Abstract
The RNA binding motif protein 5 gene (RBM5) rs2013208 single nucleotide polymorphism (SNP) has been associated with high-density lipoprotein cholesterol (HDL-C) levels in a previous genome-wide association study, but little is known about such association of the RBM5 rs2013208 SNP and serum lipid profiles in the Chinese populations. The present study was to detect the association of the RBM5 rs2013208 SNP and several environmental factors with serum lipid levels in the Jing and Han populations. Genotyping of the RBM5 rs2013208 SNP in 635 subjects of Jing and 648 participants of Han peoples was performed by polymerase chain reaction and restriction fragment length polymorphism, and then confirmed by direct sequencing. There were no significant differences in the genotypic and allelic frequencies of the RBM5 rs2013208 SNP between the two ethnic groups or between males and females. The RBM5 rs2013208G allele carriers had lower serum HDL-C levels in both Jing and Han than the G allele non-carriers. The G allele carriers in Jing had higher serum total cholesterol (TC) levels and higher apolipoprotein (Apo) A1/ApoB ratio than the G allele non-carriers (P < 0.05). Subgroup analysis according to sex showed that the G allele carriers had lower serum HDL-C levels in both Jing and Han females but not in males (P < 0.05). The G allele carriers had higher TC levels in Jing females but not in Jing males, and lower ApoA1/ApoB ratio in Jing males but not in Jing females. Serum lipid parameters were also correlated with several environmental factors in the Jing and Han populations, or in males and females in both ethnic groups. The association of the RBM5 rs2013208 SNP and serum lipid levels is different between the Jing and Han populations. These associations might have an ethnic- and/or sex-specificity.
Keywords: RNA binding motif protein 5 gene, single nucleotide polymorphism, rs2013208, lipids, environmental factors
Introduction
Cardiovascular diseases (CVD) are the leading causes of morbidity and mortality in most developed and developing countries [1,2]. It is well established that dyslipidemia is a major risk factor for CVD among several conventional risk factors such as older age, positive family history, diabetes mellitus, obesity, hypertension, tobacco use, and unhealthy diet [3-5]. Metabolic abnormalities in blood lipids, in particular low-density lipoprotein cholesterol (LDL-C) elevation and high-density lipoprotein cholesterol (HDL-C) depression, are mainly involved in the development and progression of CVD [6,7]. Epidemiological studies have consistently shown that dyslipidemia is a complex trait resulted from the joint effects of multiple genetic and environmental factors [8,9] and their interactions [10]. Through family history and twin studies, almost 40%-70% of the interindividual variation in plasma lipid phenotypes can be explained by genetic polymorphisms [10,11]. Human genetic studies of lipid levels can identify targets for new therapies for cholesterol management and prevention of heart disease [12,13].
Since 2006, genome-wide association studies (GWAS) have implicated numerous common genetic variants and respective proteins in the determination of lipid and lipoprotein levels [12,14]. It was reported that loci associated with blood lipids, accounting for ~10-12% of the total trait variance, and variants with small effects can point to pathways and therapeutic targets that enable clinically-important changes in blood lipids [12,15]. A recent study has identified 157 loci associated with lipid levels at P < 5 × 10-8, including 62 loci not previously associated with lipid levels in humans which has mentioned that the RBM5 rs2013208 SNP was associated with HDL-C levels for the first time [14]. Among the new 62 loci, two SNPs showing strongest association to coronary artery disease (CAD) near RBM5 (rs2013208, P HDL = 9 × 10-12, P CAD = 7 × 10-5) and CMTM6 (rs7640978, P LDL = 1 × 10-8, P CAD = 4 × 10-4) [14]. RBM5 (http://www.ncbi.nlm.nih.gov/gene) is a candidate tumor suppressor gene which encodes a nuclear RNA binding protein that is a component of the spliceosome A complex. The encoded protein plays a role in the induction of cell cycle arrest and apoptosis through pre-mRNA splicing of multiple target genes including the tumor suppressor protein p53. This gene is located within the tumor suppressor region 3p21.3, and may play a role in the inhibition of tumor transformation and progression of several malignancies including lung cancer. However, the biological function of the RBM5 rs2013208 SNP on serum lipid metabolism remains unclear. Importantly, the genetic variation has different magnitudes of effect in the different ethnicities but until now no GWAS has comprehensively investigated the genetic determinants of serum lipid levels in the Chinese populations. Therefore, it would be necessary to characterize the relationship between the RBM5 rs2013208 SNP and serum lipid levels in the Chinese populations.
