Abstract
DCAF16 is a DDB1-CUL4 associated factor. The expression pattern of DCAF16 in human carcinomas is largely unknown. Here, we investigated DCAF16 expression in a series of human normal epithelial tissues and carcinomas using immunohistochemistry. DCAF16 expression was detected mainly in the cytoplasm in epithelial tissues including thyroid follicles (3/3), epithelium of the prostate glands (2/8), epithelium of the gastric glands (1/2), bronchial epithelium (1/1), epithelium of the intestine (1/1), and hepatocellular epithelium (3/5). There were only 2 cases showing strong immunostaining. Nuclear expression of DCAF16 was detected in a few human carcinomas (0.8%, 9/83). Cytoplasmic DCAF16 expression was detected in human carcinomas including adenocarcinoma (80.0%, 52/65), squamous cell carcinoma (30.8%, 4/13), and urothelial carcinoma (100%, 5/5). The total positive rate of DCAF16 expression was 73.5% (61/83), higher than that in normal tissues (45.8%, 11/24) (P < 0.05). The positive rate of DCAF16 expression in adenocarcinoma (80.0%, 52/65) was higher than that in squamous cell carcinoma (30.8%, 4/13) (P < 0.05). Interestingly, we found that DCAF16 expression in human carcinomas was significantly associated with a higher degree of differentiation (P < 0.05). Our results suggest that DCAF16 is expressed in various human carcinomas including adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma and is not suitable to be used as a diagnostic marker for these cancers. In addition, DCAF16 expression in human carcinomas was elevated compared with that in normal epithelial tissues, suggesting its possible role in oncogenesis. However, the mechanism involved in elevation of DCAF16 expression and its function in human carcinoma needs to be further investigated.
Keywords: DCAF16, adenocarcinoma, squamous cell carcinoma, IHC
Introduction
DCAF16 is a damage-specific DNA binding protein 1 (DDB1)-CUL4 associated factor (DCAF) protein, and its expression and function in human carcinomas is largely unknown. DDB1-CUL4 has a complex function as an E3 ligase in the ubiquitin proteasome pathway [1-4]. DCAF proteins were recently found to be substrate-recognition receptors in the ubiquitin proteasome pathway mediated by DDB1-CUL4 [1-4]. To date, around 60 DCAF proteins have been identified [1-4]. However, the function of these proteins in human tissues, including carcinomas, remains largely unknown. Because the CUL4-DDB1 complex has been proven to play an important role in regulating various physiological processes including proliferation and cell survival, it is important to determine the roles of DCAF proteins in these processes. DCAF proteins have also been found to play important roles in developmental processes [2]. Because there is a great deal of similarity between the oncogenic process of malignant tumors and embryonic development, it is also important to know whether DCAF proteins are expressed in human carcinomas and how they function during carcinogenesis. Here, we investigated DCAF16 expression in a series of human normal epithelial and carcinoma tissues as well as their pathological and diagnostic implications.
Materials and methods
Tissue samples
The specimens were obtained from patients between 2012 and 2016 following surgical resection at the First Affiliated Hospital of China Medical University. The diagnoses were confirmed following the criteria for classification of breast cancer by the World Health Organization. The histological types are listed in Table 1. There were 24 cases of normal epithelial tissues and 83 cases of human carcinomas, including 65 cases of adenocarcinoma, 13 cases of squamous cell carcinoma, and 5 cases of urothelial carcinoma. This study was approved by the institutional Ethics Committee of China Medical University.
Table 1.
