Abstract
This study was aimed to assess serum microRNA-30d-5p (miR-30d-5p) expression in patients with esophageal squamous cell carcinoma before and after operation, exploring its associations with clinical pathological parameters. A total of 30 esophageal cancer patients who underwent radical resection and were pathologically confirmed with esophageal squamous cell carcinoma in the First Affiliated Hospital of Anhui Medical University, from April to May in 2013, were enrolled, alongside 19 healthy controls. The expression levels of miRNA in serum from patients with esophageal squamous cell carcinoma before and after operation were assessed by microarrays and real-time PCR (RT-PCR). The associations of miR-30d-5p expression with clinical pathological parameters were determined. Serum hsa-miR-30d-5p levels in patients with esophageal squamous cell carcinoma (study group) were significantly associated with the tumor depth of invasion, lymph node metastasis, tumor location and length, histopathological type and degree of differentiation, as well as history of smoking and drinking (P<0.05). Moreover, changes of serum miRNA levels were more prominent than that of thymidine kinase 1 (TK1). There were significant differences in hsa-miR-30d-5p expression levels between the study and control groups (P<0.05). These results indicated that microRNA-30d-5p is a potential marker of esophageal squamous cell carcinoma, with high expression having a certain promoting role in the occurrence and development of esophageal cancer.
Keywords: MicroRNA-30d-5p, esophageal squamous cell carcinoma, microarray, real-time PCR, thymidine kinase 1
Introduction
Esophageal cancer is a common malignant tumor and comprises two pathological types, i.e. esophageal squamous cell carcinoma and adenocarcinoma. It is the main esophageal squamous cell carcinoma in China, accounting for about 90% of all esophageal cancers [1]. Esophageal cancer has high incidence in China, with morbidity and mortality rankingfifth and fourth, respectively, among various malignant tumors countrywide [2]. Male and female mortality and morbidity in China all rank first in the world [3]. Due to the lack of specific early symptoms or effective tumor markers, the patients are mostly diagnosed in the middle and late stages. Although treatment methods have progressed greatly in recent years, most patients still succumb to local recurrence, disease progression, distant metastasis, and resistance to adjuvant therapy; moreover, patient prognosis is quite poor, with a recurrence rate of 46% within 1 year after operation [4], and a 5-year survival rate of only 23% [5].
MicroRNAs (miRNAs) are recently described non-coding small RNAs with approximately 22 nucleotides. They regulate the expression of target genes by promoting target mRNA degradation or inhibiting translation, and play important roles in cell differentiation, proliferation, and apoptosis, as well as development, metabolism, and immune regulation [6]. Silencing of coding genes by miRNAs can be achieved by the following three ways: 1) blocking the translation of mRNAs; 2) mRNA degradation (similar to RNAi); 3) induction of DNA promotormethylation. Currently, thousands of miRNAs have been described in human cells, and different human organs and tissues have specific miRNA expression profiles. Increasing evidence clearly shows that miRNAs play more important roles in the life process envisioned, participating in critical life activities such as development, hematopoiesis, and organogenesis, as well as cell proliferation, apoptosis and differentiation [7].
Although the miRNA expression profile of the tumor tissue is associated with carcinogenesis and patient prognosis, current detection techniquesare complicated and cause important trauma, limiting their application for clinical diagnosis. Since 2008, scientists acknowledge that miRNA shave long-term stability in the circulating blood, and show resistance to RNase degradation; moreover, various treatment methods such as boiling, repeated freezing and thawing cycles, exposure to acid-base environments, and long-term preservation do not decrease serum miRNA levels, making them more suitable tumor biomarkers than proteins [8]. Compared with tissues, the circulating blood can be obtained and assessed more easily, and is convenient for clinical application. The-refore, serum miRNAs can be used as valuable tumor markers, with high clinical applicability.
Materials and methods
Clinical data
A total of 30 patients pathologically diagnosed with esophageal squamous cell carcinoma that underwent esophagectomy for esophageal cancer in the First Affiliated Hospital of Anhui Medical University in April-May 2013 were enrolled. They included 26 malesand 4 females aged from 36 to 73 years, averaging 62 years old. Meanwhile, 19 healthy individuals in the same period were enrolled as the control group, including 12 males and 7 females of 45 to 74 years (average age of 61 years). All patients received surgery, whose pathological confirmed for esophageal squamous carcinoma. All the included patients had not received treatments like preoperative chemotherapy or radiation therapy. This study was approved by the Ethics Committee in our hospital, and informed consent was obtained from patients themselves for sample collection.
