Abstract
MicroRNAs (miRs), acting as tumor suppressor or oncogenes genes, play a critical role in controlling tumor invasion, metastasis and survival via regulating a variety of targets. MiR-148a has been observed low expressed in several types of human cancers, and overexpression of miR-148a inhibits tumorigenesis. However, the molecular mechanisms of miR-148a-mediated these effects are largely elusive. Therefore, the aim of this study was to evaluate the biological function and molecular insight on miR-148a mediated roles in breast cancer cell. In the present study, we demonstrated that low miR-148a expression was observed in breast cancer cells compared to the normal human breast cells. Transfection with miR-148a inhibited growth, migration, invasion, and induced apoptosis in MDA-MB-231 cells. Ubiquitin-specific protease 4 (USP4) and BIM was the potential target of miR-148a. Indeed, miR-148a overexpression decreased expression of USP4 and increased BIM expression. Additionally, we revealed that miR-148a exerts its pro-apoptotic functions through upregulation of BIM, and miR-148a exerts its anti-invasive functions through downregulation of USP4. We therefore suggested that miR-148a is a tumor suppressor, which could be a promising therapeutic target for breast cancer.
Keywords: Breast cancer, metastasis, apoptosis, microRNA-148a, ubiquitin-specific protease 4, BIM
Introduction
Breast cancer, originating from healthy mammary gland cells, is a malignant tumor. It is the most frequent among women aged between 50 and 70 years [1]. Despite the advances that have been made in the treatment of breast cancer, the major cause of death from breast cancer continues to be metastasis. Up to date, the underlying mechanisms of metastasis for breast cancer are not completely understood, further investigation of this mechanism is urgently needed.
MicroRNAs (miRs) are tiny, regulatory RNA molecules, approximately 18-25 nucleotides in length that post-transcriptionally modulate gene expression [2]. These miRs can regulate multiple genes, resulting in either translational repression or degradation of mRNAs [3]. miRNAs are now widely believed to play an essential role in many malignancies, acting as either tumor suppressors or oncogenes [4-6].
Low miR-148a expression has been reported in tissues of human breast cancer and undifferentiated gastric cancer [7,8]. Low miR-148 expression was also identified in human cancer cell lines established from metastatic lymph nodes of colon, melanoma, and head and neck cancer, suggesting that low miR-148a is responsible for the development of metastasis [9]. However, the function and targeting genes of miR-148a in breast cancer cells have not yet been documented.
It has been reported that Ubiquitin-specific protease 4 (USP4) was over-expressed in metastatic breast cancers as compared to the normal breast samples [10]. Targeting USP4 inhibited TGF-β-induced migration in vitro in breast cancer MDA-MB-231 cells, and in vivo in a zebrafish xenograft invasion and metastasis model [10]. Previous study has found that mir-148a could suppress tumor metastasis and invasion by regulating specific targeted USP4 [11].
Bcl-2-like protein 11 (BIM) has been identified as a critical pro-apoptotic factor in solid tumors [12]. Kim et al. found that over-expression of mir-148a could suppress BIM expression, resulting in the inhibition of glioma apoptosis. On the contrary, down- regulation of mir-148a could induce BIM expression and promoting cell apoptosis [13].
In the present study, we investigated effects of miR-148a on invasion and apoptosis breast cancer cells. Our results demonstrated that miR-148a plays multiple tumor suppressive roles. We identified USP4 as a direct target of miR-148a, which mediate invasion suppressive effect of miR-148a in breast cancer cells. In addition, we also identified BIM as a direct target of miR-148a, which mediate pro-apoptosis effect of miR-148a in breast cancer cells. We thus provided evidence that miR-148a has potentially therapeutic target for treatment of breast cancer.
Materials and methods
Cell line and culture
MDA-MB-231, MCF-7, FCY20202 human breast cancer cells and MCF10A normal breast cell were maintained in RPMI 1640 medium (Biochrom, Shanghai, China) and substituted with 10% FCS, 2 mmol Lglutamine (Gibco, Invitrogen, Hangzhou, China), 6 ng/ml insulin (Gibco; Invitrogen Corp, Hangzhou, China), 3.75 ng/ml hydrocortisone (Sigma, Shanghai, China). All the cells were cultured in a humidified chamber with 5% CO2 at 37°C. All experiments used logarithmically growing cells.