China is a multiethnic country of 56 ethnic groups, the custom of every ethnic group is not identical. Han is the dominant ethnic group and Jing is a native minority existing 28199 people among the 55 minority groups according to the sixth national census statistics of China in 2010. In the early 16th century, the Jing ancestors emigrated from Vietnam to China, now most of them live in the so called “Three Islands of Jing Nationality”, Dongxing City, Guangxi Zhuang Autonomous Region, People’s Republic of China [16,17]. Jing is unique in Chinese ethnic minorities living in the nation of the sea, the way of life is single. Jing nationality is a relatively conservative and isolated minority, and preserves their custom of intra-ethnic marriage, which suggests that there are lots of differences between Jing and Han (as well as the other landlocked nationalities) nationality in diet custom and culture characteristics. To our knowledge, the association of RBM5 rs2013208 SNP and serum lipid profiles has not been previously reported in this population. Thus, the present study was to detect the association of the RBM5 rs2013208 SNP and serum lipid levels in the Jing and Han populations.
Materials and methods
Subjects
A total of 648 unrelated subjects (245 males, 37.81% and 403 females, 62.19%) of Han nationality and 635 unrelated participants (244 males, 38.43% and 391 females, 61.57%) of Jing nationality were randomly selected from our previous stratified randomized samples. All participants were rural agricultural (Han) and/or fishery workers (Jing) living in Jiangping Down, Dongxing City, Guangxi Zhuang Autonomous Region, People’s Republic of China. The participants’ age ranged from 27 to 92 years with a mean age of 57.44±12.55 years in Han and 56.22±12.99 years in Jing, respectively. All participants were healthy and had no evidence of diseases related to atherosclerosis, CAD and diabetes. None of them were using lipid-lowering medication. The present study was approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University (No: Lunshen-2011-KY-Guoji-001; Mar. 7, 2011). Informed consent was obtained from all participants.
Epidemiological survey
The survey was carried out using internationally standardized methods [18]. A standard questionnaire collecting the information on demographics, socioeconomic status, and lifestyle factors was obtained from all the subjects. The alcohol information included questions about the number of liangs (about 50 g) of rice wine, corn wine, rum, beer, or liquor consumed during the preceding 12 months. Alcohol consumption was classified as groups of grams of alcohol per day: < 25 and ≥ 25. Smoking status was categorized into groups of cigarettes per day: < 20 and ≥ 20. In the physical examination, several parameters such as blood pressure, height, weight, waist circumference were measured, and body mass index (BMI, kg/m2) was calculated from the height and weight measurements.
Biochemical parameter
A fasting venous blood sample of 5 ml was drawn from the participants after an overnight (at least 12 hours) fast. A part of the sample (2 mL) was collected into glass tubes and allowed to clot at room temperature, and used to determine serum lipid levels. Another part of the sample (3 mL) was transferred to tubes with anticoagulate solution (4.80 g/L citric acid, 14.70 g/L glucose, and 13.20 g/L tri-sodium citrate) and used to extract DNA. The levels of total cholesterol (TC), triglyceride (TG), HDL-C and LDL-C in the samples were determined by enzymatic methods with commercially available kits. Serum apolipoprotein (Apo) A1 and ApoB levels were assessed by the immuneturbidimetric immunoassay [19,20].