Experimental methods
Microarrays were used to assessserum miRNA expression levels in patients (3 cases) with esophageal squamous cell carcinoma before and after the operation; miRNAs (hsa-miR-30d-5p and hsa-miR-483-5p) with important changes were screened.
Preliminary real-time PCR (qRT-PCR) was used to verify the expresssion of the above miRNAs of interest (9 cases). A new miRNA (onco-miRNA) potentially related to esophageal squamous cell carcinoma was selected, with inductive or inhibitive roles in tumors: hsa-miR-30d-5p.
Then, sample size was increased. qRT-PCR was used to validate the changes of candidate onco-miRNAs in preoperative and postoperative serum samples from 27 cases with esophageal squamous cell carcinoma. Furthermore, serum TK1 detection was performed for these 27 cases before and after the operation.
Serum expression levels of candidate onco-miRNAs were compared between patients with esophageal squamous cell carcinoma (27 cases) and the healthy control group (19 cases).
Major instruments and reagents
Total Nucleic Acid Isolation Kit was used for FFPE (Ambion); μParafloTM miRNA microfluidic chip was utilized for hybridization; laser scanning was performed, and data were analyzed with the Array-Pro image analysis software (Media Cybernetics).
Experimental procedure
Preparation of blood samples
2 ml of peripheral blood were obtained from each patient undergoing esophagectomy before and after operation, as well as from healthy controls. After centrifugation for 10 min, serum samples were collected and stored at -80°C until use.
RNA extraction and quality control, and microarray detection of miRNAs
Expression profile chip for microRNAs (LC.Bio tech) was used to perform monochrome and real-time PCR to validate differences in the expressed genes.
Serum RNA extraction
Total Nucleic Acid Isolation Kit for FFPE (Ambion, Cat. No. AM1975) was used, according to the manufacturer’s instructions. RNA samples were stored at -80°C for later use.
Quality control of RNA samples
QC was performed for total RNA samples with a kit from LC.Biotech. Samples must have clear bandsas detected by electrophoresis without significant dispersion or smearing. Total RNA was assessed by Nanodrop; samples were considered for the assays with OD260/280≥1.8 and OD260/230≥1.5.
Microarray experiments
Microarray experiments were performed byLC Sciences, using 4-8 μg of total RNA per sample. Poly (A) tail was added to the total RNA 3’ end by Poly (A) polymerase, with an oligonucleotide tag attached to the poly (A) tail for subsequent fluorescent labeling. On the microfluidic chip, each probe is composed of a chemically modified nucleotide sequence complementary to the target microRNA (from miRBase, http://www.mirbase.org/) and a spacer segment consisting of polyethylene glycol. Hybridization was performed using 100 μL of 6×SSPE buffer containing 25% formamide at a hybridization temperature of 34°C. After hybridization, Cy3 was used for staining. A laser scanner (GenePix 4000B, Molecular Device) was used to collect hybridization images, and the Array-Pro image analysis software (Media Cybernetics) was utilized for image digitalization. Background was subtracted first, and LOWESS was employed for filtering (Locally-Weighted Regression) to normalize the signals.
Determination of serum TK1 concentration
2 ml of peripheral blood were obtained from each patient undergoing esophagectomy before and after operation, as well as from healthy controls. After centrifugation for 10 min, serum samples were collected and stored at -80°C until use.
Serum TK1 was analyaed by an ECL dot blot assay. The procedure was performed according to the manufacturer’s protocol (commercial kit; SSTK, Shenzhen, China) as described elsewhere.
Data analysis
All data were analyzed using the SPSS 11.0 software. Continuous data were presented as X̅ ± S and categorical data as percentile. MiRNA expression levels in esophageal squamous cell carcinoma and their associations with clinicopathological features were assessed by paired t test. Preoperative and postoperative amounts of miRNA and TK1 inserumwere evaluated by Kolmogorov-Smirnov and Shapiro-Wilk tests. P<0.05 was considered statistically significant.