MiR148a transfection
MiR-148a mimics (miR-148a) from GenePharma was used for the overexpression. Negative-control mimics (GenePharma) was transfected as matched controls (NC). Primer sequences were as follows: miR-148a, 5’-CUCAGUAGGGAAGACCUUUGCU-3’ (sense) and 5’-GAAAUGGCUUCUCCCUAAGGUU-3’ (antisense); control mimics (NC), 5’-UUCGUCCAACGUGACUCUGTT-3’ (sense) and 5’-ACGAGUCACGUGUCGAAGATT-3’ (antisense); MDA-MB-231 were seeded in six-well plates, grown to 50-80% confluence. Cells were transfected with miR-148a and negative control (NC) at a final concentration of 50 nM were accomplished with Lipofectamine 2000 (Invitrogen, Hangzhou, China), following the manufacturer’s protocol. For stable miR-148a transfection, we infected cells for 24 h and added 0.1% puromycin into medium 72 h after transfection.
USP4 cDNA and BIM siRNA transfection
The pcDNA3.1 and pCDNA3.1-USP4 cDNA (USP4 cDNA) plasmids was constructed in our laboratory [14]. pcDNA3.1 or (USP4 cDNA) plasmids BIM siRNA or control siRNA were transiently transfected into MDA-MB-231 cells for 72 h using Lipofectamine 2000 according to the manufacturer’s instructions. Gene expression were confirmed by western blot analysis.
RNA extraction and real-time quantitative PCR
Total miRNA was extracted from cells in different groups using a mirVana miR Isolation Kit (Ambion, Shanghai, China) according to the manufacturer’s instructions. cDNA was systems using a TaqMan MicroRNA Reverse Transcription Kit (Shanghai, China). MiR-148a expression was quantified using a miR-specific TaqMan miR Assay Kit (Applied Biosystems). Real-time PCR (qRT-PCR) was performed using the Applied Biosystems 7500 Sequence Detection System. miR-148a expression was quantified based on the comparative threshold (Ct); the relative miR-148a expression levels were calculated as 2-(Ct miR-148a-Ct U6) after normalization with reference to the quantification of U6 small nuclear RNA expression.
Western blot analysis
Cells in different groups were washed with ice-cold PBS. The whole cell extracts were prepared from whole cell lysates. Total protein concentration was measured. Cell lysates containing 30 ug total protein were analyzed by western blot assay using primary antibodies: anti-USP4 and anti-BIM. Chemoluminescent detection (Upstate, Lake Placid, NY) was done in accordance with the manufacturer’s instructions.
MTT assay
MDA-MB-231 cells were seeded in six-well plates. When the cells were 50-80% confluence, the cells were transiently transfected with miR-148a or NC, or co-transfected with miR-148a and BIM siRNA. After transfection for 72 hs, 50 μl MTT (Sigma-Aldrich Corp., Hangzhou, China) in PBS was added to each well. After 3 h of incubation, the viability of cultured MDA-MB-231 cells was determined by MTT assay as the manufacture’s instruction.
Clonogenic assay
A total of 2,000 cells each (MDA-MB-231 cells, stable miR-148a transfected MDA-MB-231 cells or NC transfected MDA-MB-231 cells were seeded into 100-mm plates in triplicates. 14 days later, the cells were fixed with 4% paraformaldehyde and stained with crystal violet for 30 min. The number of colonies with diameters of more than 1.5 mm were counted.
Flow cytometric analysis
The cells were seeded in 6-well plates at 30-50% confluency. After transient transfection of MDA-MB-231 cells with miR-148a, or NC, or co-transfected with miR-148a and BIM siRNA or control siRNA for 72 h, the cells were stained using the annexin V FITC Apoptosis detection kit (Beijing Biosea Biotechnology Co., Ltd., Beijing, China), and analyzed by flow cytometry according to the manufacture’s instruction. Data analysis was performed using WinMDI 2.9 software (Scripps Research Institute, La Jolla, CA).
