 48 or 72 h. D. Analysis of apoptosis in Hep-2 cells transfected with indicated constructs by flow cytometry after annexin-V/PI staining. *P < 0.05.
Discussion
Low expression of SFRP1 is frequently observed in cancers and correlates with poor prognosis [12,18]. Several mechanisms have been suggested to explain the downregulation of SFRP1 in malignant diseases [18,19]. It has been reported that the SFRP1 promoter region is hypermethylated in nasopharyngeal carcinoma and such epigenetic modification is linked to downregulation of SFRP1 [18]. microRNA-27a-mediated repression of SFRP1 is detected in glioma tissues [19]. A previous study has shown that methylation-mediated silencing of SFRP1 frequently occurs in laryngeal carcinoma tissues [14]. Consistently, in this study, we demonstrated that treatment with the demethylating agent significantly induced the expression of SFRP1 in laryngeal carcinoma cell lines. Our data provide additional evidence that SFRP1 is epigenetically silenced in laryngeal carcinoma.
SFRP1 has exhibited growth-suppressive activity in many cancer types such as nasopharyngeal carcinoma [18] and glioma [20]. In agreement with these studies, we found that restoration of SFRP1 significantly suppressed cell proliferation and colony formation of laryngeal carcinoma cells in vitro and tumorigenesis in vivo. Therefore, SFRP1 acts as a tumor suppressor in laryngeal carcinoma. Its downregulation therapeutically contribute to the development and progression of laryngeal carcinoma, although it remains unclear whether it is a central mechanism.
Our data further demonstrated that ectopic expression of SFRP1 significantly facilitated apoptosis in laryngeal carcinoma cells, as determined by annexin-V and PI double staining. Caspase-3 is a key mediator for the execution of apoptosis [21]. Measurement of caspase-3 levels revealed that SFRP1 overexpression enhanced the activation of caspase-3 in laryngeal carcinoma cells. Moreover, we noted that SFRP1 overexpression was associated with loss of Δψm and increased release of cytochrome c from the mitochondria to the cytosol. Cytochrome c is capable of initiating the activation of caspase cascade through interaction with apoptotic protease activating factors [22]. These data suggest that SFRP1-induced apoptosis involves the mitochondrial apoptotic pathway. The pro-apoptotic activity of SFRP1 is also detected in nasopharyngeal carcinoma [18] and hepatocellular carcinoma [23].
Our data also identified a link between SFRP1 expression and cisplatin sensitivity, revealing that SFRP1 overexpression renders laryngeal carcinoma cells more sensitive to cisplatin. Mechanistically, SFRP1 overexpression prevented the induction of NHE1 after exposure to cisplatin. NHE1 is involved in a pH-dependent H+ efflux and thus plays a pivotal role in intracellular pH homeostasis [10]. We found that cisplatin treatment stimulated the expression of NHE1, which accounts for intracellular acidification and apoptosis. Cisplatin-mediated promotion of NHE1 was compromised in the presence of SFRP1. Moreover, overexpression of NHE1 rescued laryngeal carcinoma cells from SFRP1-mediated growth suppression and apoptosis. NHE1 has shown the ability to orchestrate multiple aspects of tumor biology [9,11]. For instance, upregulation of NHE1 promotes gastric cancer cell proliferation, migration and invasion [9]. NHE1 contributes to imatinib resistance in chronic myeloid leukemia cells by inducing heme oxygenase-1 [24]. Downregulation of NHE1 suppresses the aggressive potential of triple-negative breast cancer cells [25]. Consequently, NHE1 is suggested to be a promising anticancer target. Collectively, we provide evidence that inhibition of NHE1 is an important mechanism by which SFRP1 suppresses cell growth and enhances cisplatin sensitivity in laryngeal carcinoma cells. However, the signaling pathway involved in the downregulation of NHE1 by SFRP1 needs to be further clarified.
In conclusion, our data demonstrate that SFRP1 functions as a tumor suppressor in laryngeal carcinoma. Overexpression of SFRP1 evokes mitochondrial apoptotic death and increases the sensitivity to cisplatin in laryngeal carcinoma cells, which is likely ascribed to downregulation of NHE1. These findings raise a rationale for restoration of SFRP1 in the treatment of laryngeal carcinoma.
