Abstract
Objective: To study the effects of angiopoietin-like 2 (Angptl2) on atherosclerotic calcification in aortic artery of ApoE-/- mice. Methods: Twelve 6-week-old male mice were randomly divided into control group (n=6) and interventional group (n=6), the control group were fed with high fat diet and the interventional group were fed with high fat diet and at the eighth week interventional group mice were infused (intravenously) with purified recombinant Angptl-2 once a week for one month. All mice were sacrificed when the mice were 16 weeks old, blood was collected and plasma triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDLC) were measured, aortic sections were stained with hematoxylin and eosin (HE) or Von Kossa and were observed under microscope. Calcium content and alkaline phosphatase activity of aorta were measured to measure the degree of vascular calcification. The expressions of Runx2 protein and mRNA levels in aortic sections of mice were detected by immunohistochemistry, Western Blot and qRT-PCR respectively. Results: The plasma TG, TC and LDLC level in interventional group was significantly higher than that in control group and the expression of Runx2 in aortic had the similar results. HE staining demonstrated significant thickening of the intima, with typical atherosclerotic plaque formation in interventional group mice, and Von Kossa staining showed spotty black clumps of aortic calcification under the fibrous cap plaque, while control group had atherosclerotic plaques without significant calcium deposits formation; The quantitative analysis showed that aortic vascular wall calcium and alkaline phosphatase activity were significantly higher in the intervention group than that of the control group (P<0.01). Conclusions: Angptl-2 could increase ApoE-/- mice plasma lipid level, it also facilitate the expression of Runx2, calcium content and ALP activity in aortic and then accelerate atherosclerotic calcification. Our experiments demonstrated that Angptl2 could accelerate atherosclerotic calcification. It reminded us that by controlling or decreasing the Anglt-2 level in plasma could help inhibit atherosclerotic calcification and then provides a new target to prevent coronary heart disease.
Keywords: Angiopoietin-like 2, ApoE-/- mice, intimal calcification, atherosclerosis
Introduction
Angiosteosis may reduce the compliance of diabetes, nephrosis and blood vessels of elderly people, as well as increase mortality. Some scholars believed that the level of arterial calcification is one of the optimal index for predicting cardiovascular mortality [1]. Current studies believe that inflammatory reaction plays an important role in the development of atherosclerosis and even the formation of angiosteosis [2]. However, the specific physiopathologic mechanism of angiosteosis is yet to be illuminated thoroughly. Angiopoietin-like proteins 2 (Angptl-2) is a member of the recently discovered angiopoietin-like proteins family. As a species of circulatory glycoproteins, Anpgtl2 is widely distributed in tissues and organs, including cardiovascular system, lung as well as kidney, etc. [3]. Fafhat et al [4] reported that Angptl2 is capable of inducing leukocytes and macrophages to gather on the vascular wall, increasing the level of cholesterol circulating in the blood so that the formation of atheromatous plaque is quickened during the development of atherosclerosis. Documents reporting whether Angptl2 promotes the development of atherosclerotic intimal calcification as a powerful pro-inflammatory factor are yet to be found. The purpose of this paper is to study the impacts of Angptl2 on the atherosclerotic intimal calcification of ApoE-/- mice.
Materials and methods
Main materials
Twelve 6-week-old male ApoE-/- mice were purchased from the Department of Laboratory Animal at Peking University Health Science Centre (batch number: SCXK(BJ)2011-00012). High-fat fodder (15% fat, 1.25% cholesterol, 0.5% cholic acid) was bought from Jiangsu Xietong Medical Bio-engineering Ltd. Recombinant Angptl2 were sourced from R&D in the United States. Trizol mRNA extract was from Sangon company. Runx2 (core-binding factor α1) antibodies were provided by Proteintech Group. Reverse transcription kits were bought from TaKa Ra company. PCR upstream and low-stream primers were synthesized by Shanghai Generay Co., Ltd. Goat-anti-rabbit-HRP marked secondary antibodies were purchased from CW Biotech. Von Kossa staining kits were from Shanghai Genmed. Lastly, calcium ion detection kits, protein quantification kits and alkaline phosphatase testing kits were all sourced from BioAssay Systems.
