Abstract
Many miRNAs are reported to be involved in tumor development. The underlying mechanism of miRNAs driving multiple myeloma (MM) progress remains elusive. This study is to investigate the effect of miR-125b, a brain-enriched microRNA, on mitogen-activated protein kinase 7 (MKK7) in vivo. We found significantly up-regulation of miR-125b and deregulation of MKK7 in MM. The inverse correlation was further confirmed in multiple myeloma samples and cell lines. In addition, MKK7 was identified as a downstream target gene of miR-125b, which could bind to the 3’ UTR of MKK7. Overexpression of miR-125b was associated with decreased MKK7 expression and miR-125b antagonisminhibited cell proliferation and clonogenicity. Taken together, our results demonstrated that MKK7 could function as an important tumor suppressor neutralized by miR-125b in MM, suggesting that miR-125b may be a novel potential molecular therapeutic target in the treatment of MM.
Keywords: miR-125b, multiple myeloma, metastasis, MKK7
Introduction
Multiple myeloma (MM) is a heterogeneous disease characterized by infiltration in the bone marrow of malignant plasma cells. It ranks as the second most common but as yet incurable hematologic malignancy with survival duration ranging from a few months to more than 10 years [1,2]. Although its mortality decreases along with the advancement of high-dose therapy and novel agents such as bortezomib, thalidomide, and lenalidomide, the long-term prognosis remains unsatisfactory [3]. For example, the 5-year survival rate is only 30 to 40%, mainly due to the development of drug resistance [4], highlighting an urgent requirement for more effective therapies. It has been generally accepted that microRNAs (miRNAs) have an important role in almost all biological processes, but have also been linked to a variety of human diseases such as MM [5].
miRNAs are small non-coding RNAs of 19-25 nucleotides, which regulate gene expression via base pairing to partially or fully complementary sites in the 3’untranslated region (UTR) of target mRNAs [6]. Differences in miRNA expression define the signatures of various cancers; miRNAs may play an important role in cancer pathogenesis [7]. Indeed, aberrant miRNA expression has been demonstrated in MM, which contributes to carcinogenesis and cancer development by promoting oncogene [8,9]. Previous studies have shown that miR-125b which is elevated in several types of cancers might be a novel class of oncogene [10,11]. Further understanding of the expression and the function of miRNAs in cancers might be a valuable strategy for cancer treatment.
MKK7 is an important tumor suppressor gene involved in several cancer-related signaling pathways, which participates in regulating cells proliferation, differentiation, and apoptosis [12]. Activation of MKK7 could inhibit lung cancer cells development [13]. Inactivation of MKK7 driven mammary tumors markedly accelerate and could cause distinct phenotypic abnormalities in mice [14]. In addition, several studies also indicate that MKK7 acts as a suppressor in tumors migration, invasion, and metastasis [15-17].
Here, we report for the first time a miRNA that directly regulates MKK7. We identified miR-125b as a negative regulator of MKK7 in MM cell lines. Low expression of miR-125b inhibited MM cell growth in vivo. Furthermore, MKK7 was identified as a downstream target gene of miR-125b, which bound to the 3’ UTR of MKK7. Our report provided new insights into the function of miR-125b during development and tumorigenesis.
Materials and methods
Patient samples and cell lines
Fifteen MM and eight healthy control samples were collected from the 1st Affiliated Hospital of Nanchang University (Nanchang, China). The study was approved with the Local Research Ethics committee. Written informed consent obtained from all patients. Human MM cell lines (MM1S, U266, and RPMI-8226) and normal plasma cells (nPCs) were cultured in RPMI-1640 (Gibco, CA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a 5% CO2 atmosphere. When cells reached 80% confluency, cells at the logarithmic growth phase were collected.
RNA isolation and quantitative RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, CA, USA). The concentration and purity of extracted RNA were measured at 260 and 280 nm optical densities. cDNA was synthesized from RNAs using gene specific primers (Applied bio-systems, Beijing, China) with the M-MLV Reverse Transcriptase kit (GeneCopoeia, Rockville, MD), following the manufacturers protocol. To determine MKK7 and miR-125b expression levels, real-time PCR was performed with SYBR Green (Takara, Osaka, Japan). RNU6B (U6) and β-actin was used as internal control. PCR conditions were as follows: 95°C for 5 min, 95°C for 45 sec, 55°C for 15 sec, 72°C for 50 sec, for 40 cycles. Samples were analyzed in triplicate and gene expression was quantified by normalizing target gene expression to that of the internal control using the 2-ΔΔCt formula. The primer sequences used were listed in Table 1.