Acknowledgements
This study was supported by the Natural Science Foundation of Jiangsu Province of China (BK20161388).
Disclosure of conflict of interest
None.
References
- 1.Shuman AG, Larkin K, Thomas D, Palmer FL, Fins JJ, Baxi SS, Lee N, Shah JP, Fagerlin A, Patel SG. Patient reflections on decision making for laryngeal cancer treatment. Otolaryngol Head Neck Surg. 2017;156:299–304. doi: 10.1177/0194599816683377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki H, Hanai N, Nishikawa D, Fukuda Y, Hasegawa Y. Auris Nasus Larynx. Complication and surgical site infection for salvage surgery in head and neck cancer after chemoradiotherapy and bioradiotherapy. Auris Nasus Larynx. 2017;44:596–601. doi: 10.1016/j.anl.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Lian M, Shi Q, Fang J, Feng L, Ma H, Wang H, Zhang L, Wang H, Ma Z, Liu H. In vivo gene expression profiling for chemosensitivity to docetaxel-cisplatin-5-FU (TPF) triplet regimen in laryngeal squamous cell carcinoma and the effect of TPF treatment on related gene expression in vitro. Acta Otolaryngol. 2017;137:765–772. doi: 10.1080/00016489.2016.1272001. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Tang Q, Li S, Yang X. Inhibition of HAX-1 by miR-125a reverses cisplatin resistance in laryngeal cancer stem cells. Oncotarget. 2016;7:86446–86456. doi: 10.18632/oncotarget.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eskiizmir G, Tanyeri Toker G, Celik O, Gunhan K, Tan A, Ellidokuz H. Predictive and prognostic factors for patients with locoregionally advanced laryngeal carcinoma treated with surgical multimodality protocol. Eur Arch Otorhinolaryngol. 2017;274:1701–1711. doi: 10.1007/s00405-016-4411-9. [DOI] [PubMed] [Google Scholar]
- 6.Gdovin MJ, Kadri N, Rios L, Holliday S, Jordan Z. Focal photodynamic intracellular acidification as a cancer therapeutic. Semin Cancer Biol. 2017;43:147–156. doi: 10.1016/j.semcancer.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Kant S, Kumar A, Singh SM. Bicarbonate transport inhibitor SITS modulates pH homeostasis triggering apoptosis of Dalton’s lymphoma: implication of novel molecular mechanisms. Mol Cell Biochem. 2014;397:167–178. doi: 10.1007/s11010-014-2184-2. [DOI] [PubMed] [Google Scholar]
- 8.Hardonnière K, Huc L, Sergent O, Holme JA, Lagadic-Gossmann D. Environmental carcinogenesis and pH homeostasis: not only a matter of dysregulated metabolism. Semin Cancer Biol. 2017;43:49–65. doi: 10.1016/j.semcancer.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Xie R, Wang H, Jin H, Wen G, Tuo B, Xu J. NHE1 is upregulated in gastric cancer and regulates gastric cancer cell proliferation, migration and invasion. Oncol Rep. 2017;37:1451–1460. doi: 10.3892/or.2017.5386. [DOI] [PubMed] [Google Scholar]
- 10.Parks SK, Cormerais Y, Durivault J, Pouyssegur J. Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget. 2017;8:10225–10237. doi: 10.18632/oncotarget.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong D, Zhu W, Shi Y, Pointer KB, Clark PA, Shen H, Kuo JS, Hu S, Sun D. Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H+ extrusion, cell migration and survival. Carcinogenesis. 2014;35:2014–2024. doi: 10.1093/carcin/bgu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Wu Y, Fang Z, Yan Q, Zhang S, Sun R, Khaliq J, Li Y. Low expression of RBMS3 and SFRP1 are associated with poor prognosis in patients with gastric cancer. Am J Cancer Res. 2016;6:2679–2689. [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J, Kang X, Halifu Y, Zeng X, Jin T, Zhang M, Luo D, Ding Y, Zhou Y, Yakeya B, Abudu D, Pu X. Secreted frizzled-related protein promotors are hypermethylated in cutaneous squamous carcinoma compared with normal epidermis. BMC Cancer. 2015;15:641. doi: 10.1186/s12885-015-1650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paluszczak J, Hemmerling D, Kostrzewska-Poczekaj M, Jarmuż-Szymczak M, Grenman R, Wierzbicka M, Baer-Dubowska W. Frequent hypermethylation of WNT pathway genes in laryngeal squamous cell carcinomas. J Oral Pathol Med. 2014;43:652–657. doi: 10.1111/jop.12178. [DOI] [PubMed] [Google Scholar]