Laboratory animal grouping and sample acquisition
The mice were fed in a specific-pathogen-free barrier, where they ate and drank freely with temperatures between 19 and 22°C, as well as relative humidity between 50% and 70%. The mice were adaptive my fed with ordinary fodder for one week prior to the introduction of high-fat feed. They were randomly divided into two groups, namely the treatment (n=6) and the control group (n=6). While being fed with high-fat fodder, the mice of the treatment group were given intravenous injections of recombinant human Angptl2 100 μg during the eighth week, whereas the control group was intravenously injected with normal saline 100 μg. Both injections were operated once a week, for four weeks in a row. During the 16th week of the experiment, all mice had to fast for 12 hours and then had abdominal anesthesia with 0.5-1.0 mL 1% pentobarbital. Blood was taken from their eye sockets afterwards. Following a 3 kr/min centrifuge that lasted for ten minutes, serum was separated and the mice were given euthanasia. The vessels from the aortic root to the aortic arch were taken out. Some of the aortas were fixed with 10% neutral formalin after the adipose tissues on the outer membrane were removed. Such aortas were then wrapped with paraffin and sliced, being used for the chemical analysis of HE staining and immunologic tissues. The other aortas were placed in clean EP tubes and frozen at -70°C for Western blot as well as qRT-PCR.
Testing the lipid levels of ApoE-/- mouse serum
After taken blood from the eye sockets of the ApoE-/- mice, they were then left static under room temperature for one hour. Subsequently, they were centrifuged in a speed of 3 kr/min at 4°C for 10 minutes. Afterwards, the mouse serum was separated. The levels of triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDLC) and low density lipoprotein cholesterol (LDLC) of the mouse serum were tested with BECKMANCOULTE R Au2007.
HE and Von Kossa staining on the aortas
Blood vessels of the mouse aortas were fixed with 10% neutral formalin and wrapped with paraffin for serial sections. After routine dewaxing and dehydration, hematoxylin and eosin (HE) staining, the blood vessels were placed under the light microscope for observation as well as photography. Von Kossa staining: the paraffin wrapped sections of mouse aorta were dewaxed and dehydrated. They were then placed into the 2% silver nitrate buffer in accordance with the user instruction. After being exposed directly to strong sunshine for 40 minutes, the vessels were washed three times with distilled water. As soon as the distilled water was all dried up, photographic fixing took place with 5% sodium sulphate for 2 minutes. Afterwards, the blood vessels were washed with distilled water again for 3 minutes and restained with neutral magenta for another 3 minutes. Finally, having been dehydrated, transparentised and sealed, the blood vessels were placed under light microscope to be observed for the deposition of calcium salt in the aorta.
Content of calcium and the activity of alkaline phosphatase in mouse aorta tissues
Measuring the content of calcium: approximately 5 mg aorta tissues that had been stored at -70°C were dried and placed into 1 mmol/L diluted hydrochloric acid at 37°C to be decalcified for 12 hours. Following that, the content of calcium was measured according to the instruction of the kit. The protein content was measured by BCA. Eventually, the calcium content was standardized by the protein content.
The measurement of alkaline phosphatase (ALP) activity: approximately 5 mg aorta tissues were extracted and made into homogenate. Next, mixed it with 12000 g phosphate as well as centrifuged for 10 minutes. Following that, the supernatant was extracted for the quantified test of protein with BCA kit. The remaining was used to test the ALP activity based on the instruction of the kit. Finally, the ALP activity was standardized by the protein content.