Table 1.
The primer sequences
| Name | Primer | Sequence |
|---|---|---|
| Homo β-actin | Forward | 5’-AGCGAGCATCCCCCAAAGTT-3’ |
| Reverse | 5’-GGGCACGAAGGCTCATCATT-3’ | |
| Homo MKK7 | Forward | 5’-GAGAAGCACGGTGTCATCCA-3’ |
| Reverse | 5’-AGGGAAACTGTCCTGTTGCCA-3’ | |
| Homo U6 | Forward | 5’-CGCTTCGGCAGCACATATAC-3’ |
| Reverse | 5’-AAATATGGAACGCTTCACGA-3’ | |
| mir125b-5p | Forward | 5’-TGCGCTCCCTGAGACCCTAAC-3’ |
| Reverse | 5’-CCAGTGCAGGGTCCGAGGTATT-3’ |
Plasmid construction and transfection
The 5’-flanking regions of the pre-miR-125b-5p and 3’ UTR sequences of MKK7 containing the putative miR-125b target sites were isolated using specific PCR primers. For luciferase assays, the MKK7 3’ UTR sequence was inserted into the pGL3 luciferase reporter vector (Madison, USA). Target sites were mutated using the QuickChange Site-Directed Mutagenesis (Santa Clara, USA). The miR-125b mimics and the miR-125b inhibitor were synthesized by (Genepharma, Shanghai, China). The sequences used are shown in Table 2. Transfection of cells was performed using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. The cells were added and cultured in complete medium. Cells in the logarithmic growth phase were seeded in 12-well plates at a density of 1×105 cells, and were divided into three groups: miR-125b inhibitor group (transfected with anti-miR-125b), scramble group (transfected with small interfering RNA negative control), and blank group (untransfected cells). Three wells were set for each group. The transfection concentration was 100 nmol/l, and 6 h after transfection, the medium was replaced with normal medium. After cultured for 24 h, DMEM containing 10% FBS and G418 was added for selection, and clones were obtained after two weeks of screening, followed by culturing of the clones for the subsequent experiments.
Table 2.
The sequences of RNAs
| Name | Sense (5’-3’) | Anti-sense (5’-3’) |
|---|---|---|
| has-mircoRNA 125b mimic | UCCCUGAGACCCUAACUUGUGA | ACAAGUUAGGGUCUCAGGGAUU |
| has-mircoRNA 125b inhibitor | UCACAAGUUAGGGUCUCAGGGA | |
| has-mircoRNA 125b inhibitor NC | CAGUACUUUUGUGUAGUACAA | |
| has-mircoRNA 125b mimic NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Cell proliferation and migration assays
For cell proliferation assays, cells were plated at individual wells of a 96-well plate (1500 per well) and examined 48 h later after transfection using the cell counting Kit-8 (CCK7) (Dojindo, Shanghai, China). Optical density (OD) values were determined at a wavelength of 450 nm. For the migration assays, a transwell chamber was placed in a 24-well cell culture plate. Following suspension and dilution of serum-free medium, 5×104 cells were inoculated in each chamber containing a volume of 200 µl, and 600 µl complete medium was added to the lower chamber. After several hours of incubation, cells adhering to the lower membrane were stained with CCK (Shanghai Qiaoxing Trading Corporation, Shanghai, China) for 10 min, and under an optical microscope (magnification, ×200), eight fields of view were randomly selected to perform the averaged cell count. Two independent experiments were carried out in triplicate.
Luciferase reporter experiments
The pGL3-MKK7-3’ UTR-WT/MUT vector was co-transfected with control plasmid or miR-125b-expressing plasmid into U226 or RPMI-8226 cells using Lipofectamine 2000 (Thermo Fisher Scientific,Waltham, MA, USA). Firfly and Renilla luciferase activities were measured consecutively 24 h after transfection using the Dual-luciferase assay kit.