Results
Microarray data for the 3 cases
Microarray analysis was used to compare preoperative (Group 1) and postoperative (Group 2) miRNA expression levels in serum from patients with esophageal squamous cell carcinoma in a small sample (3 cases). A total of 2555 miRNAs were detected, of which 143 had signal values ≥500. MiRNAs with significant changes were screened, i.e. those with ratios of Group 1 to Group 2>2 (ratio of log2>1) or <0.5 (ratio of log2<-1) (Table 1). Then, chip data were imported into Cluster 3.0 for cluster analysis (Figure 1). Two miRNAs with the most significant changes postoperatively were selected, including hsa-miR-30d-5p (downregulated) and hsa-miR-483-5p (upregulated).
Table 1.
Preoperative and postoperative microarray data for the 3 cases of esophageal cancer
| Reporter Name | Group 1 Mean | Group 2 Mean | Log2 (G1/G2) Mean | Target Sequence (5’ to 3’) |
|---|---|---|---|---|
| hsa-miR-30d-5p | 1,107 | 385 | 6.47 | UGUAAACAUCCCCGACUGGAAG |
| hsa-miR-30c-5p | 754 | 537 | 5.45 | UGUAAACAUCCUACACUCUCAGC |
| hsa-miR-10b-5p | 213 | 118 | 5.15 | UACCCUGUAGAACCGAAUUUGUG |
| hsa-miR-30b-5p | 1,004 | 592 | 4.24 | UGUAAACAUCCUACACUCAGCU |
| hsa-miR-451a | 52,810 | 31,444 | 3.73 | AAACCGUUACCAUUACUGAGUU |
| hsa-miR-30a-5p | 1,216 | 659 | 2.73 | UGUAAACAUCCUCGACUGGAAG |
| hsa-miR-150-5p | 220 | 97 | 2.5 | UCUCCCAACCCUUGUACCAGUG |
| hsa-miR-16-5p | 8,727 | 7,414 | 2.28 | UAGCAGCACGUAAAUAUUGGCG |
| hsa-miR-181a-5p | 627 | 471 | 2.08 | AACAUUCAACGCUGUCGGUGAGU |
| hsa-miR-122-5p | 2,193 | 241 | 1.76 | UGGAGUGUGACAAUGGUGUUUG |
| hsa-miR-93-5p | 809 | 561 | 1.67 | CAAAGUGCUGUUCGUGCAGGUAG |
| hsa-miR-140-3p | 815 | 526 | 1.5 | UACCACAGGGUAGAACCACGG |
| hsa-miR-17-5p | 1,249 | 960 | 1.34 | CAAAGUGCUUACAGUGCAGGUAG |
| hsa-miR-425-5p | 686 | 514 | 1.01 | AAUGACACGAUCACUCCCGUUGA |
| hsa-miR-486-5p | 3,092 | 2,134 | 0.97 | UCCUGUACUGAGCUGCCCCGAG |
| hsa-miR-652-3p | 246 | 185 | 0.93 | AAUGGCGCCACUAGGGUUGUG |
| hsa-miR-638 | 1,605 | 709 | 0.85 | AGGGAUCGCGGGCGGGUGGCGGCCU |
| hsa-miR-151b | 514 | 457 | 0.46 | UCGAGGAGCUCACAGUCU |
| hsa-miR-320e | 539 | 730 | -0.4 | AAAGCUGGGUUGAGAAGG |
| hsa-miR-483-5p | 767 | 2,347 | -1.12 | AAGACGGGAGGAAAGAAGGGAG |
| hsa-let-7e-5p | 175 | 471 | -1.88 | UGAGGUAGGAGGUUGUAUAGUU |
Figure 1.