Migration and invasion assays
The stable miR-148a transfected MDA-MB-231 cells or NC transfected MDA-MB-231 cells at a density of 3×104 cells/well were seeded in the upper chambers in serum-free media. The lower chamber was filled with 20% FBS as chemoattractant. To detect the effect of USP4 in miR-148a induced invasion, the stable miR-148a transfected MDA-MB-231 cells or NC transfected MDA-MB-231 cells were transfected with USP4 cDNA or control plasmid for 48 h, then the cells were seeded in the upper chambers in serum-free media. The lower chamber was filled with 20% FBS as chemoattractant. The invading cells were fixed with 4% paraformaldehyde (Santa Cruz, USA), and stained with a fluorescent dye. Fluorescence was measured with ~480 nm excitation wavelength and ~560 nm emission wavelength.
Statistical analysis
Student’s t-test was used to assess significance of differences among the different groups. Differences were expressed as mean ± SD. with P values <0.05 being considered as statistically significant.
Results
MiR-148a was down-regulated in breast cancer cells
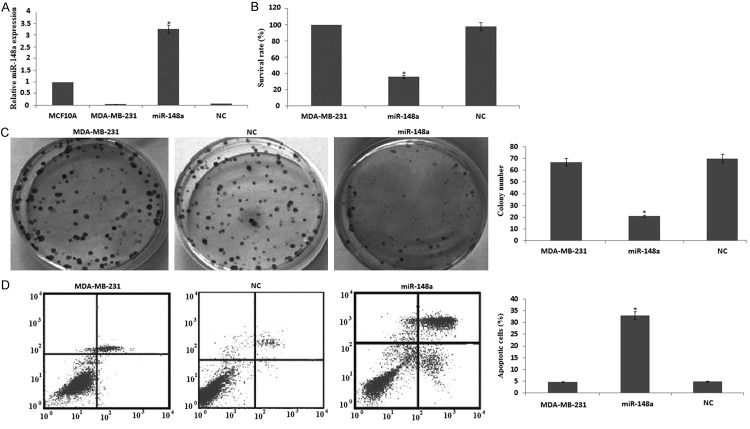
We investigated expression levels of miR-148a in MDA-MB-231, MCF-7, FCY20202 human breast cancer cells and MCF10A normal breast cell. miR-148a expression was significantly decreased in breast cancer cells, and miR-148a was significantly increased in MCF10A normal breast cell (Figure 1). Of the three breast cancer cells, MDA-MB-231 cells had the lowest levels of miR-148a expression, we used MDA-MB-231 cells for further study.
Figure 1.
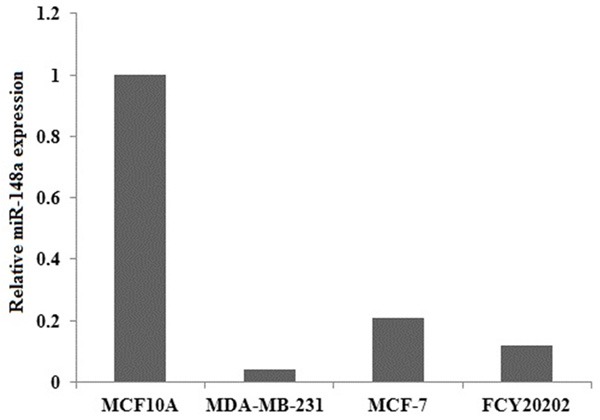
MiR-148a expression levels in breast cancer cell lines. Total RNA containing miRNA was extracted from MDA-MB-231, MCF-7, FCY20202 human breast cancer cells and MCF10A normal breast cells and subjected to quantitative RT-PCR for miR-148a. Data are expressed as mean ± S.D. (n=3).
Expression of miR-148a inhibited MDA-MB-231 cell growth
MDA-MB-231 cells were transfected with miR-148a or NC for 72 hs, miR-148a expression was significantly increased by qRT-PCR assay (Figure 2A). MTT assay revealed that miR-148a significantly inhibited cell growth 3 days after transfection (Figure 2B). Colony formation assay showed the number of colonies formed was reduced in MDA-MB-231/miR-148a cells compared with NC/MDA-MB-231 cells (Figure 2C). These results suggest that miR-148a inhibits cell growth of MDA-MB-231 cells. Next, we examined whether the inhibition of cell growth was also accompanied by the induction of apoptosis induced by miR-148a.