Flow cytometric analysis of the cell cycle
The cells in each group were collected at 24, 48, and 72 h following transfection, and cold PBS was used to wash cells three times. The cells were resuspended in 500 µl pre-cooled binding buffer, and the concentration was adjusted to 5×106 ml. Then, 100 µl of the cell suspension was added to flow cytometry tubes and 5 µl Annexin V-fluorescein isothiocyanate (Beyotime Institute of Biotechnology, Jiangsu, China) was added. Following mixing, the samples were incubated at room temperature in the dark for 15 min, and 5 min prior to the measurements, 5 µl 10 mg/l propidium iodide (PI) dye (Beyotime Institute of Biotechnology, Jiangsu, China) was added. Flow cytometry (FACScan; BD Biosciences, NJ, USA) was used to determine the cell cycle distribution. Each sample was repeated three times. Cell Quest FCS 3.0 software (BD Biosciences, NJ, USA) was used for data analysis.
Western blotting
Whole cell protein extracts were prepared from MM cell lines. The cells in the logarithmic growth phase and with 80% confluency were collected, and radio immunoprecipitation assay lysis buffer containing protease inhibitors (Beyotime Institute of Biotechnology, Jiangsu, China) was used for conventional extraction of total cellular protein. A bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) was used for protein quantification. After separated by 10% SDS-PAGE, the proteins were transferred to a nitrocellulose membrane (Beyotime Institute of Biotechnology, Jiangsu, China). Then 5% skim milk was added, and the membranes were agitated in a sealed container for 1 h. The membranes were incubated overnight at 4°C using the following antibodies: anti-MKK-7 (1:300), and anti-JNK (1:1000), anti-p-JNK (1:500) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Anti-β-actin (1:200, Abcam, Cambridge, MA) was used as a loading control. After washed with phosphate-buffered saline with Tween 20 (PBS-T) for 3 times, the membranes were incubated with secondary antibodies (1:2000; Santa Cruz Biotechnology, Dallas, USA) for 2 h at room temperature. Then, the membranes were developed with enhanced chemiluminescence detection kit (Sigma-Aldrich, St. Louis, USA) for imaging. Image lab (Bio-Rad, Hercules, USA) software was used to acquire and analyze imaging signals.
Statistical analysis
Each experiment was performed at least three times and values are presented as mean ± s.d. Student’s test and two-tailed test were used for analysis using SPSS 19.0 software (SPSS Inc, Chicago, IL, USA). P values less than 0.05 was considered as statistically significant.
Result
miR-125b was over-expressed in MM plasma and cell lines
In order to evaluate miR-125b expression condition in MM plasma and cell lines, qRT-PCR was performed. It was found that the plasma level of miR-125b was upregulated in patient MM cells, compared with the healthy control subjects (Figure 1A). Consistent with these data, the expression of miR-125b in the U266, MM1s, and RPMI-8226 MM cell lines was also significantly increased compared with normal healthy bone marrow-derived plasma cells (Figure 1B). These results confirmed that miR-125b was up-regulated in MM plasma and cell lines, and suggested its important roles in MM development.
Figure 1.

Determination of miR-125b expression in human multiple myeloma (MM) cell lines and clinical samples via qRT-PCR. A. qRT-PCR detection of miR-125b in plasma cells derived from bone marrow of healthy donors (Normal) and MM patients. B. Expression of miR-125b in the MM cell lines MM.1S, U266 and RPMI-8226, compared to normal plasma cells (nPCs). Each sample was analyzed in triplicate and error bars represent standard deviation. *P < 0.05.
miR-125b regulated the proliferation and migration of MM cells
To investigate the effect of miR-125b on cell growth, miR-125b inhibitor and the corresponding negative control were synthesized and transfected into MM cells, respectively.Cell proliferation was determined by CCK8. As shown in Figure 2A, the viability of U266 and RPMI-8226 cells was inhibited by the transfection of miR-125b inhibitor. Furthermore, we determined whether miR-125b inhibitor could inhibit the migration of MM cells in a trans-well assay. Consistent with CCK8 assays, trans-well assay also demonstrated that the migration rate of cells transfected with miR-125b inhibitor was inhibited (Figure 2B). Flow cytometry showed that 48 h following transfection of U266 and RPMI-8226 cells with miR-125b inhibitor, the apoptotic rates were (11.7±1.97%) in the U266 group and (8.97±1.56%) in the RPMI-8226 group (Figure 2C). Compared with the two control groups, the apoptotic rate in the miR-125b inhibitor group was significantly higher (P < 0.05) (Figure 2D). The findings indicated that miR-125b regulated the proliferation and migration of MM cells.