Histological type and case number of the samples
| Histological type of the samples | Number |
|---|---|
| Normal tissues | 24 |
| Epithelium of the intestine | 1 |
| Thyroid follicles | 3 |
| Mucoid epithelium of uterine cervix | 2 |
| Epithelium of the prostate glands | 8 |
| Epithelium of the breast duct | 2 |
| Epithelium of the gastric glands | 2 |
| Hepatocellular epithelium | 5 |
| Bronchial epithelium | 1 |
| Carcinoma | |
| Adenocarcinoma | 65 |
| Intrahepatic cholangiocarcinoma | 6 |
| Thyroid papillary carcinoma | 3 |
| Gastric adenocarcinoma | 5 |
| Hepatocellular carcinoma | 4 |
| Adenocarcinoma of the intestine | 5 |
| Endometrioid adenocarcinoma of the uterine | 4 |
| Adenocarcinoma of the lung | 8 |
| Acinar adenocarcinoma of the prostate | 18 |
| Invasive ductal carcinoma of the breast | 3 |
| Serous adenocarcinoma of the ovary | 9 |
| Squamous cell carcinoma | 13 |
| Esophagus | 2 |
| Uterine cervix | 3 |
| Lung | 8 |
| Urothelial carcinoma | 5 |
Immunohistochemical staining
Immunohistochemical staining was performed using UltraSensitive™ S-P kits (Maixin Biotechnology, Fuzhou, Fujian, China) as described previously [5]. The sections were incubated with DCAF16 rabbit polyclonal primary antibody (1:200, Abcam) overnight at 4°C. Immunohistochemical evaluation was performed as described previously [5]. A final score of 2 or more than 2 was defined as positive.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Pearson’s chi-square test was used to analyze the association between DCAF16 expression and pathological factors. McNemar’s test was used to compare DCAF16 expression between carcinoma and non-tumor tissues. P values < 0.05 were considered statistically significant.
Results
Expression of DCAF16 in normal epithelial tissues
DCAF16 expression in normal tissues is shown in Figure 1. The immunostaining is mainly detected in the cytoplasm. The total positive rate of DCAF16 expression in normal tissues was 45.8% (11/24). However, there were only 2 cases showing strong immunostaining. The details of DCAF16 expression in normal tissues are shown in Table 2. Immunostaining of DCAF16 was detected in thyroid follicles (3/3), epithelium of the prostate glands (2/8), epithelium of the gastric glands (1/2), bronchial epithelium (1/1), epithelium of the intestine (1/1), and hepatocellular epithelium (3/5).
Figure 1.

Expression of DCAF16 in normal epithelial tissues. Immunostaining was detected in thyroid follicles (A), epithelium of the prostate glands (B), epithelium of the intestine (C), hepatocellular epithelium (D), epithelium of the gastric glands (E), and bronchial epithelium (F).
Table 2.
Cytoplasmic DCAF16 expression in normal tissues
| Cytoplasmic DCAF16 expression | Total | ||
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| Histological type | |||
| Thyroid follicles | 0 | 3 | 3 |
| Mucoid epithelium of uterine cervix | 2 | 0 | 2 |
| Epithelium of the prostate glands | 6 | 2 | 8 |
| Epithelium of the breast duct | 2 | 0 | 2 |
| Epithelium of the gastric glands | 1 | 1 | 2 |
| Bronchial epithelium | 0 | 1 | 1 |
| Epithelium of the intestine | 0 | 1 | 1 |
| Hepatocellular epithelium | 2 | 3 | 5 |
| Total | 13 | 11 | 24 |
Expression of DCAF16 in human carcinomas
DCAF16 expression in human carcinomas including adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma were investigated. Immunostaining was detected mainly in the cytoplasm of the cancer cells. A few cases of cancer also showed nuclear expression of DCAF16 (Figure 2). The positive rate of nuclear DCAF16 expression in human carcinomas was 10.8% (9/83). The positive rate of cytoplasmic expression of DCAF16 was 73.5% (61/83), significantly higher than in the nucleus (P < 0.05). The details of cytoplasmic expression of DCAF16 in human carcinomas are shown in Table 3. DCAF16 expression was detected in all types of carcinoma including adenocarcinoma (80.0%, 52/65), squamous cell carcinoma (30.8%, 4/13), and urothelial carcinoma (100%, 5/5) (Figure 3).
Figure 2.

Nuclear expression of DCAF16 in human carcinoma. DCAF16 expression was detected in the nucleus of cancer cells in a few human carcinomas (A-F). (A) Thyroid papillary carcinoma; (B) Adenocarcinoma of the intestine; (C) Squamous cell carcinoma; (D) Urothelial carcinoma; (E) Adenocarcinoma of the lung; (F) Acinar adenocarcinoma of the prostate.
Table 3.