Figure 2.
Effects of miR-148a on cell growth and apoptosis in MDA-MB-231 cells. MDA-MB-231 cells were transfected with miR-148a or NC for 72 hrs. A. miR-148a expression was detected by quantitative RT-PCR assay; B. Viable cell number was counted 3 days after transfection using MTT assay. C. miR-148a or NC stably transfected cells were seeded onto 6-well plates and cultured for 14 days. After fixation and staining, colonies were counted. D. Cell apoptosis was detected by flow cytometry using the Annexin V-FITC apoptosis detection kit. Data are expressed as mean ± S.D. (n=3). *P<0.05, compared with cells transfected with a negative controls.
Expression of miR-148a induces MDA-MB-231 cell apoptosis
MDA-MB-231 cells were transfected with miR-148a or NC for 72 hs. After treatment, the apoptotic cells were measured. The results showed that the induction of apoptosis was significantly increased with miR-148a transfection (Figure 2D). These results demonstrated that miR-148a could induce apoptosis in MDA-MB-231 cells. To further understand the molecular mechanism of miR-148a-induced apoptosis in MDA-MB-231 cells, the cell survival pathway was investigated.
Apoptosis-enhancing effect of miR-148a is mediated through activating BIM
MDA-MB-231 cells were transfected with miR-148a or NC for 72 hs. BIM protein was significantly increased by western blot assay (Figure 3A). We co-transfected miR-148a and BIM siRNA or control siRNA into MDA-MB-231 cells for 72 h, and detected BIM protein expression, survival and apoptosis. We found that co-transfected miR-148a and BIM siRNA or control siRNA blocked BIM protein expression (Figure 3A), reversed survival (Figure 3B) and inhibited apoptosis (Figure 3C) in BIM siRNA -treated cells. These results provide mechanistic support in favor of our claim that the apoptosis-inducing effect by miR-148a is partly mediated through the activation of BIM.
Figure 3.
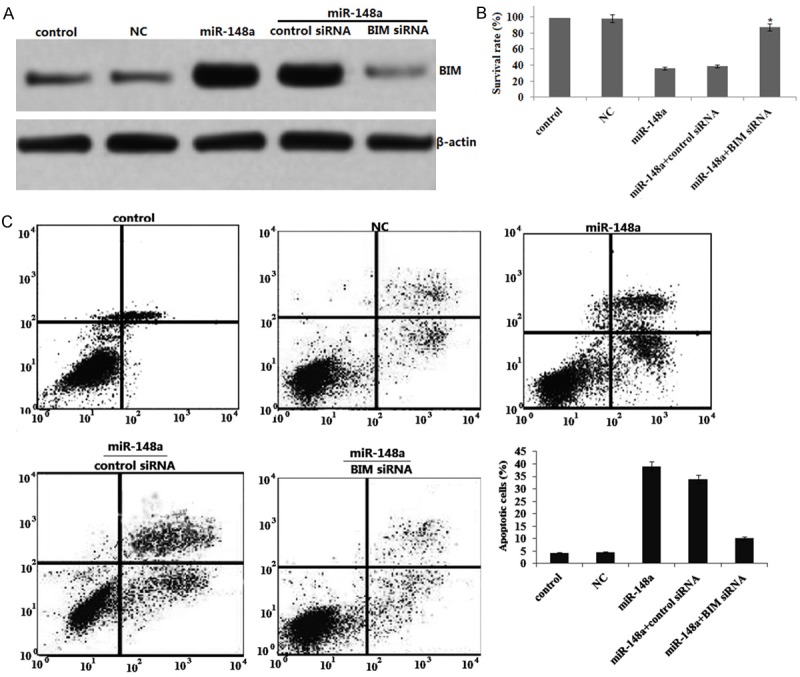
Targeting BIM inhibits miR-148a-induced apoptosis and survival inhibition in MDA-MB-231 cells. MDA-MB-231 cells were transfected with miR-148a or NC or co-transfected miR-148a and BIM siRNA or control siRNA for 72 hrs. A. BIM expression was detected by western blot assay; B. Viable cell number was counted 3 days after transfection using MTT assay. C. Cell apoptosis was detected by flow cytometry using the Annexin V-FITC apoptosis detection kit. Data are expressed as mean ± S.D. (n=3). *P<0.05, compared with cells transfected with a negative controls.