Figure 2.
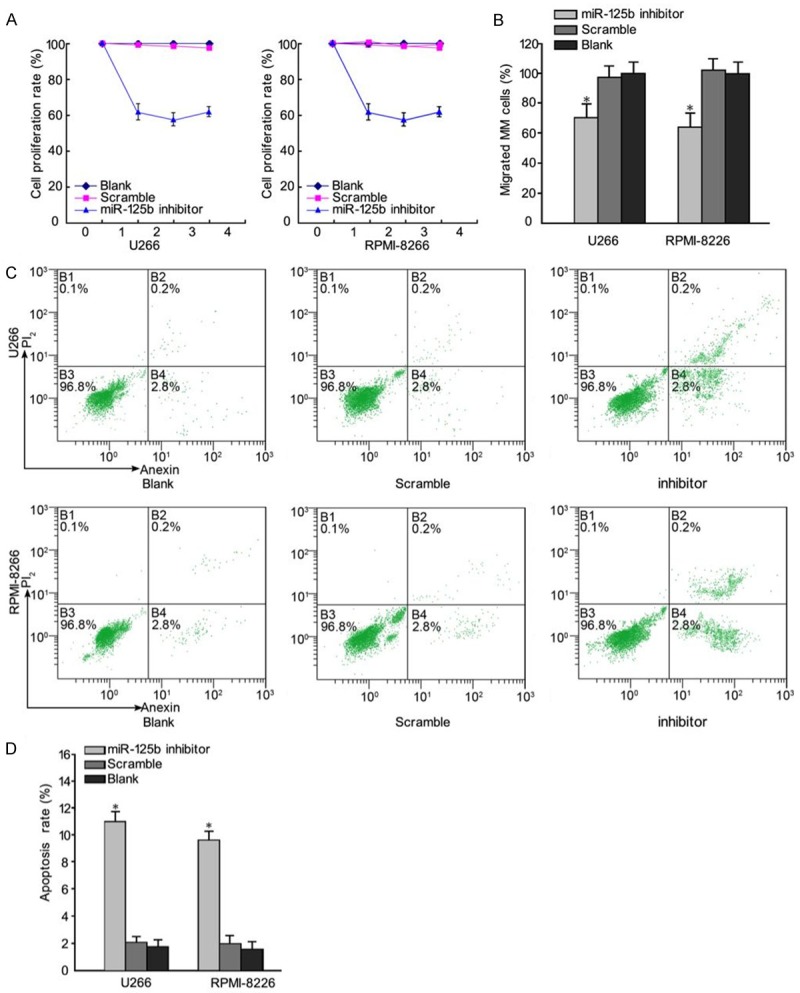
Regulatory effects of miR-125b inhibitor on MM cell proliferation and migration. A. The CCK7 assay showed that U266 and RPMI-8226 cells transfected with miR-125b inhibitor grew slower than cells transfected with a control (Scramble) and blank cells. B. Transwell migration assays of U266 and RPMI-8226 cells were performed after transfection with miR-125b inhibitor, a negative control (Scramble) and blank cells. Cells transfected with miR-125b inhibitor exhibited significantly lower motility than control-treated cells and blank. C. Cell cycle analysis. D. Compared with the two control groups, the apoptotic rate in the miR-125b inhibitor group was significantly increased. Data represent means ± SD (n = 3). *P < 0.05.
MKK7 was directly targeted by miR-125b
In order to further explore the mechanisms by which miR-125b regulated MM cell growth and metastasis, candidate targets of miR-125b was identified using TargetScan program. Among the identified genes, we chose to investigate MKK7 as a possible target of miR-125b (Figure 3A). To demonstrate that miR-125b binds to the 3’ UTR of MKK7, we performed a vector containing the 3’ UTR of MKK7. As expected, miR-125b directly bound to MKK7 3’ UTR, by which it remarkably reduced luciferase activities whereas cells with mutant MKK7 3’ UTR displayed much higher luciferase activities (Figure 3B). Moreover, our RT-PCR results demonstrated that MKK7 mRNA levels were reduced in U266 and RPMI-8226 MM cells infected with miR-125b, and increased in MM cell lines infected with antagomiR-125b (Figure 3C). In consistent with these results, miR-125b decreased the protein of MKK7 in U266 and RPMI-8226 MM cells (Figure 3D). The results suggested that miR-125b could regulate MKK4 in MM cells.