Expression of Runx2 determined by qRT-PCR and Western blot
The carotid artery frozen at -70°C was ground on ice and had its total RNAs extracted. The RNAs were then amplified according to the manufacturer’s instruction. The upstream primer of Runx2 was 5’-AAG TGT TCT GTG GTCTCT GAG TTGA-3’, and the low-stream primer was 5’-GCT GTA TGGTGA GGC TGG TAGG-3’. The upstream primer of GAPDH was 5’-GGT GAA GGT CGG TGT GAA CG-3’, whereas the low-stream primer was 5’-CTC GCT CCT GGA AGA TGG TG-3’. The reaction condition was: pre-denatured at 95°C for 30 s, and then at 95°C for 5 s, as well as 58°C for 30 s, cycling 40 times in a row. Each group repeated such cycle for 6 times. The result was analyzed by 2-ΔΔCt.
The carotid artery frozen at -70°C was ground on ice and had its total proteins extracted by ultrasound. The proteins were quantified by Bradford method. After loading 50 μg of the sample, the rest was placed into 10% polyacrylamide gel for electrophoresis. The proteins were transferred onto a PVDF film and sealed for 1 hour with 5% skimmed milk. Subsequently, TBST diluted rabbit-anti-mouse polyclonal antibodies Runx2 (1:1000) and β-actin (1:2000) were added in and vibrated at room temperature for 4 hours. Afterwards, the mixture was vibrated and washed with TBST for 15 minutes and three times in a row. As the washing was completed, goat-anti-rabbit secondary antibodies marked with HRP (1:1000) were mixed in and incubated on the table concentrator at room temperature for 1 hour. Again, they were washed with TBST for 15 minutes and three times in a row. Finally, exposure liquids A and B were evenly mixed in a 1:1 ratio and dropped on the PVDF film. The ELC coloration system was used for fixing and color rendering as well as observing stripes.
Expression of Runx2 detected by immunohistochemical method
After conventionally dehydrating the paraffin sections and repairing the antigens, 5% BSA confining liquid was added in, following by 30-minute incubation at room temperature. Next, rabbit-anti-mouse Runx2 monoclonal antibodies (1:300) were mixed in and placed at 4°C overnight. HRP marked secondary antibodies were added in and incubated at 37°C for 40 minutes before the addition of drops of DAB color-substrate solution. After restraining with hematoxylin, 1% hydrochloric acid solution was utilized for differentiating the sections. It was then dehydrated with gradient ethanol and dried. After being transparentised with dimethylbenzene, the sections were sealed and placed under the light microscope to be observed. The immunohistochemical result was identified based on both the positive cell density and the immunity coloration intensity. Five power fields were randomly selected for each section, with 100 cells in each field counted, so that the positive cell percentage of Runx2 could be calculated. If positive cells were less than 5%, the score would be 0; 5%-25% scored 1; 26%-49% scored 2; 50%-75% scored 3; and more than 75# scored 4. Coloration intensity scores ranged from 0 to 3: 0 for non-computation within cells; 1 for light yellow; brownish yellow for 2, and brown for 3. By adding up two scores, four levels were derived, namely negative (-) for 0-1, weak positive (+) for 2-3, medium positive (++) for 4-5, strong positive for over 5.
Statistical analysis
All measurement data were presented as x±s and analyzed using SPSS 16.0 software. Both groups were comparatively tested with independent sample t, P<0.05, indicating that the difference was statistically significant.
Results
Blood lipid levels of mice
The contents of TC, TG and LDLC in the serum of the treatment group mice, which were treated with Angptl2 significantly increased in comparison with the control group. Such difference was statistically significant (P<0.05, Table 1).
Table 1.
Levels of TC, TG, LDLC, HDLC in each group (x±s)
| TC (mmol/L) | TG (mmol/L) | HDLC (mmol/L) | LDLC (mmol/L) | |
|---|---|---|---|---|
| Control Group | 24.91±2.80 | 2.50±0.17 | 5.62±0.29 | 15.87±1.24 |
| Intervention Group | 35.50±1.18 | 3.35±0.26 | 5.39±0.27 | 20.16±1.76 |
HE and Von Kossa staining on aorta
After HE staining on the aorta, thickening was identified on the intima of the ApoE-/- mice of the control group. Also, atherosclerosis occurred and projected towards the vessel lumen, the size of which shrank (Figure 1A). In terms of the treatment group, the intima thickening was more significant, accompanied by a large number of atherosclerotic plaques. Numerous foam cells were discovered in the plaques and the lumen was more obviously narrow (Figure 1B). The result of Von Kossan staining showed that the intima and plaques of the mice in the control group contain a little calcified deposition (Figure 2A), whereas black residue was obviously seen from the mouse intima of the treatment group, with more significant black calcified deposition within the plaques (Figure 2B).