DNA amplification and genotyping
Genomic DNA was isolated from peripheral blood leukocytes using the phenol-chloroform method. The extracted DNA was stored at -20°C until analysis. Genotyping of the RBM5 rs2013208 SNP was performed by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). PCR amplification was performed using 5’-CTTCGGGATTCACGCTCATC-3’ and 5’-ACTCTAGGCTTGACAAAATGCA-3’ (Sangon, Shanghai, People’s Republic of China) as the forward and reverse primer pairs; respectively. Each amplification reaction was performed in a total volume of 25 μL, containing 10 × PCR buffer (1.8 mM MgCl2) 2.5 μL, 1 U Taq polymerase, 2.5 mmol/L of each dNTP (Tiangen, Beijing, People’s Republic of China) 2.0 μL, 20 pmol/L of each primer and 50 ng of genomic DNA, processing started with 95°C for 7 min and followed by 45 s of denaturing at 95°C, 35 s of annealing at 58°C and 1 min of elongation at 72°C for 30 cycles. The amplification was completed by a final extension at 72°C for 7 min. Then 10 U of BsuRI enzyme was added directly to the PCR products (10 μL) and digested at 37°C overnight. After restriction enzyme digestion of the amplified DNA, genotypes were identified by electrophoresis on 2% ethidium-bromide stained agarose gels and visualizing with ultraviolet illumination. Genotypes were scored by an experienced reader blinded to the epidemiological and lipid results. Six samples (AA, AG and GG genotypes in two; respectively) detected by the PCR-RFLP were also confirmed by direct sequencing. The PCR product was purified by low melting point gel electrophoresis and phenol extraction, and then the DNA sequences were analyzed in Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., People’s Republic of China. The methods of DNA amplification and genotyping were referred to our previous studies [20,21].
Diagnostic criteria
The normal values of serum TC, TG, HDL-C, LDL-C, ApoA1 and ApoB levels, and the ratio of ApoA1 to ApoB in our Clinical Science Experiment Center were 3.10-5.17, 0.56-1.70, 1.16-1.42, 2.70-3.10 mmol/L, 1.20-1.60, 0.80-1.05 g/L, and 1.00-2.50; respectively. The individuals with TC > 5.17 mmol/L and/or TG > 1.70 mmol/L were defined as hyperlipidemia [22,23]. Hypertension was assessed according to the criteria outlined by the 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension [24,25]. The categories of normal weight, overweight and obesity were defined as a BMI of < 24, 24-28 and > 28 kg/m2; respectively. The diagnostic criteria of overweight and obesity were according to the Cooperative Meta-analysis Group of China Obesity Task Force [26].
Statistical analyses
Epidemiological data were recorded on a pre-designed form and managed with Excel software. Data analysis was performed using the software SPSS version 16.0 (SPSS Inc., Chicago, Illinois). Qualitative variables are expressed as raw counts and percentages. Quantitative variables are presented as the mean ± standard deviation, except serum TG levels, which were presented as medians and interquartile ranges. Allele frequency was determined via direct counting, and the standard goodness-of-fit test was used to test the Hardy-Weinberg equilibrium. Difference in genotype distribution between the groups was obtained using the chi-square test. The difference in general characteristics between Jing and Han was tested by the Student’s unpaired t-test. The association between genotypes and serum lipid parameters was tested by analysis of covariance (ANCOVA). Age, sex, BMI, cigarette smoking, and alcohol consumption were adjusted for the statistical analysis. Multivariable linear regression analyses with stepwise modeling were used to determine the correlation between genotypes (AA = 1, AG/GG = 2) and several environmental factors with serum lipid levels in males and females of Han and Jing populations. Two sided P value < 0.05 was considered statistically significant.
Results
General and biochemical characteristics of the subjects
Table 1 shows the general characteristics and serum lipid levels between the Jing and Han populations. The levels of weight, waist circumference, BMI, TG, glucose were higher in Jing than in Han, but the ApoA1/ApoB ratio, the percentages of subjects consuming alcohol, the levels of ApoA1, HDL-C were lower in Jing than in Han (P < 0.05-0.001). The values of gender ratio, age, systolic blood pressure, diastolic blood pressure, pulse pressure, LDL-C, TC, ApoB and the percentages of smoking were not different between the two ethnic groups (P > 0.05 for all).
Table 1.