Figure 3.
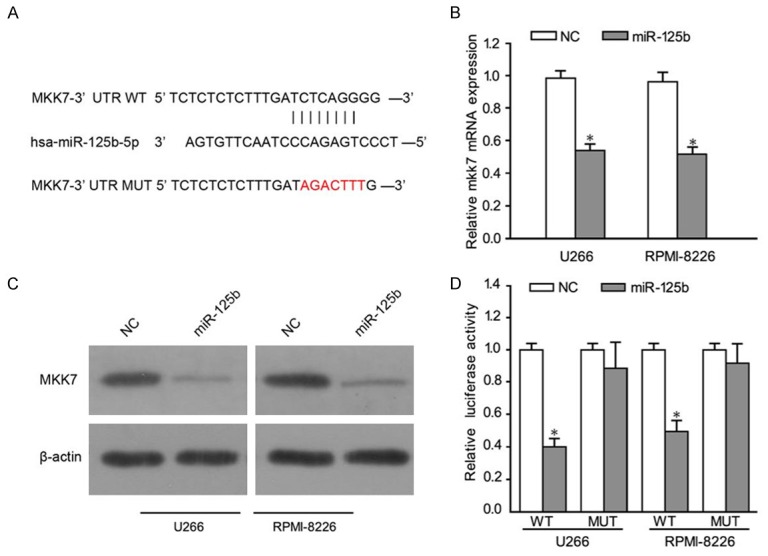
miR-125b targets MKK7 by binding to its 3’ UTR. A. The wild-type binding sites and corresponding mutated sequences for miR-125b within MKK7 3’ UTR. B. qRT-PCR analysis of MKK7 expression in U266 and RPMI-8226 cells. C. Western blot analysis of MKK7 expression in the indicated cells. D. Luciferase reporter assays in U266 and RPMI-8226 cells. A firefly luciferase construct containing either the WT or mutant target site (MUT) of MKK7 3’ UTR was generated and transfected as described in Materials and methods. Luciferase activities were analyzed as the relative activity of firefly to Renilla. Data represent means ± SD (n = 3). *P < 0.05.
The effect of miR-125b on MKK7/JNK signal pathway
MKK7 is involved in activating JNK signaling pathway to induce cell apoptosis. As MKK7/JNK signal pathway is important in many aspects of cancer development, including metastatic processes, we evaluated the effect of miR-125b on JNK molecules involved in the MKK7/JNK signal pathway. The phosphorylation pattern of JNK was studied by western blot analysis. The results demonstrated that cells transfected with miR-125b inhibited the JNK expression (Figure 4).
Figure 4.
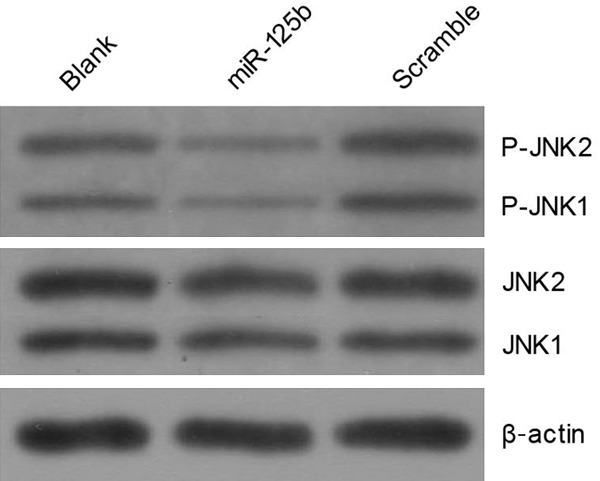
miR-125b inhibits the MKK7/JNK signal pathway in multiple myeloma cell lines.