Figure 1.
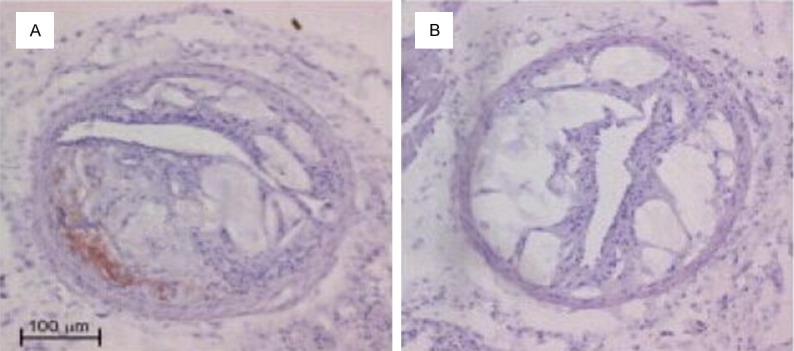
Hematoxylin and eosin staining in ApoE-/- mice aorta (×200).
Figure 2.
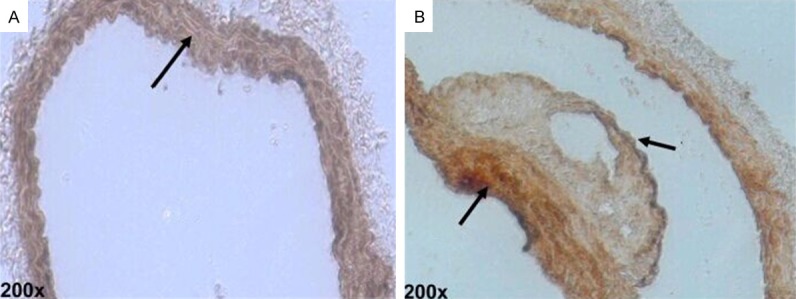
Representative photomicrographs of calcium deposition in mice aorta after Von Kossa staining (black calcium particles).
Effects of Angptl-2 on the calcium content and alkaline phosphatase activity of mouse aorta
In comparison with the control group, the calcium content of mouse aorta of the treatment group increased by 9.22 times (P<0.05), and the alkaline phosphatase activity increased by 8.58 times (P<0.05, Table 2).
Table 2.
The levels of calcium content activity of alkaline phosphatase in different groups
| Calcium content (μmol/g) | ALP Activity (U/g) | |
|---|---|---|
| Control Group | 101.12±6.97 | 152.42±5.37 |
| Intervention Group | 932.18±92.57 | 1307.60±48.16 |
Difference in the expression of Runc2 in the vascular plaques of mice
The outcomes of immunohistochemical staining revealed that there was a small amount of Runx2 expression in the vascular wall and plaques of the control group mice. By contrast, in the mice treated with Angotl2, the Runx2 expression of the vascular wall significantly increased (P<0.05, Figure 3).
Figure 3.
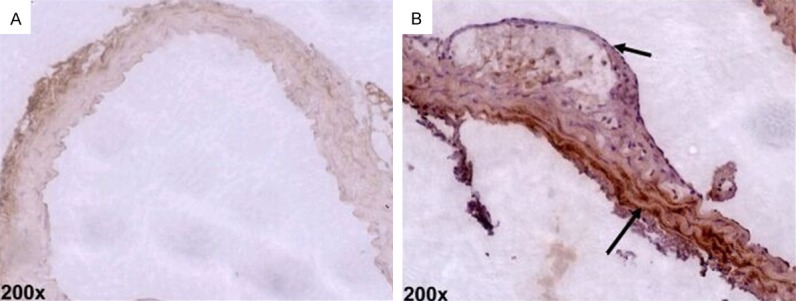
Runx2 expression in aortic plaque by immunohistochemical analysis (×200, n=6).