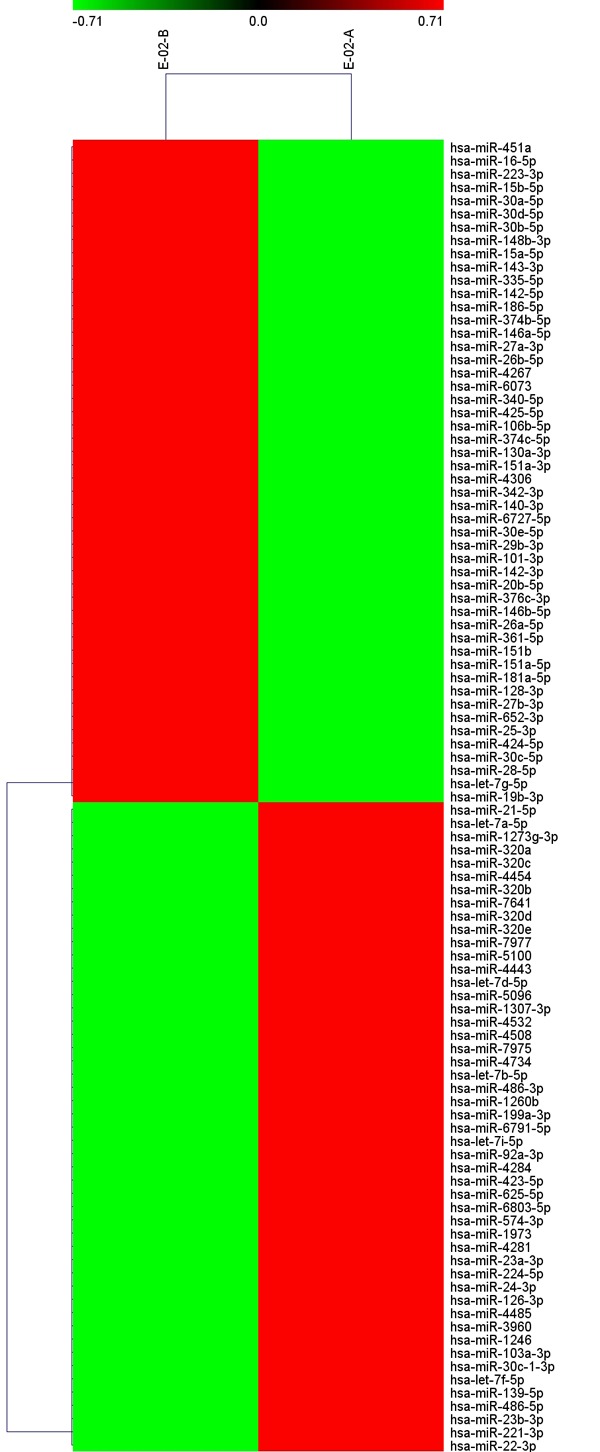
Chip data imported into Cluster 3.0 for cluster analysis. Notes: FDR<0.05; Signal>500.
Preoperative and postoperative qRT-PCR of 9 cases with esophageal cancer
qRT-PCR validation was performed for the above two miRNAs of interest in 9 cases before (Group 1) and after (Group 2) the operation (Table 2). There was no obvious difference in mean hsa-miR-483-5p levels before and after the operation (P>0.05). In contrast, the trend of hsa-miR-30d-5p expresion before and after the operation was conistent with microarray data, with statistical significance (P=0.047). Therefore, hsa-miR-30d-5p was selected for futhrer assessment as a new potential onco-miR for esophageal squamous cell carcinoma.
Table 2.
Validation by qRT-PCR of serum miRNA levels in 9 patients before and after the operation
| Assay Name | Preoperative (X̅ ± S) | Postoperative (X̅ ± S) | t | P |
|---|---|---|---|---|
| has-miR-30d-5p | 24.719±1.982 | 22.679±1.751 | 2.181 | 0.047 |
| has-miR-483-5p | 29.120±1.439 | 29.233±1.013 | 0.183 | 0.858 |
Validation by qRT-PCR in 27 cases with esophageal cancer before and after operation
Quantitative RT-PCR was used to validate the changes of hsa-miR-30d-5p in serum samples from 27 cases with esophageal squamous cell carcinoma before (Group 1) and after (Group 2) the operation (P<0.05) (Supplementary Figure 1). The qRT-PCR results were consistent with microarray data, with statistical significance.
Associations of hsa-miR-30d-5p expression in cancer tissues with clinicopathological features of patients with esophageal squamous cell carcinoma
Paired t test was performed for the expression of preoperative and postoperative hsa-miR-30d-5p as well as the histopathological parameters of the 27 cases. The results demonstrated that the expression ofserum hsa-miR-30d-5p in the patients with esophageal squamous cell carcinoma was correlated with clinicopathological features such as gender, age, depth of tumor invasion, lymph node metastasis, tumor location and length, pathological type and differentiation of tumor, together with smoking and drinking history (P<0.05) (Table 3).
Table 3.