Discussion
Precious studies have shown that miR-125b plays essential roles in gastric cancer [18], liver cancer [19], and acute myeloid leukemia [20], indicating that miR-125b may function as either tumor suppressor or oncogene. Due to the function of miRNAs in cancers, therapeutic modulation of miRNAs might be a valuable strategy for cancer treatment. We have shown that miR-125b was upregulated in MM plasma and cells. However, the underling mechanism for miR-125b in MM is still unknown.
In this study, we firstly reported that miR-125b might function as oncogene and identified its targets in MM. In addition, we quantified the expression level of miR-125b both in tumor plasma and in cell lines and found that endogenous miR-125b expression was relatively high in MM samples when compared with the noncancerous samples. Based on the endogenous expression level of miR-125b, we chose U266 and RPMI-8226 for subsequent loss-of-function studies. Our results showed that the viability of U266 and RPMI-8226 cells was marked inhibited by the transfection of miR-125b inhibitor. In addition, we also found thatcells transfected withmiR-125b inhibitor could inhibit the migration and promote the apoptosis of MM cells. These data suggested that up-regulation of miR-125b in MM was related to the development and progressionof the disease. Additionally, by using luciferase reporter experiments, MKK7 was identified as the potential downstream target of miR-125b in MM.
MKK7, is identified as a tumor suppressor gene, plays critical roles in tumors migration, invasion, and metastasis [21]. MKK7 activates the JNK signaling pathway, and the activated JNK in turn phosphorylates a variety of target proteins [22]. In this study, we found that the expression of MKK7 decreased after over expression of miR-125b, suggesting that MKK7 can be regulated by miR-125b. These data were further confirmed by a luciferase activity assay. Our results showed that MKK7 was a direct target of miR-125b. The luciferase reporter assay disclosed that miR-125b binds efficiently to the predicted binding site within the jagged 3’ UTR. MKK7 and JNK protein levels were further demonstrated to be markedly decreased by miR-125b overexpression in MM. These findings suggested that the molecular mechanism by which miR-125b regulates the proliferation and migration of MM cells may partly be attributed to the suppression of JNK pathway.
In conclusion, we reported that miR-125b regulated the proliferation and migration of MM cells by targeting MKK7. Our study provided novel insights on the regulation of MKK7 by miRNAs in the development and progress of MM. Moreover, our findings could offer the rationale for the design of novel miRNA based therapeutic strategies leading to MKK7 activation in MM.
Acknowledgements
This study was supported byInternational Collaboration Fund from National Science and Technology Committee of China (No. 2011DFA32820). Innovation fund project in Jiangxi province (No. YC2016-B018). The National Natural Science Fund Project (No. 81460037).
Disclosure of conflict of interest
None.
References
- 1.Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle RA, Caers J, Hillengass J, San Miguel J, van de Donk NW, Einsele H, Bladé J, Durie BG, Goldschmidt H, Mateos MV, Palumbo A, Orlowski R. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127:2955–62. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdi J, Chen G, Chang H. Erratum: Drug resistance in multiple myeloma: latest findings and new concepts on molecular mechanisms. Oncotarget. 2015;6:7364. doi: 10.18632/oncotarget.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43:732–41. doi: 10.1016/j.exphem.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau P, LeLeu X, Hulin C, Klein SK, Sonneveld P, Siegel D, Bladé J, Goldschmidt H, Jagannath S, Miguel JS, Orlowski R, Palumbo A, Sezer O, Rajkumar SV, Durie BG International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–57. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro A, Díaz T, Tovar N, Pedrosa F, Tejero R, Cibeira MT, Magnano L, Rosiñol L, Monzó M, Bladé J, Fernández de Larrea C. A serum microRNA signature associated with complete remission and progression after autologous stem-cell transplantation in patients with multiple myeloma. Oncotarget. 