Expression of Runx2 mRNA and protein in the vascular plaques of mice
The results of qRT-PCR showed that micet hat were intravenously injected with Angptl2 had an increase in their Runx2 mRNA expressions (P<0.05). In addition, the outcomes of Western Blot also demonstrated that the Runx2 protein expression in the aorta of the treated mice were significantly increased, compared to the control group (P<0.05, Figure 4).
Figure 4.
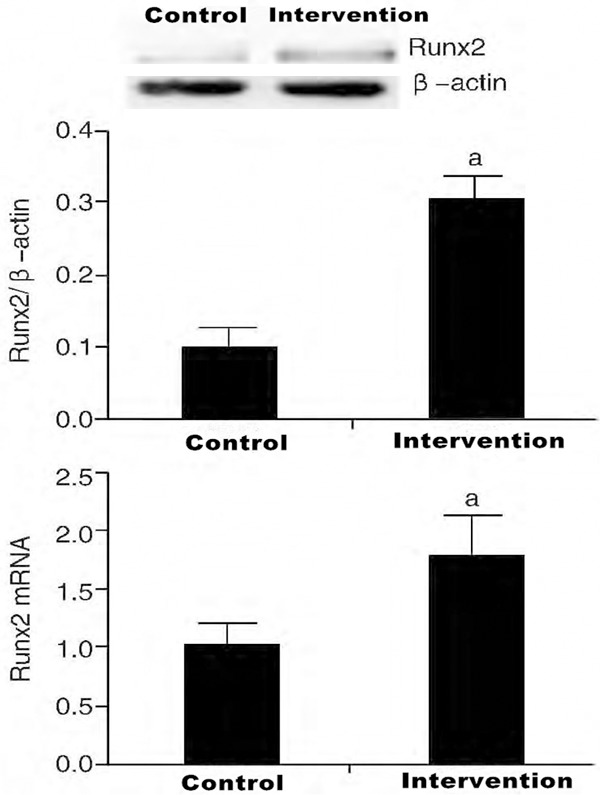
Expression of Runx2 protein and mRNA in aortic artery by intervention of angiopoietin-like 2 (n=6).
Discussion
Angiosteosis is highly correlated to kidney failure, diabetes and advanced ages, etc. Clinically, angiosteosis is found in 80% blood vessel damages and 90% coronary arterial diseases. The stability of the calcified fibrous plaque tends to be relatively weak. Hence, the plaques break easily, largely increasing the probability of acute coronary syndrome (ACS) happening in CHD patients. Furthermore, angiosteosis also drastically enhances the difficulty of operating clinical interventional surgery [5].