Comparison of demography, lifestyle and serum lipid levels between the Jing and Han Chinese
| Parameter | Han | Jing | t (x 2) | P |
|---|---|---|---|---|
| Number | 648 | 635 | ||
| Male/Female | 245/403 | 244/391 | 0.052 | 0.820 |
| Age (year) | 57.44±12.55 | 56.22±12.99 | -1.71 | 0.088 |
| Height (cm) | 156.21±7.75 | 156.94±7.73 | 1.68 | 0.093 |
| Weight (kg) | 55.50±9.37 | 57.87±9.89 | 4.42 | 0.000 |
| Body mass index (kg/m2) | 22.67±3.16 | 23.42±3.18 | 4.17 | 0.000 |
| Waist circumference (cm) | 77.24±8.98 | 79.89±8.85 | 5.31 | 0.000 |
| Cigarette smoking [n (%)] | ||||
| Non-smoker | 551 (85.0) | 549 (86.5) | ||
| < 20 cigarettes/day | 23 (3.5) | 19 (3.0) | ||
| ≥ 20 cigarettes/day | 74 (11.4) | 67 (10.6) | 0.600 | 0.741 |
| Alcohol consumption [n (%)] | ||||
| Non-drinker | 543 (83.8) | 563 (88.7) | ||
| < 25 g/day | 23 (3.5) | 39 (6.1) | ||
| ≥ 25 g/day | 82 (12.7) | 33 (5.2) | 25.240 | 0.000 |
| Systolic BP (mmHg) | 132.28±19.25 | 132.13±21.65 | -0.133 | 0.895 |
| Diastolic BP (mmHg) | 80.22±10.06 | 80.22±10.07 | -1.55 | 0.12 |
| Pulse pressure (mmHg) | 51.18±15.15 | 51.91±17.96 | 0.784 | 0.433 |
| Glucose (mmol/L) | 6.65±1.11 | 7.32±0.38 | 14.628 | 0.000 |
| Total cholesterol (mmol/L) | 4.93±0.88 | 4.92±0.94 | -0.229 | 0.819 |
| Triglyceride (mmol/L) | 1.32 (0.63) | 1.44 (0.73) | -3.99 | 0.000 |
| HDL-C (mmol/L) | 1.81±0.51 | 1.76±0.44 | -2.130 | 0.033 |
| LDL-C (mmol/L) | 2.86±0.44 | 2.82±0.45 | -1.724 | 0.085 |
| Apolipoprotein (Apo) A1 (g/L) | 1.32±0.20 | 1.29±0.23 | -2.432 | 0.015 |
| ApoB (g/L) | 1.04±0.24 | 1.05±0.24 | 0.806 | 0.420 |
| ApoA1/ApoB | 1.34±0.38 | 1.30±0.38 | -2.13 | 0.034 |
BP, blood pressure; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B. The quantitative variables were presented as mean ± standard deviation and their difference between the groups was determined by the t-test. The values of triglyceride were presented as median (interquartile range), and the difference between the groups was determined by the Wilcoxon-Mann-Whitney test. The difference in percentage of cigarette smoking and alcohol consumption between the groups was determined by Chi-square-test.
Results of genotyping
After the genomic DNA of the samples was amplified by PCR and imaged by 2% agarose gel electrophoresis, the purpose gene of 379-bp nucleotide sequences was seen in all samples (Figure 1). The genotypes identified were named according to the presence or absence of the enzyme restriction sites. The absence of the cutting site indicates the A allele cannot be cut, while its presence indicates the G allele can be cut. Thus, GG genotype is homozygote for the presence of the site (156- and 223-bp), AG genotype is heterozygote for the presence and absence of the site (156-, 223- and 379-bp), and AA genotype is homozygote for the absence of the site (379-bp; Figure 2).
Figure 1.