Cytoplasmic DCAF16 expression in human carcinomas
| Cytoplasmic DCAF16 expression | Total | ||
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| Histological type | |||
| Adenocarcinoma | 13 | 52 | 65 |
| Adenocarcinoma of the intestine | 0 | 5 | 5 |
| Intrahepatic cholangiocarcinoma | 1 | 5 | 6 |
| Hepatocellular carcinoma | 2 | 2 | 4 |
| Thyroid papillary carcinoma | 0 | 3 | 3 |
| Serous adenocarcinoma of the ovary | 4 | 5 | 9 |
| Acinar adenocarcinoma of the prostate | 2 | 16 | 18 |
| Invasive ductal carcinoma of the breast | 2 | 1 | 3 |
| Gastric adenocarcinoma | 1 | 4 | 5 |
| Endometrioid adenocarcinoma of the uterine | 0 | 4 | 4 |
| Adenocarcinoma of the lung | 1 | 7 | 8 |
| Squamous cell carcinoma | 9 | 4 | 13 |
| Urothelial carcinoma | 0 | 5 | 5 |
| Total | 22 | 61 | 83 |
Figure 3.
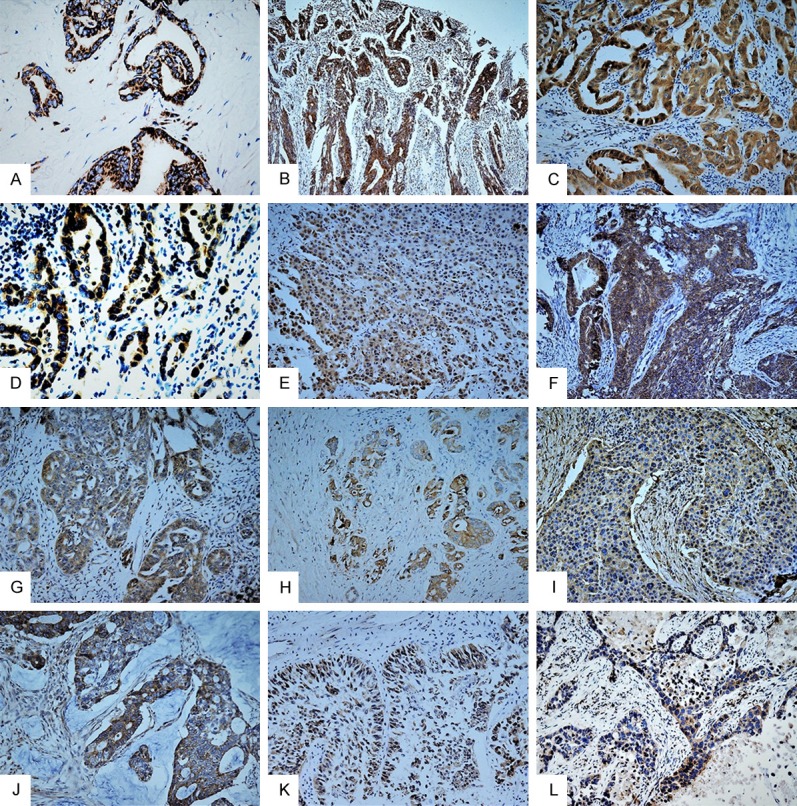
Expression of DCAF16 in human carcinomas. DCAF16 was generally expressed in human carcinomas including adenocarcinomas (A-J), urothelial carcinoma (K), and squamous cell carcinoma (L). (A) Thyroid papillary carcinoma; (B) Adenocarcinoma of the intestine; (C) Intrahepatic cholangiocarcinoma; (D) Adenocarcinoma of the lung; (E) Hepatocellular carcinoma; (F) Endometrioid adenocarcinoma of the uterine; (G) Serous adenocarcinoma of the ovary; (H) Acinar adenocarcinoma of the prostate; (I) Invasive ductal carcinoma of the breast; (J) Gastric adenocarcinoma.