At present, it is mostly agreed that angiosteosis is a process of initiative regulation, including the phenotypic switch from vascular smooth muscle cells into osteoblasts and the crystallized deposition of hydroxyl phosphate [6], etc. The increased phosphate, advanced glycation end products, bone morphogenetic proteins, inflammatory factors, apoptotic bodies and the oxidative stress factors of vascular smooth muscle cells in the plasma were all capable of facilitating the phenotypic switch from vascular smooth muscle cells into osteoblasts [7]. ALP is a functional market of osteoblasts. It is not only an important component of matrix vesicle, but also an important enzyme during the formation of calcium salt. During biological calcification, ALP is able to hydrolyse a variety of phosphate ester linkages, as well as increase the concentration of phosphate in local hydroxyapatite crystals, providing the development of calcium phosphate crystallization with raw materials. Moreover, hyper-phosphate can induce cell calcification as well [8]. Runx2 is a factor that plays an important role in the switch from smooth muscle cells into osteo-like cells and the deposition of calcium phosphate. The expression of Runx2 may increase when it is stimulated by inflammatory factors. Also, bone morphogenetic protein 2 (BMP2) can cause an increase in the expression of Runx2 by inducing the transcription factors in the mesenchymal stem cells [9]. Runx2 not only regulates the differentiation of osteoblasts, but also adjusts the osteogenesis of mature osteoblasts by facilitating the synthesis of extracellular matrix proteins [10]. Runx2 proteins worked with other proteins as compounds to influence the response elements of the target genes in order to fulfill their regulation function. With gene knockout technology, Enomoto et al found that the osteoblasts of Runx2-/- mice cannot effectively differentiate, and neither intramembranous nor endochondral ossification happens. The absence of Runx2 prevents the bone loss presented due to the maturity of osteoblasts. A domestic study proves that pioglitazone may inhibit the calcification of vascular smooth muscle cells by inhibiting Runx2 expression [11]. All the above information indicates that Runx2 is essential in bone formation. Multiple researches suggest that angiosteosis is very similar to bone formation, which indirectly proves that Runx2 promotes blood vessel calcification. Previous studies often stressed on inducing angiosteosis with factors like hyper-phosphate, which is affected by the imbalance of phosphate. Hence, the results were bound to be influenced. Atherosclerosis patients tend to develop angiosteosis easily, and the expression of inflammatory factors induced by high fat is often considered as the important cause of blood vessel calcification [12].
This paper induced the development of atherosclerosis by providing ApoE-/- mice with high fat diets. The effects of high fat diets on the calcification of atherosclerotic plaques were observed through Angptl2, which is an inflammatory factor. Results revealed that the ApoE-/- mice developed atherosclerotic plaques after being fed with high fat fodder, and the atherosclerosis became worse after being tested with exogenous Angptl2 [13]. In addition, mice that were treated with exogenous Angptl2 had more significant calcification in the atherosclerotic plaques, accompanied by increases in the calcium contents, ALP activity and Runx2 expression in the blood vessels. This proved that Angptl2 may facilitate the intramembranous calcification of atherosclerotic plaques. The plaques may become unstable as it is calcified; Tazume et al [14] reported that patients with higher levels of Angptl2 in plasma are faced with more risk of encountering cardiovascular accidents, an important factor of which may be the plaque calcification promotion ability of Angptl2. This study stated that Angptl2 not only promotes atherosclerosis, but also facilitates the calcification of atherosclerotic blood vessels. As an inflammatory factor, Angptl2 is capable of significantly promote the development of atherosclerosis in mice. It is mainly secreted by intravascular endothelial cells, but mostly combined with vascular smooth muscle cells [15]. Such physiological characteristic of Angptl2 brings a drastic confusion. Regarding why it is highly compatible with vascular smooth muscle cells and what mechanism it uses to affect the physiological and pathological process of the latter, further exploration is needed to find out the answers. In comparison with stable ones, vulnerable plaques contain more new vessels. Numerous physiopathologic evidence revealed that new vessels in plaques are closely related to the rapid and unstable development of plaques. Some reports claimed that Angpt can promote angiogenesis by activating c-Jun N-terminal kinase (JNK) [16]. This means that facilitating angiogenesis is a crucial reason that Angptl2 promotes the development and deterioration of atherosclerosis [17-19].
The result showed that Angptl2 can increase blood fat levels and hyperlipidaemia is highly correlated to the instability of atherosclerosis [20]. To conclude, Angptl2 is capable of facilitating the development of atherosclerosis. This study proved for the first time that Angptl2 may facilitate atherosclerotic intimal calcification, which also demonstrated how Angptl2 promotes the development of coronary heart disease (CHD) from the perspective of blood vessel calcification. Such conclusion also indicated that Angptl2 may work as a potential biological index for clinically assessing the hazardous degrees of CHD patients with kidney failure, diabetes and older ages, etc. In addition, attempts can be made to control or block the bioactivity of Angptl2 by maintaining and reducing its level in CHD patients. In this way, the development of CHD can be obstructed so that a new strategy for preventing such disease can be developed.