- 15.Dahl E, Wiesmann F, Woenckhaus M, Stoehr R, Wild PJ, Veeck J, Knüchel R, Klopocki E, Sauter G, Simon R, Wieland WF, Walter B, Denzinger S, Hartmann A, Hammerschmied CG. Frequent loss of SFRP1 expression in multiple human solid tumours: association with aberrant promoter methylation in renal cell carcinoma. Oncogene. 2007;26:5680–5691. doi: 10.1038/sj.onc.1210345. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Fei HJ, Yang Y, Jiang XS, Yan M, Zeng Z, Wu J, Song LJ, Tian H, Fu GH. Expression of AE1/p16 promoted degradation of AE2 in gastric cancer cells. BMC Cancer. 2016;16:716. doi: 10.1186/s12885-016-2751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh Y, Zhou Y, Shi X, Zhang S, Umbach AT, Salker MS, Lang KS, Lang F. Alkaline cytosolic pH and high sodium hydrogen exchanger 1 (NHE1) activity in Th9 cells. J Biol Chem. 2016;291:23662–23671. doi: 10.1074/jbc.M116.730259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren XY, Zhou GQ, Jiang W, Sun Y, Xu YF, Li YQ, Tang XR, Wen X, He QM, Yang XJ, Liu N, Ma J. Low SFRP1 expression correlates with poor prognosis and promotes cell invasion by activating the Wnt/β-catenin signaling pathway in NPC. Cancer Prev Res (Phila) 2015;8:968–977. doi: 10.1158/1940-6207.CAPR-14-0369. [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Xie D, Xie J, Wan Y, Ma L, Qi X, Yang S. MiR-27a regulates Wnt/beta-catenin signaling through targeting SFRP1 in glioma. Neuroreport. 2015;26:695–702. doi: 10.1097/WNR.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 20.Kierulf-Vieira KS, Sandberg CJ, Grieg Z, Günther CC, Langmoen IA, Vik-Mo EO. Wnt inhibition is dysregulated in gliomas and its re-establishment inhibits proliferation and tumor sphere formation. Exp Cell Res. 2016;340:53–61. doi: 10.1016/j.yexcr.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Savitskaya MA, Onishchenko GE. Mechanisms of apoptosis. Biochemistry (Mosc) 2015;80:1393–1405. doi: 10.1134/S0006297915110012. [DOI] [PubMed] [Google Scholar]
- 22.Lawal AO, Marnewick JL, Ellis EM. Heme oxygenase-1 attenuates cadmium-induced mitochondrial-caspase 3- dependent apoptosis in human hepatoma cell line. BMC Pharmacol Toxicol. 2015;16:41. doi: 10.1186/s40360-015-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang GX, Liu W, Cui YF, Zhong XY, Tai S, Wang ZD, Shi YG, Li CL, Zhao SY. Reconstitution of secreted frizzled-related protein 1 suppresses tumor growth and lung metastasis in an orthotopic model of hepatocellular carcinoma. Dig Dis Sci. 2010;55:2838–2843. doi: 10.1007/s10620-009-1099-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma D, Fang Q, Wang P, Gao R, Wu W, Lu T, Cao L, Hu X, Wang J. Induction of heme oxygenase-1 by Na+-H+ exchanger 1 protein plays a crucial role in imatinib-resistant chronic myeloid leukemia cells. J Biol Chem. 2015;290:12558–12571. doi: 10.1074/jbc.M114.626960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amith SR, Wilkinson JM, Fliegel L. KR-33028, a potent inhibitor of the Na+/H+ exchanger NHE1, suppresses metastatic potential of triple-negative breast cancer cells. Biochem Pharmacol. 2016;118:31–39. doi: 10.1016/j.bcp.2016.08.010. [DOI] [PubMed] [Google Scholar]