Pathological and diagnostic implications of DCAF16 in human carcinomas
The statistical analysis is shown in Table 4. The total positive rate of DCAF16 expression in human carcinomas was significantly higher compared to normal tissues (P < 0.05). We also investigated the association between DCAF16 expression and the differentiation of carcinomas. Interestingly, we found that DCAF16 expression was significantly associated with higher differentiation of the carcinomas (P < 0.05). The positive rate of DCAF16 expression in carcinomas with high differentiation was 87.5% (28/32), higher than in those with moderate or low differentiation (64.7%, 33/51). This indicates that there is a possibility that DCAF16 may play an important role in oncogenesis of human carcinomas. Analysis of the association between DCAF16 expression and histological type of carcinoma shows that there is differential expression of DCAF16 between adenocarcinoma and squamous cell carcinoma. The positive rate of DCAF16 expression in adenocarcinoma (80.0%, 52/65) was significantly higher than that in squamous cell carcinoma (30.8%, 4/13) (P < 0.05). However, the positive rate of DCAF16 expression in squamous cell carcinoma was 30.8%. DCAF16 was expressed in human carcinomas including adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma, and our results suggest it is not a good marker for differentiating between adenocarcinoma and squamous cell carcinoma.
Table 4.
Association between cytoplasmic DCAF16 expression and histological type and differentiation status of human carcinomas
| Tissue type | Carcinoma type | Differentiation | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Normal tissues | Carcinomas | AC* | SCC | UC | High | Moderate and low | |
| DCAF16+ | 11 | 61 | 52 | 4 | 5 | 28 | 33 |
| DCAF16- | 13 | 22 | 13 | 9 | 0 | 4 | 18 |
| P < 0.05 | P < 0.05 | P < 0.05 | |||||
AC: Adenocarcinoma;
SCC: Squamous cell carcinoma; UC: Urothelial carcinoma; P values were obtained using the Spearman Correlation Test.
Discussion
DCAF proteins were recently identified as substrate receptors in the ubiquitin pathway involving DDB-CUL4 ligase. Dysfunction of the ubiquitin-proteasome system has been found in many diseases, including cancer and non-tumor diseases including Parkinson’s disease [6,7]. The proteins of the cullin family are associated with E3 ligases in the ubiquitin pathways and play various functions including cell proliferation, survival, and oncogenesis [8]. Cullin-1 was found to promote cancer cell proliferation in many malignant tumors [9,10].
Approximately 60 DCAF proteins have been identified, the expression and functions of which are largely unknown [1]. DCAF proteins were shown to bind to DDB1 and thus play important roles in the ubiquitin pathway mediated by DDV-CUL4 ligase [1-4]. The CUL4-DDB1 complex functions in many developmental and physiological processes [1-4,11]. Zhang et al. showed that DCAF1 is essential for plant embryogenesis [2] and Yu et al. showed that DCAF1 is essential for primordial follicle maintenance through interactions with DDB-CUL4 ligase [12]. DCAF1 was also found to play critical roles in methylation-dependent ubiquitination [13]. Peng’s study shows that DCAF7 interacts with the DDB-CUL4 complex and mediates DNA ligase I ubiquitylation; thus, playing important roles in regulating genomic stability [14]. RAF1, another DCAF protein, was found to function in histone methylation and promote siRNA amplification [15].
Here, we found that DCAF16 was expressed in some human epithelial tissues including thyroid follicles, epithelium of the prostate glands, epithelium of the gastric glands, bronchial epithelium, epithelium of the intestine, and hepatocellular epithelium. However, the level of immunostaining was generally low. There were only 2 cases showing strong immunostaining. DCAF16 expression in human carcinomas was elevated compared to normal tissues. This indicates DCAF16 may have a role in oncogenesis. DCAF16 expression was detected in human carcinomas including adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma. However, we do not suggest its use as a diagnostic marker for these carcinomas. We did find that DCAF16 expression was more frequent in adenocarcinoma than squamous cell carcinoma, although the implications of this finding are not known yet. What is interesting is that we found that DCAF16 expression in human carcinomas was significantly associated with higher differentiation of the tumors. However, the function of DCAF16 in these carcinomas needs to be further investigated. What is to be noted is that DCAF proteins commonly interact with DDB1 through the WD40 domain. However, DCAF16 does not have this domain. How DCAF16 interacts with DDB1 and functions in the ubiquitin pathway needs to be further investigated.
Conclusion
DCAF16 is expressed in human carcinomas including adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma, and we do not suggest using it as a diagnostic marker for these tumors. DCAF16 expression in carcinomas was elevated compared with that in normal epithelial tissues, which indicates its possible roles in oncogenesis, but its function in human cancers remains to be determined.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 81472599 to Chuifeng Fan, MD).