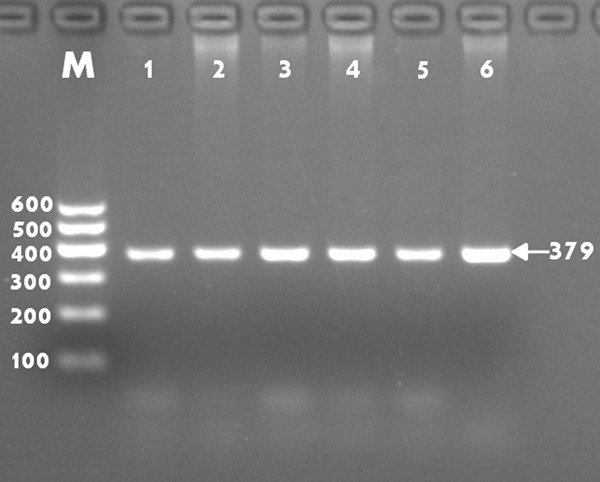
Electrophoresis of PCR products of the samples. Lane M, 100 bp marker ladder; lanes 1-6, samples. The 379 bp bands are the target genes.
Figure 2.
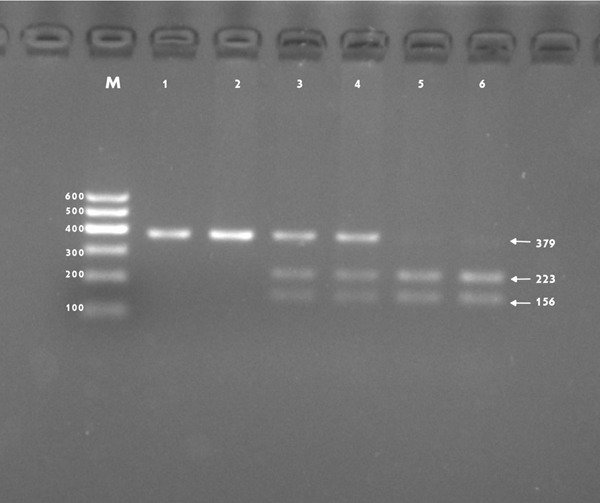
Genotyping of the RBM5 rs2013208 SNP. Lane M is the 100 bp Marker Ladder; lanes 1 and 2, AA genotype (379-bp); lanes 3 and 4, AG genotype (156-, 223- and 379-bp); and lanes 5 and 6, GG genotype (156- and 223-bp).
Nucleotide sequences
The results were separated into AA, AG and GG genotypes of the RBM5 rs2013208 SNP by PCR-RFLP and the genotypes were further confirmed by direct sequencing (Figure 3); respectively.
Figure 3.
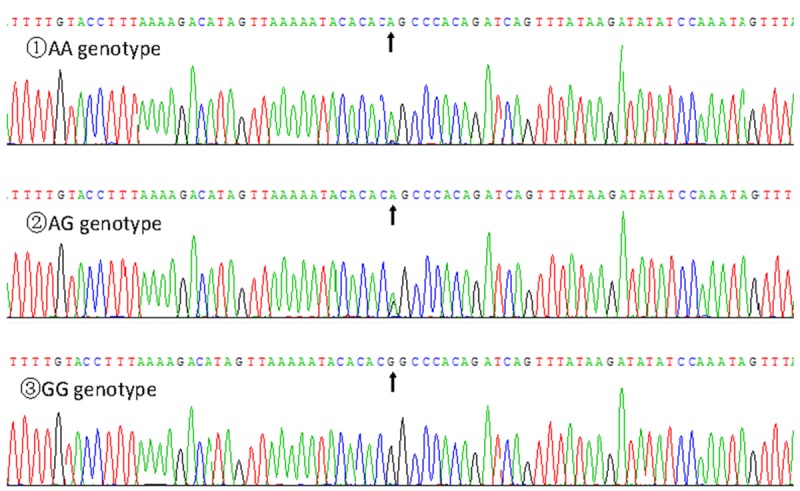
A part of the RBM5 rs2013208 SNP sequence.
Genotypic and allelic frequencies
The genotypic and allelic frequencies of the rs2013208 SNP in the both ethnic groups are shown in Table 2. The frequency of RBM5 rs2013208-G allele was 14.4% in Han and 13.6% in Jing (P > 0.05). There was no significant difference in either genotypic or allelic frequencies between Han and Jing, or between males and females of the both ethnic groups.