Comparison of preoperative and postoperative hsa-miR-30d-5p in different groups
| Characteristics | Preoperative (x̅ ± s) | Postoperative (x̅ ± s) | t | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 23.963±0.691 | 21.857±3.462 | 3.198 | 0.004 |
| Female | 22.078±5.899 | 18.560±1.022 | 1.186 | 0.321 |
| Age (years) | ||||
| <60 | 22.108±2.220 | 20.020±2.095 | 1.653 | 0.174 |
| ≥60 | 24.042±3.924 | 21.675±3.681 | 2.939 | 0.008 |
| T | ||||
| <3 | 22.607±3.880 | 21.340±3.243 | 0.958 | 0.361 |
| ≥3 | 24.424±3.524 | 21.388±3.725 | 4.314 | 0.001 |
| Lymphatic metastasis | ||||
| None | 24.873±3.841 | 22.513±3.723 | 2.166 | 0.048 |
| Have | 22.197±3.074 | 19.937±2.611 | 2.860 | 0.016 |
| Position | ||||
| Upper and Middle | 23.988±3.372 | 20.886±2.704 | 3.412 | 0.004 |
| Lower | 23.241±4.291 | 22.069±4.411 | 1.176 | 0.267 |
| Length | ||||
| <3.5 cm | 23.967±4.246 | 22.003±0.753 | 1.976 | 0.067 |
| ≥3.5 cm | 23.272±2.912 | 20.444±1.211 | 3.126 | 0.011 |
| Type | ||||
| Ulcer-infiltrating | 24.376±3.792 | 21.630±3.521 | 3.423 | 0.003 |
| Others | 21.707±2.834 | 20.621±3.476 | 0.823 | 0.442 |
| Differentiation | ||||
| Moderately and poorly | 23.788±3.785 | 20.716±2.843 | 4.237 | <0.001 |
| High | 23.224±3.747 | 24.238±4.823 | 0.989 | 0.378 |
| Cigarette smoking | ||||
| No | 22.323±4.718 | 20.021±2.617 | 1.314 | 0.237 |
| Yes | 24.160±3.303 | 21.840±3.66 | 3.175 | 0.005 |
| Drinking | ||||
| No | 22.663±3.723 | 19.737±2.526 | 2.060 | 0.073 |
| Yes | 24.194±3.705 | 22.184±3.649 | 2.610 | 0.018 |
| Total | 23.684±3.712 | 21.368±3.471 | 3.367 | 0.002 |
Associations of serum thymidine kinase 1 (TK1) with clinicopathological features in patients with esophageal squamous cell carcinoma
Paired t test was performed to assess preoperative/postoperative TK1 levels and clinicopathologic parameters in 27 patients. Interestingly, serum TK1 in patients with esophageal squamous cell carcinoma was associated with gender, age, depth of tumor invasion, pathological type, and degree of differentiation, as well as history of smoking and drinking (P<0.05) (Table 4).
Table 4.
Comparison of serum TK1 before and after the operation in different groups
| Characteristics | Preoperative (X̅ ± S) | Postoperative (X̅ ± S) | t | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 2.086±0.484 | 1.803±0.129 | 2.227 | 0.037 |
| Female | 1.388±0.389 | 1.140±0.322 | 4.294 | 0.023 |
| Age (years) | ||||
| <60 | 2.180±0.645 | 1.704±0.612 | 1.365 | 0.244 |
| ≥60 | 1.938±0.506 | 1.705±0.646 | 2.123 | 0.046 |
| T | ||||
| <3 | 1.839±0.627 | 1.499±0.588 | 2.323 | 0.043 |
| ≥3 | 2.081±0.444 | 1.846±0.634 | 1.513 | 0.151 |
| Lymphatic metastasis | ||||
| None | 2.008±0.500 | 1.701±0.610 | 2.115 | 0.053 |
| Have | 1.951±0.583 | 1.709±0.678 | 1.427 | 0.181 |
| Position | ||||
| Upper and Middle | 1.917±0.445 | 1.700±0.499 | 1.667 | 0.116 |
| Lower | 2.078±0.643 | 1.711±0.808 | 1.917 | 0.084 |
| Length | ||||
| <3.5 cm | 1.840±0.527 | 1.620±0.619 | 1.752 | 0.100 |
| ≥3.5 cm | 2.190±0.480 | 1.827±0.651 | 1.829 | 0.097 |
| Type | ||||
| Ulcer-infiltrating | 2.035±0.511 | 1.701±0.541 | 3.057 | 0.006 |
| Others | 1.834±0.591 | 1.716±0.886 | 0.413 | 0.694 |
| Differentiation | ||||
| Moderately and poorly | 1.924±0.543 | 1.792±0.609 | 1.305 | 0.206 |
| High | 2.242±0.409 | 1.318±0.625 | 4.123 | 0.015 |
| Cigarette smoking | ||||
| No | 1.646±0.522 | 1.433±0.482 | 2.232 | 0.067 |
| Yes | 2.101±0.490 | 1.800±0.656 | 2.098 | 0.049 |
| Drinking | ||||
| No | 1.890±0.666 | 1.760±0.796 | 0.967 | 0.362 |
| Yes | 2.029±0.461 | 1.677±0.551 | 2.390 | 0.029 |
| Total | 1.983±0.529 | 1.704±0.628 | 2.566 | 0.016 |
Comparison of preoperative and postoperative changes of hsa-miR-30d-5p and TK1
In patients with esophageal squamous cell carcinoma, preoperative and postoperative differences between the two detection methods were statistically significant (P<0.05); more prominent changes were obtained for serumhsa-miR-30d-5p compared with TK1 (Table 5).