Disclosure of conflict of interest
None.
References
- 1.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–80. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Feng S, Chen F, Chen H, Wang J, McCall C, Xiong Y, Deng XW. Arabidopsis DDB1-CUL4 associated factor1 forms a nuclear E3 ubiquitin ligase with DDB1 and CUL4 that is involved in multiple plant developmental processes. Plant Cell. 2008;20:1437–55. doi: 10.1105/tpc.108.058891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biedermann S, Hellmann H. WD40 and CUL4-based E3 ligases: lubricating all aspects of life. Trends Plant Sci. 2011;16:38–46. doi: 10.1016/j.tplants.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Seo KI, Lee JH, Nezames CD, Zhong S, Song E, Byun MO, Deng XW. ABD1 is an Arabidopsis DCAF substrate receptor for CUL4-DDB1-based E3 ligases that acts as a negative regulator of abscisic acid signaling. Plant Cell. 2014;26:695–711. doi: 10.1105/tpc.113.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan C, Zhao Y, Liu D, Zhang X, Wang E. Detection of Brk expression in non-small cell lung cancer: clinicopathological relevance. Tumour Biol. 2011;32:873–80. doi: 10.1007/s13277-011-0188-z. [DOI] [PubMed] [Google Scholar]
- 6.Su C, Niu P. Low doses of single or combined agrichemicals induces α-synuclein aggregation in nigrostriatal system of mice through inhibition of proteasomal and autophagic pathways. Int J Clin Exp Med. 2015;8:20508–15. [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y, Zhang W, Xu J, Wang H, Zhang Z, Chu C, Liu X, Zou Q. UCH-L1 involved in regulating the degradation of EGFR and promoting malignant properties in drug-resistant breast cancer. Int J Clin Exp Pathol. 2015;8:12500–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Sui J, Zhang F, Zhang C. Cullin family proteins and tumorigenesis: genetic association and molecular mechanisms. J Cancer. 2015;6:233–42. doi: 10.7150/jca.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, He D, Xu H, Liu J, Qu L, Tong S. Cullin-1 promotes cell proliferation via cell cycle regulation and is a novel in prostate cancer. Int J Clin Exp Pathol. 2015;8:1575–83. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou YH, Xia J, Xu WH, Zhu X, Wu XH, Hua D, Xing C. Cullin-1 promotes cell proliferation in human breast cancer and is related to diabetes. Int J Biol Markers. 2016;31:e375–e381. doi: 10.5301/jbm.5000215. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Buckley SM, Cimmino L, Guillamot M, Strikoudis A, Cang Y, Goff SP, Aifantis I. The CUL4-DDB1 ubiquitin ligase complex controls adult and embryonic stem cell differentiation and homeostasis. Elife. 2015:4. doi: 10.7554/eLife.07539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Xu YW, Sha QQ, Fan HY. CRL4DCAF1 is required in activated oocytes for follicle maintenance and ovulation. Mol Hum Reprod. 2015;21:195–205. doi: 10.1093/molehr/gau103. [DOI] [PubMed] [Google Scholar]
- 13.Lee JM, Lee JS, Kim H, Kim K, Park H, Kim JY, Lee SH, Kim IS, Kim J, Lee M, Chung CH, Seo SB, Yoon JB, Ko E, Noh DY, Kim KI, Kim KK, Baek SH. EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol Cell. 2012;48:572–86. doi: 10.1016/j.molcel.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Peng Z, Liao Z, Matsumoto Y, Yang A, Tomkinson AE. Human DNA ligase I interacts with and is targeted for degradation by the DCAF7 specificity factor of the Cul4-DDB1 ubiquitin ligase complex. J Biol Chem. 2016;291:21893–902. doi: 10.1074/jbc.M116.746198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buscaino A, White SA, Houston DR, Lejeune E, Simmer F, de Lima Alves F, Diyora PT, Urano T, Bayne EH, Rappsilber J, Allshire RC. Raf1 is a DCAF for the Rik1 DDB1-like protein and has separable roles in siRNA generation and chromatin modification. PLoS Genet. 2012;8:e1002499. doi: 10.1371/journal.pgen.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]