Table 2.
Comparison of the genotype and allele frequencies of the RBM5 rs2013208 SNP in the Han and Jing populations [n (%)]
| Group | n | Genotype | Allele | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| AA | AG | GG | A | G | ||
| Han | 648 | 470 (72.5) | 169 (26.1) | 9 (1.4) | 1109 (85.6) | 187 (14.4) |
| Jing | 635 | 477 (75.1) | 143 (22.5) | 15 (2.4) | 1097 (86.4) | 173 (13.6) |
| x2 | 3.587 | 0.346 | ||||
| P | 0.166 | 0.556 | ||||
| Han | ||||||
| Male | 245 | 177 (72.2) | 67 (27.3) | 1 (0.4) | 421 (85.9) | 69 (14.1) |
| Female | 403 | 293 (72.7) | 102 (25.3) | 8 (2.0) | 688 (85.4) | 118 (14.6) |
| x2 | 2.975 | 0.077 | ||||
| P | 0.226 | 0.781 | ||||
| Jing | ||||||
| Male | 244 | 182 (74.6) | 55 (22.5) | 7 (2.9) | 419 (85.9) | 69 (14.1) |
| Female | 391 | 295 (75.4) | 88 (22.5) | 8 (2.0) | 678 (86.7) | 104 (13.3) |
| x2 | 0.445 | 0.180 | ||||
| P | 0.800 | 0.671 | ||||
Genotypes and serum lipid levels
As shown in Tables 3 and 4, the levels of TC and the ratio of ApoA1 to ApoB were different between the AA and AG/GG genotypes in Jing (P < 0.05 for each) but not in Han, the G allele carriers had higher TC levels and ApoA1/ApoB ratio than the G allele non-carriers. The G allele carriers had lower serum HDL-C levels than the G allele non-carriers in both Han and Jing. Subgroup analyses showed that the G allele carriers in Han females but not in Han males had lower HDL-C levels than the G allele non-carriers (P < 0.05). The G allele carriers in Jing females but not in Jing males had higher TC levels than the G allele non-carriers (P < 0.001). The G allele carriers in Jing males but not in Jing females had lower ApoA1/ApoB ratio than the G allele non-carriers (P < 0.05). There was no significant difference in the remaining serum lipid parameters between the genotypes in Jing, Han, males, or females (P > 0.05 for all).
Table 3.
Comparison of the genotypes and serum lipid levels in the Han and Jing populations
| Genotype | n | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ApoA1 (g/L) | ApoB (g/L) | ApoA1/ApoB |
|---|---|---|---|---|---|---|---|---|
| Han | ||||||||
| AA | 470 | 4.93±0.87 | 1.33 (0.62) | 1.84±0.54 | 2.87±0.43 | 1.32±0.20 | 1.03±0.24 | 1.35±0.38 |
| AG/GG | 178 | 4.94±0.91 | 1.30 (0.67) | 1.74±0.43 | 2.86±0.44 | 1.32±0.20 | 1.06±0.25 | 1.31±0.36 |
| F | 0.138 | -0.530 | 3.937 | 0.002 | 1.254 | 1.696 | 1.474 | |
| P | 0.710 | 0.596 | 0.048 | 0.969 | 0.263 | 0.193 | 0.225 | |
| Jing | ||||||||
| AA | 477 | 4.86±0.94 | 1.43 (0.76) | 1.78±0.45 | 2.83±0.44 | 1.29±0.24 | 1.05±0.24 | 1.28±0.37 |
| AG/GG | 158 | 5.11±0.90 | 1.52 (0.66) | 1.67±0.43 | 2.80±0.46 | 1.30±0.18 | 1.04±0.26 | 1.34±0.42 |
| F | 5.586 | -1.214 | 4.984 | 2.136 | 0.001 | 2.010 | 6.376 | |
| P | 0.018 | 0.225 | 0.026 | 0.144 | 0.972 | 0.157 | 0.012 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B. The value of triglyceride was presented as median (interquartile range), the difference between the genotypes was determined by the Wilcoxon-Mann-Whitney test.