Disclosure of conflict of interest
None.
References
- 1.Yamada S, Tokumoto M, Tatsumoto N, Taniguchi M, Noguchi H, Nakano T, Masutani K, Ooboshi H, Tsuruya K, Kitazono T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am J Physiol Renal Physiol. 2014;306:F1418–428. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 2.Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory control for osteblast growth and differentiation: role of Runx/Cbfa/AML Factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 3.Komori T. Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab. 2003;21:193–197. doi: 10.1007/s00774-002-0408-0. [DOI] [PubMed] [Google Scholar]
- 4.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–230. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 5.Aikawa E, Nahrendorf M, Fiqueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 6.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietinlike protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Farhat N, Thorin-Trescases N, Mamarbachi M, Villeneuve L, Yu C, Martel C, Duquette N, Gayda M, Nigam A, Juneau M, Allen BG, Thorin E. Angiopoietin-like 2 promotes atherogenesis in mice. J Am Heart Assoc. 2013;2:e000–201. doi: 10.1161/JAHA.113.000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297:H802–810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T, Nakashima Y, Kunitomo R, Kaneko Y, Moriyama S, Sakaguchi H, Okamoto K, Hara M, Yoshinaga T, Yoshimura K, Aoki H, Araki K, Hao H, Kawasuji M, Oike Y. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32:1400–409. doi: 10.1161/ATVBAHA.112.247866. [DOI] [PubMed] [Google Scholar]
- 10.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic Intermittent Hypoxia Induces Atherosclerosis via Activation of Adipose Angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tjeerdema N, Georgiadi A, Jonker JT, van Glabbeek M, Alizadeh Dehnavi R, Tamsma JT, Smit JW, Kersten S, Rensen PC. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2:e000034. doi: 10.1136/bmjdrc-2014-000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, Yamabi M, Yano W, Faits T, Yabusaki K, Zhang H, Mlynarchik AK, Inoue K, Mizuno K, Aikawa M. Angiopoietin like Protein 2 (ANGPTL2) promotes adipose tissue macrophage and t lymphocyte accumulation and leads to insulin resistance. PLoS One. 2015;10:e0131176. doi: 10.1371/journal.pone.0131176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novák J, Bienertová-Vašků J, Kára T, Novák M. MicroRNAs Involved in the Lipid Metabolism and Their Possible Implications for Atherosclerosis Development and Treatment. Mediators Inflamm. 2014;2014:275867. doi: 10.1155/2014/275867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotter TN, Li M, Pan Q, Peker D, Rowan PD, Li J, Zhan F, Suva LJ, Javed A, Yang Y. Myeloma cell-derived Runx2 promotes myeloma progression in bone. Blood. 2015;125:3598–3608. doi: 10.1182/blood-2014-12-613968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrokhi E, Samani KG, Chaleshtori MH, Tabatabaiefar MA. Effect of Oxidized Low Density Lipoprotein on the Expression of Runx2 and SPARC Genes in Vascular Smooth Muscle Cells. Iran Biomed J. 2015;19:160–164. doi: 10.7508/ibj.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Human miR-221 /222 in Physiological and Atherosclerotic Vascular Remodeling. Biomed Res Int. 2015;2015:354517. doi: 10.1155/2015/354517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lannoy M, Slove S, Jacob MP. The function of elastic fibers in the arteries: beyond elasticity. Pathol Biol (Paris) 2014;62:79–83. doi: 10.1016/j.patbio.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Lichtman A, Hansson G. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenzie NC, Staines KA, Zhu D, Genever P, Macrae VE. miRNA-221 and miRNA-222 synergistically function to promote vascular calcification. Cell Biochem Funct. 2014;32:209–216. doi: 10.1002/cbf.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbone C, Piro G, Fassan M, Tamburrino A, Mina MM, Zanotto M, Chiao PJ, Bassi C, Scarpa A, Tortora G, Melisi D. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis. Oncotarget. 2015;6:13822–13834. doi: 10.18632/oncotarget.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]