Table 5.
Comparison of preoperative and postoperative changes between the two indicators
| Normal Test | X̅ ± S | t | P | ||
|---|---|---|---|---|---|
|
| |||||
| P K-S | P S-W | ||||
| hsa-miR-30d-5p | 0.200 | 0.253 | 2.316±3.573 | 2.927 | 0.007 |
| TK1 | 0.200 | 0.535 | 0.278±0.563 | ||
Notes: K-S: Kolmogorov-Smirnov test; S-W: Shapiro-Wilk test.
Levels of hsa-miR-30d-5p in the study (27 cases with esophageal squamous cell carcinoma) and control (19 healthy individuals) groups
Serum expression levels of hsa-miR-30d-5p were significantly different between the study and control groups (P<0.05). Preoperative and postoperative expression levels were also significantly different (t=2.916, P=0.007) (Table 6).
Table 6.
Levels of hsa-miR-30d-5p in the study (27 cases with esophageal squamous cell carcinoma) and control (19 healthy individuals) groups
| X̅ ± S | t | P | |
|---|---|---|---|
| Control group | 20.912±0.516 | ||
| Preoperative study groupa | 22.988±3.481 | 3.158 | 0.004 |
| Postoperative study groupb | 21.154±1.823 | 0.674 | 0.505 |
preoperative study group vs. control group;
postoperative study group vs. control group.
Follow-up deadline for all patients was January 31, 2017. There were 19/30 cases (63.3%) who died; the remaining patients were still alive (36.7%).
Discussion
In 2008, Mitchell [8] found that circulating miRNAs can be used as biomarkers of prostate cancer. They demonstrated that plasma miRNAs remain highly stable after prolonged incubation at room temperature and/or multiple freeze-thaw cycles. In addition, some miRNAs are abundant in cells and tissue-specific [9], thus having a biomarker advantage over other circulating nucleic acids. These studies supported that circulating blood miRNAs satisfy the basic requirements of circulating tumor markers, with good application prospects. Currently, the relationships between tumors and circulating miRNA shave arou-sed wide concerns. Studies have shown that miRNAs are involved in post-transcriptional regulation, controlling development and cell growth in various eukaryotes; therefore, they can be used as potential biomarkers of cancer, reflecting the development of tumor lineage and differentiation stage [10]. Various miRNAs are considered regulators of oncogenesis, tumor suppression, cancer stem cells, and metastasis [11].