MiR-148a inhibits migration and invasion in MDA-MB-231 cells
It has been recently reported that miR-148a inhibits cell motility of cell lines established from lymph node metastasis of colon, melanoma, and head and neck cancer [9]. Here we examined whether ectopic expression of miR-148a could inhibit cell migration and invasion of MDA-MB-231 cells using the Matrigel Invasion Chamber. The number of invaded and migrated cells were significantly decreased in MDA-MB-231 cells transfected with miR-148a precursor compared with those transfected with a negative control precursor (Figure 4A, 4B). These results suggest that ectopic expression of miR-148a attenuates cell migration and invasion of MDA-MB-231 cells.
Figure 4.
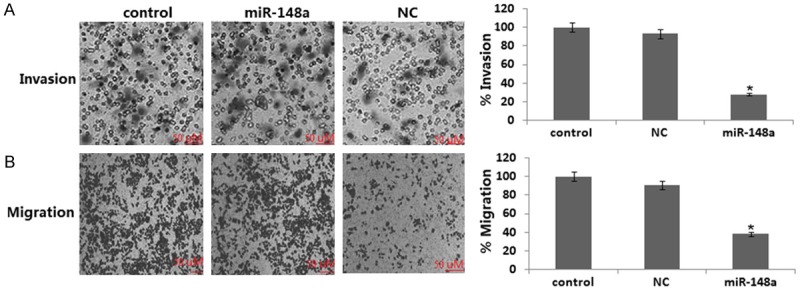
Effects of ectopic expression of miR-148a on cell migration and invasion in MDA-MB-231 cells. Transwell migration (A) and Matrigel invasion (B) assays for miR-148a-infected MDA-MB-231 at 24 h seeding. Data are expressed as mean ± S.D. (n=3). **, P<0.05, compared with cells transfected with a negative control precursor.
MiR-148a inhibits migration and invasion by inactivation of USP4
MiR-148a decreased cell migration and invasion (Figure 4) and miR-148a inhibited USP4 protein expression (Figure 5A). We wished to further confirm whether miR-148a inhibits migration and invasion by inactivation of USP4. We next transiently infected USP4 cDNA or NC to the stable miR-148a transfected MDA-MB-231 cells for 24 h. We ensured that the expression of USP4 was restored in miR-148a transfected MDA-MB-231 cells after USP4 cDNA transfection by western blot assay (Figure 5A). In addition, the levels of cell migration and invasion in stable miR-148a transfected MDA-MB-231 cells infected by USP4 cDNA were significantly higher than those infected by the miR-148a alone (Figure 5B, 5C). These data collectively suggest that during inhibition of cell migration and invasion, miR-148a down-regulates the levels of USP4.
Figure 5.
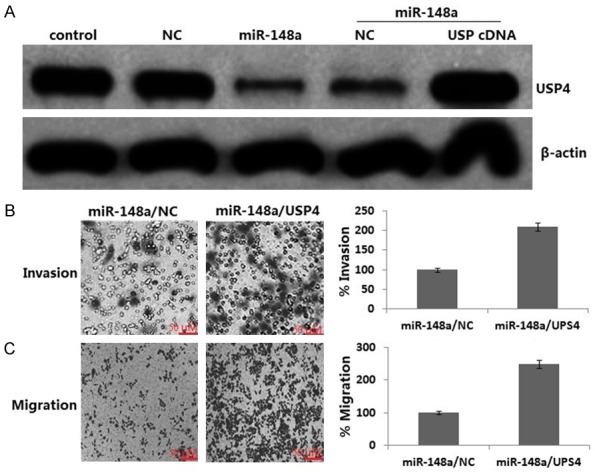
Inactivation of USP4 is necessary for miR-148a-induced inhibition of cell migration and invasion. USP4 cDNA or NC was transiently transfected into the stable miR-148a transfected MDA-MB-231 cells for 24 h. A. Western blotting analysis of USP4 in MDA-MB-231 cells; B. Transwell migration; C. Matrigel invasion. *P<0.05 for Student’s t-test.