2015;6:1874–83. doi: 10.18632/oncotarget.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Zheng P, Guo H, Li G, Han S, Luo F, Liu Y. PSMB4 promotes multiple myeloma cell growth by activating NF-kappaB-miR-21 signaling. Biochem Biophys Res Commun. 2015;458:328–33. doi: 10.1016/j.bbrc.2015.01.110. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Guo Y, Yu J, Qi J, Shi W, Wu X, Ni H, Ju S. miRNA-202 in bone marrow stromal cells affects the growth and adhesion of multiple myeloma cells by regulating B cell-activating factor. Clin Exp Med. 2016;16:307–16. doi: 10.1007/s10238-015-0355-4. [DOI] [PubMed] [Google Scholar]
- 10.Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci U S A. 2007;104:19983–8. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bousquet M, Quelen C, Rosati R, Mansat-De Mas V, La Starza R, Bastard C, Lippert E, Talmant P, Lafage-Pochitaloff M, Leroux D, Gervais C, Viguié F, Lai JL, Terre C, Beverlo B, Sambani C, Hagemeijer A, Marynen P, Delsol G, Dastugue N, Mecucci C, Brousset P. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t (2;11) (p21;q23) translocation. J Exp Med. 2008;205:2499–506. doi: 10.1084/jem.20080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu F, Yang L, Lu X, Chen J, Wu D, Wei Y, Nong Q, Zhang L, Fang W, Chen X, Ling X, Yang B, Zhang X, Zhou Y, Lu J. The MKK7 p. Glu116Lys rare variant serves as a predictor for lung cancer risk and prognosis in Chinese. PLoS Genet. 2016;12:e1005955. doi: 10.1371/journal.pgen.1005955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SY, Iordanov M, Zhang Q. c-Jun NH2-terminal kinase promotes apoptosis by down-regulating the transcriptional co-repressor CtBP. J Biol Chem. 2006;281:34810–5. doi: 10.1074/jbc.M607484200. [DOI] [PubMed] [Google Scholar]
- 14.Schramek D, Kotsinas A, Meixner A, Wada T, Elling U, Pospisilik JA, Neely GG, Zwick RH, Sigl V, Forni G, Serrano M, Gorgoulis VG, Penninger JM. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet. 2011;43:212–9. doi: 10.1038/ng.767. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J, Lv M, Li X, Li Y, Ma Y, Shen B, Zhang J. Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogenactivated protein kinase kinase 7 activity. Hepatology. 2013;57:140–51. doi: 10.1002/hep.25978. [DOI] [PubMed] [Google Scholar]
- 16.Song IS, Jun SY, Na HJ, Kim HT, Jung SY, Ha GH, Park YH, Long LZ, Yu DY, Kim JM, Kim JH, Ko JH, Kim CH, Kim NS. Inhibition of MKK7-JNK by the TOR signaling pathway regulator-like protein contributes to resistance of HCC cells to TRAIL-induced apoptosis. Gastroenterology. 2012;143:1341–51. doi: 10.1053/j.gastro.2012.07.103. [DOI] [PubMed] [Google Scholar]
- 17.Davies CC, Harvey E, McMahon RF, Finegan KG, Connor F, Davis RJ, Tuveson DA, Tournier C. Impaired JNK signaling cooperates with KrasG12D expression to accelerate pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:3344–56. doi: 10.1158/0008-5472.CAN-13-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu JG, Wang JJ, Jiang X, Lan JP, He XJ, Wang HJ, Ma YY, Xia YJ, Ru GQ, Ma J, Zhao ZS, Zhou R. MiR-125b promotes cell migration and invasion by targeting PPP1CA-Rb signal pathways in gastric cancer, resulting in a poor prognosis. Gastric Cancer. 2015;18:729–39. doi: 10.1007/s10120-014-0421-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, Cao N, Fu CJ, Yan XL, Jia YL, Wang JX, Zhao AH, Li ZW, Li YH, Xie XY, Zhang XM, Dong Y, Xu YC, He LJ, Yue W, Pei XT. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62:801–15. doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 20.Vargas Romero P, Cialfi S, Palermo R, De Blasio C, Checquolo S, Bellavia D, Chiaretti S, Foà R, Amadori A, Gulino A, Zardo G, Talora C, Screpanti I. The deregulated expression of miR-125b in acute myeloid leukemia is dependent on the transcription factor C/EBPalpha. Leukemia. 2015;29:2442–5. doi: 10.1038/leu.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HJ, Su CC, Lu HF, Yang JS, Hsu SC, Ip SW, Wu JJ, Li YC, Ho CC, Wu CC, Chung JG. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol Rep. 2010;23:665–70. [PubMed] [Google Scholar]
- 22.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]