Table 4.
Comparison of the genotypes and serum lipid levels between males and females in the Han and Jing populations
| Ethnic/Genotype | n | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ApoA1 (g/L) | ApoB (g/L) | ApoA1/ApoB |
|---|---|---|---|---|---|---|---|---|
| Han/male | ||||||||
| AA | 177 | 4.81±0.86 | 1.32 (0.63) | 1.74±0.53 | 2.83±0.43 | 1.32±0.21 | 1.04±0.23 | 1.34±0.40 |
| AG/GG | 68 | 4.90±0.86 | 1.35 (0.70) | 1.71±0.53 | 2.90±0.45 | 1.31±0.20 | 1.09±0.26 | 1.26±0.35 |
| F | 0.522 | -0.633 | 0.273 | 1.256 | 0.055 | 1.638 | 0.960 | |
| P | 0.471 | 0.527 | 0.602 | 0.264 | 0.815 | 0.202 | 0.328 | |
| Han/female | ||||||||
| AA | 293 | 5.00±0.87 | 1.35 (0.61) | 1.90±0.53 | 2.88±0.44 | 1.33±0.20 | 1.03±0.25 | 1.34±0.36 |
| AG/GG | 110 | 4.97±0.94 | 1.27 (0.67) | 1.76±0.36 | 2.84±0.44 | 1.33±0.19 | 1.05±0.25 | 1.39±0.35 |
| F | 0.089 | -0.167 | 4.351 | 0.441 | 0.287 | 0.411 | 0.209 | |
| P | 0.766 | 0.867 | 0.038 | 0.507 | 0.592 | 0.522 | 0.648 | |
| Jing/male | ||||||||
| AA | 182 | 4.82±0.90 | 1.50 (0.81) | 1.70±0.41 | 2.84±0.41 | 1.26±0.21 | 1.08±0.23 | 1.26±0.39 |
| AG/GG | 62 | 5.05±0.75 | 1.64 (1.08) | 1.59±0.43 | 2.77±0.34 | 1.28±0.18 | 1.03±0.25 | 1.22±0.34 |
| F | 1.106 | -0.735 | 0.869 | 3.072 | 1.387 | 3.123 | 4.698 | |
| P | 0.294 | 0.462 | 0.352 | 0.081 | 0.240 | 0.079 | 0.031 | |
| Jing/female | ||||||||
| AA | 295 | 4.89±0.96 | 1.38 (0.61) | 1.83±0.46 | 2.83±0.46 | 1.31±0.25 | 1.04±0.24 | 1.31±0.36 |
| AG/GG | 96 | 5.15±0.99 | 1.52 (0.34) | 1.73±0.42 | 2.81±0.53 | 1.32±0.19 | 1.04±0.27 | 1.33±0.41 |
| F | 5.488 | -0.975 | 2.635 | 0.102 | 0.197 | 0.000 | 0.924 | |
| P | 0.020 | 0.329 | 0.105 | 0.750 | 0.657 | 0.989 | 0.337 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B. The value of triglyceride was presented as median (interquartile range), the difference between the genotypes was determined by the Wilcoxon-Mann-Whitney test.
Risk factors for serum lipid parameters
The risk factors for serum lipid parameters in Jing and Han are shown in Tables 5 and 6. Multiple linear regression analyses showed that serum TC and HDL-C levels in Jing and Han, HDL-C, TC levels and ApoA1/ApoB ratio in Jing were correlated with genotypes (P < 0.05), respectively. When serum lipid data were analyzed according to gender, serum TC levels in Jing and HDL-C levels in Han were associated with the genotypes only in females but not in males. The ApoA1/ApoB ratio in Jing was associated with the genotypes only in males but not in females. Several environmental factors such as age, gender, height, weight, waist circumference, alcohol consumption and cigarette smoking, and traditional cardiovascular risk factors such as BMI, fasting blood glucose and blood pressure levels were also correlated with serum lipid parameters in the Han and Jing populations and in males and females of both ethnic groups (P < 0.05-0.001, Tables 5 and 6).