Discussion
Many studies have confirmed that miR-148a acts as tumor suppressor in different cancers. Downregulation of miR-148a stimulates tumor cell motility via activating the targets like Wnt10B in cancer-associated fibroblasts [15]. miR-148a is also known to be a potential prognostic biomarker in gastric cancer [16,17]. In human pancreatic ductal adenocarcinoma cells, miR-148a regulates cell survival by targeting CDC25B [18], and promotes apoptosis in colorectal cancer by targeting BCL2 [19], and inhibits cell motility in vitro and reduces tumor growth and inhibition of metastasis formation in xenograft models in head and neck cancer SIHN-011B cells [9]. In our study, we demonstrated that miR-148a was lowly expressed in breast cancer cells, and ectopic expression of miR-148a resulted in indusion of apoptosis and inhibition of growth, and migration and invasion in MDA-MB-231 cells, suggesting that dysregulation of miR-148a expression may contribute to metastatic potential of MDA-MB-231 cells.
BIM play an essential role in inhibiting survival of cancer cells by inducing cell apoptosis [12]. Recently, it has reported that miR-148a directly targets and upregulates BIM [13]. In the present study, we found that overexpression of miR-148a induced BIM upregulation, followed by increased apoptosis and decreased cell survival. However, targeting BIM could reverse the effect of miR-148a. It is suggested that the effects of miR-148a on cell growth and apoptosis may be mediated in part by up-regulation of BIM in MDA-MB-231 cells.
USP4 is overexpressed in various types of cancer and the increased levels of USP4 appear to play a functional role in malignant cells [20,21]. For example, USP4 promotes tumorigenesis when overexpressed in mice, suggesting an oncogenic potential for USP4 [21]. MiR-148a could suppress tumor metastasis and invasion by regulating specific targeted genes including HPIP [22], matrix metalloproteinase-7 (MMP-7) [23], ubiquitin specific protease 4 (USP4) [11], HOX transcript antisense RNA (HOTAIR) [24], OKI and S-Phase Kinase-Associated Protein 1 (SKP1) [25]. In the present study, we found that miR-148a overexpression inhibited UPS4 expression, followed by decreased cell invasion and metastasis. However, USP4 overexpression could reverse the effect of miR-148a.Therefore, miR-148a-mediated migration and invasion may be partly through down-regulation of USP4 in MDA-MB-231 cells.
In conclusion, we found for the first time that miR-148a markedly inhibited USP4 expression and upregulated BIM expression, leading to decreased cell invasion and increased cell apoptosis in MDA-MB-231 cells. Without a doubt, further in-depth investigation is necessary to fully elucidate the molecular insight on miR-148a in human breast cancer cells. In summary, our findings reveal the critical role of miR-148a in cell invasion and survival and indicate that upregulation of miR-148a may be a promising novel approach for the treatment of breast cancer.
Disclosure of conflict of interest
None.