Regarding esophageal squamous cell carcinoma, Feber et al [12] found that miR-2 And miR-93 are up-regulated, and miR-205 and miR-203 down-regulated, while assessing by microRNA microarrays 10 esophageal adenocarcinoma, 10 esophageal squamous cell carcinoma, and 9 normal esophageal epithelium cases. In addition, miR-194, miR-192 and miR-200c were up-regulated in esophageal adenocarcinoma, while miR-342 in esophageal squamous cell carcinoma was downregulated. Mathe et al [13] analyzed 70 pairs of esophageal squamous cell carcinoma and adjacent normal tissue, which found that the expression of miR-21 in esophageal squamous cell carcinoma was up-regulated while miT-375 was downregulated. Wijnhoven et al [14] assessed samples of normal esophageal squamous epithelium, normal gastric mucosa epithelium, Barrett’s esophagus with intestinal metaplasia, and esophageal adenocarcinoma, and found increased levels of miR-21, miR-143, miR-145, miR-194 and miR-215 in columnar epithelium; meanwhile, miR-203 and miR-205 amounts in normal squamous epithelium were higher than those of columnar epithelium. Zhang et al [15] analyzed 290 cases with esophageal squamous cell carcinoma and 140 healthy controls, and 25 serum miRNAs in patients with esophageal squamous cell carcinoma showed an increasing trend compared with control values, as assessed by Solexa high-throughput sequencing. Quantitative RTPCR confirmed that 7 serum miRNAs (miR10a, miR-22, miR-140, miR-223, miR-133a and miR-127-3p) could be used as tumor markers for esophageal squamous cell carcinoma. Meanwhile, the 7 miRNAs could clearly distinguish patients with stage I/II esophageal squamous cell carcinoma from healthy controls. Thus, the 7 miRNAs could be used as serum tumor markers for the diagnosis of esophageal squamous cell carcinoma.
In this study, microarray data showed that there were 2555 serum miRNAs in patients with esophageal carcinoma before and after operation, with 143 miRNA sat high levels. Among them, a total of 10 miRNAs with more than 2 fold change between preoperative and postoperative specimens were screened. A literature review revealed that two miRNA shave not been reported so far in esophageal squamous cell carcinoma: hsa-miR-483-5p and hsa-miR-30d-5p. Hsa-miR-30d is a precursor of hsa-miR-30d-5p, and hsa-miR-30d-5p is one of the stem-loop structures. Has-miR-30d has been widely studied, with roles in various tumors, regulating metastasis, apoptosis, proliferation, and differentiation in tumor cells [16]. Studies have shown that hsa-miR-30d is significantly down-regulated in central nervous system tumors [17] and lung squamous cell carcinoma compared with respective normal tissues [18]. Few studies have assessed hsa-miR-30d-5p, and demonstrated that hsa-miR-30d-5p is significantly down-regulated in non-small cell lung cancer (NSCLC), inhibiting the growth, motility and distribution of cells. In addition, cyclin E2 usually shows an increasing trend in NSCLC tissues, and may be a direct target of miR-30d-5p. This study demonstrated that the mir-30d-5p/CCNE2 axis may contribute to proliferation and migration in lung cancer cells, suggesting that miR-30d-5p may serve as a potential therapeutic target for the treatment of NSCLC [19]. Furthermore, hsa-miR-30d-5 in the circulating blood is also related to diseases such as myocardial infarction [20], but reports assessing the relationship between hsa-miR-30d-5p in the circulating blood and tumors are scarce.
In this study, serum hsa-miR-30d-5p levels in patients with esophageal squamous cell were obviously associated with clinicopathological features such as age, gender, depth of tumor invasion, lymph node metastasis, tumor location and length, histological type and differentiation, TNM staging, and history of smoking and drinking. Thus, this miRNA should be considered a useful marker for evaluating the prognosis of patients with esophageal cancer. Meanwhile, serum hsa-miR-30d-5p in patients with esophageal squamous cell carcinoma was significantly higher than in healthy controls, and significantly reduced in patients postoperatively; postoperative amounts were close to healthy control levels. Therefore, hsa-miR-30d-5p constitutes a potential circulating tumor markerin the clinical diagnosis and monitoring of postoperative efficacy.
Thymidine kinase 1 (TK1) is the key enzyme in the S phase of cell proliferation. Its concentrations are extremely low or undetectable in non-proliferating cells and healthy human serum, but increaseby 2~100 times in case of abnormal cell proliferation (such as in tumor cells) [21]. TK1, as a kinetic marker of abnormal proliferation, has high sensitivity and specificity, with an important prognostic value [22]. An analysis of patients with esophageal squamous cell carcinoma demonstrated that preoperative and postoperative changes of hsa-miR-30d-5p and TK1 were statistically significant. Interestingly, associations of hsa-miR-30d-5p with various pathological characteristics were more pronounced than those of TK1. These findings indicated that hsa-miR-30d-5p may bea more sensitive reference index thanserum TK1 in predicting precancerous lesions, screening early malignant tumors, and performing early diagnosis.