Authors’ contribution
ZL and WHB designed and evaluating the manuscript. ZL and CWH performed the transfection, FCM assay and MTT assay. WHB performed the western blot assay. ZL wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Alphandéry E. Perspectives of breast cancer thermotherapies. J Cancer. 2014;5:472–479. doi: 10.7150/jca.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croce CM, Calin GA. MiRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.O’Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali AS, Ali S, Ahmad A, Philip PA, Sarkar FH. MicroRNAs in cancer invasion and metastasis. In: Cho WCS, editor. MicroRNAs in cancer translational research. 1st edition. New York, NY, USA: Springer; 2011. pp. 389–413. [Google Scholar]
- 6.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, Kreipe H. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J Pathol. 2008;214:17–24. doi: 10.1002/path.2251. [DOI] [PubMed] [Google Scholar]
- 8.Katada T, Ishiguro H, Kuwabara Y, Kimura M, Mitui A, Mori Y, Ogawa R, Harata K, Fujii Y. MicroRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–542. [PubMed] [Google Scholar]
- 9.Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, Gallagher WM, Eccles SA, Croce CM, Esteller M. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, ten Dijke P. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol. 2012;14:717–726. doi: 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- 11.Heo MJ, Kim YM, Koo JH, Yang YM, An J, Lee SK, Lee SJ, Kim KM, Park JW, Kim SG. MicroRNA-148a dysregulation discriminates poor prognosis of hepatocellular carcinoma in association with USP4 overexpression. Oncotarget. 2014;5:2792–2806. doi: 10.18632/oncotarget.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bean GR, Ganesan YT, Dong Y, Takeda S, Liu H, Chan PM, Huang Y, Chodosh LA, Zambetti GP, Hsieh JJ, Cheng EH. PUMA and BIM are required for oncogene inactivation-induced apoptosis. Sci Signal. 2013;6:ra20. doi: 10.1126/scisignal.2003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Zhang Y, Skalski M, Hayes J, Kefas B, Schiff D, Purow B, Parsons S, Lawler S, Abounader R. MicroRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to regulate EGFR and apoptosis in glioblastoma. Cancer Res. 2014;74:1541–1553. doi: 10.1158/0008-5472.CAN-13-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao WH, Liu XP, Meng SL, Gao YW, Wang Y, Ma ZL, Wang XG, Wang HB. USP4 promotes invasion of breast cancer cells via Relaxin/TGF-β1/Smad2/MMP-9 signal. Eur Rev Med Pharmacol Sci. 2016;20:1115–22. [PubMed] [Google Scholar]
- 15.Aprelikova O, Palla J, Hibler B, Yu X, Greer YE, Yi M, Stephens R, Maxwell GL, Jazaeri A, Risinger JI, Rubin JS, Niederhuber J. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene. 2013;32:3246–3253. doi: 10.1038/onc.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Song Y, Wang Z, Yue Z, Xu H, Xing C, Liu Z. Altered expression of miR-148a and miR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg. 2010;14:1170–1179. doi: 10.1007/s11605-010-1202-2. [DOI] [PubMed] [Google Scholar]
- 17.Guo SL, Peng Z, Yang X, Fan KJ, Ye H, Li ZH, Wang Y, Xu XL, Li J, Wang YL, Teng Y, Yang X. MiR-148a promoted cell proliferation by targeting p27 in gastric cancer cells. Int J Biol Sci. 2011;7:567–574. doi: 10.7150/ijbs.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liffers ST, Munding JB, Vogt M, Kuhlmann JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA, Tannapfel A. MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest. 2011;91:1472–1479. doi: 10.1038/labinvest.2011.99. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Li Y, Huang Q, Ren X, Hu H, Sheng H, Lai M. MiR-148a promotes apoptosis by targeting Bcl-2 in colorectal cancer. Cell Death Differ. 2011;18:1702–1710. doi: 10.1038/cdd.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao N, Li H, Luo J, Wang R, Chen H, Chen J, Wang P. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem J. 2012;441:979–986. doi: 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 21.Luna-Vargas MP, Faesen AC, van Dijk WJ, Rape M, Fish A, Sixma TK. Ubiquitin-specific protease 4 is inhibited by its ubiquitin-like domain. EMBO Rep. 2011;12:365–372. doi: 10.1038/embor.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K, Ding L, Zhang H, Cheng L, Fu H, Song Y, Jiang Y, Liu J, Wang R, Du N, Ye Q. Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis. J Clin Invest. 2013;123:630–645. doi: 10.1172/JCI64265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto N, Naito Y, Oue N, Sentani K, Uraoka N, Zarni Oo H, Yanagihara K, Aoyagi K, Sasaki H, Yasui W. MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and indicates tumor invasiveness and poor prognosis. Cancer Sci. 2014;105:236–243. doi: 10.1111/cas.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao S, He H, Chen Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J Transl Med. 2015;13:131. doi: 10.1186/s12967-015-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Pan JQ, Luo L, Ning XJ, Ye ZP, Yu Z, Li WS. NF-κB induces miR-148a to sustain TGF-β/Smad signaling activation in glioblastoma. Mol Cancer. 2015;14:2. doi: 10.1186/1476-4598-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]