In conclusion, the expression profiles of miRNAs in the circulating blood of patients with esophageal squamous cell carcinoma were significantly different pre- and post-operation. In addition, hsa-miR-30d-5p expression was significantly higher in patients with esophageal squamous cell carcinoma than in the healthy control group, with a significant decreasing trend one month after the operation compared with preoperative values. Therefore, hsa-miR-30d-5p should be considered a potential tumor biomarker, with good clinical application prospect in the early diagnosis as well as patient prognosis in esophageal squamous cell carcinoma.
Acknowledgements
This study was funded by National Natural Science Foundation of China (grants number. 81572430 and 81402040).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Zhou MG, Wang XF, Hu JP, Li GL, Chen WQ, Zhang SW, Wan X, Wang LJ, Xiang C, Hu YS, Yang GH. [Geographical distribution of cancer mortality in China, 2004-2005] . Zhonghua Yu Fang Yi Xue Za Zhi. 2010;44:303–308. [PubMed] [Google Scholar]
- 4.Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616–1623. doi: 10.1002/cncr.11228. [DOI] [PubMed] [Google Scholar]
- 5.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 6.Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 7.Shruti K, Shrey K, Vibha R. Micro RNAs: tiny sequences with enormous potential. Biochem Biophys Res Commun. 2011;407:445–449. doi: 10.1016/j.bbrc.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32:326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 10.Braicu C, Calin GA, Berindan-Neagoe I. MicroRNAs and cancer therapy-from bystanders to major players. Curr Med Chem. 2013;20:3561–3573. doi: 10.2174/0929867311320290002. [DOI] [PubMed] [Google Scholar]
- 11.George GP, Mittal RD. MicroRNAs: potential biomarkers in cancer. Indian J Clin Biochem. 2010;25:4–14. doi: 10.1007/s12291-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feber A, Xi L, Luketich JD, Pennathur A, Landreneau RJ, Wu M, Swanson SJ, Godfrey TE, Litle VR. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. discussion 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, Braun R, Reimers M, Kumamoto K, Hughes D, Altorki NK, Casson AG, Liu CG, Wang XW, Yanaihara N, Hagiwara N, Dannenberg AJ, Miyashita M, Croce CM, Harris CC. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res. 2009;15:6192–6200. doi: 10.1158/1078-0432.CCR-09-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijnhoven BP, Hussey DJ, Watson DI, Tsykin A, Smith CM, Michael MZ South Australian Oesophageal Research Group. MicroRNA profiling of Barrett’s oesophagus and oesophageal adenocarcinoma. Br J Surg. 2010;97:853–861. doi: 10.1002/bjs.7000. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L, Zhang Y, Li Y, Qiu H, Xing J, Liang Z, Ren B, Yang C, Zen K, Zhang CY. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. mir-30d regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells. Biochem Biophys Res Commun. 2013;431:617–622. doi: 10.1016/j.bbrc.2012.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi YH, Murakami Y. Principal component analysis based feature extraction approach to identify circulating microRNA biomarkers. PLoS One. 2013;8:e66714. doi: 10.1371/journal.pone.0066714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137:557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang L, Liu L, Huang S, Zhao Y, He X. MicroRNA-30d-5p inhibits tumour cell proliferation and motility by directly targeting CCNE2 in non-small cell lung cancer. Cancer Lett. 2015;362:208–217. doi: 10.1016/j.canlet.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Ward JA, Esa N, Pidikiti R, Freedman JE, Keaney JF, Tanriverdi K, Vitseva O, Ambros V, Lee R, McManus DD. Circulating cell and plasma microRNA profiles differ between non-ST-segment and ST-segment-elevation myocardial infarction. Fam Med Med Sci Res. 2013;2:108. doi: 10.4172/2327-4972.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Li H, Pendleton AR, Robison JG, Monson KO, Murray BK, O’Neill KL. Thymidine kinase 1 immunoassay: a potential marker for breast cancer. Cancer Detect Prev. 2001;25:8–15. [PubMed] [Google Scholar]
- 22.Huang S, Lin J, Guo N, Zhang M, Yun X, Liu S, Zhou J, He E, Skog S. Elevated serum thymidine kinase 1 predicts risk of pre/early cancerous progression. Asian Pac J Cancer Prev. 2011;12:497–505. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