Table 5.
The risk factors for serum lipid parameters in the Han and Jing populations
| Lipid | Risk factor | B | Std.error | Beta | t | P |
|---|---|---|---|---|---|---|
| Han and Jing | ||||||
| TG | Waist circumference | 0.040 | 0.005 | 0.419 | 8.807 | 0.000 |
| Cigarette smoking | 0.250 | 0.037 | 0.185 | 6.737 | 0.000 | |
| Weight | -0.018 | 0.004 | -0.206 | -4.295 | 0.000 | |
| Glucose | 0.099 | 0.025 | 0.103 | 3.925 | 0.000 | |
| Diastolic blood pressure | 0.009 | 0.002 | 0.105 | 3.914 | 0.000 | |
| TC | Glucose | 0.153 | 0.028 | 0.151 | 5.502 | 0.000 |
| Age | 0.006 | 0.002 | 0.086 | 3.039 | 0.002 | |
| Height | -0.011 | 0.003 | -0.098 | -3.525 | 0.000 | |
| Diastolic blood pressure | 0.007 | 0.002 | 0.082 | 2.982 | 0.003 | |
| Genotype | 0.122 | 0.056 | 0.059 | 2.161 | 0.031 | |
| HDL-C | Waist circumference | -0.016 | 0.002 | -0.301 | -10.687 | 0.000 |
| Gender | 0.169 | 0.036 | 0.171 | 4.647 | 0.000 | |
| Alcohol consumption | 0.120 | 0.025 | 0.149 | 4.854 | 0.000 | |
| Cigarette smoking | -0.073 | 0.024 | -0.097 | -3.063 | 0.002 | |
| Genotype | -0.085 | 0.029 | -0.078 | -2.954 | 0.003 | |
| Diastolic blood pressure | 0.003 | 0.001 | 0.061 | 2.255 | 0.024 | |
| Height | 0.005 | 0.002 | 0.077 | 2.234 | 0.026 | |
| LDL-C | Glucose | 0.037 | 0.014 | 0.075 | 2.695 | 0.007 |
| Height | -0.004 | 0.002 | -0.070 | -2.485 | 0.013 | |
| Diastolic blood pressure | 0.005 | 0.001 | 0.106 | 3.842 | 0.000 | |
| Age | 0.003 | 0.001 | 0.081 | 2.857 | 0.004 | |
| ApoA1 | Body mass index | -0.013 | 0.002 | -0.188 | -6.940 | 0.000 |
| Alcohol consumption | 0.057 | 0.011 | 0.159 | 5.264 | 0.000 | |
| Gender | 0.059 | 0.013 | 0.133 | 4.422 | 0.000 | |
| Glucose | -0.015 | 0.006 | -0.062 | -2.298 | 0.022 | |
| ApoB | Waist circumference | 0.006 | 0.001 | 0.223 | 7.933 | 0.000 |
| Age | 0.001 | 0.001 | 0.078 | 2.658 | 0.008 | |
| Height | -0.004 | 0.001 | -0.134 | -3.537 | 0.000 | |
| Gender | -0.047 | 0.018 | -0.093 | -2.563 | 0.010 | |
| ApoA1/ApoB | Body mass index | 0.072 | 0.032 | 0.601 | 2.264 | 0.024 |
| Glucose | -0.040 | 0.011 | -0.093 | -3.488 | 0.001 | |
| Age | -0.002 | 0.001 | -0.056 | -1.934 | 0.053 | |
| Waist circumference | -0.007 | 0.002 | -0.158 | -2.908 | 0.004 | |
| Alcohol consumption | 0.058 | 0.019 | 0.090 | 3.058 | 0.002 | |
| Gender | 0.095 | 0.030 | 0.122 | 3.228 | 0.001 | |
| Height | 0.030 | 0.009 | 0.602 | 3.178 | 0.002 | |
| Weight | -0.036 | 0.013 | -0.919 | -2.807 | 0.005 | |
| Han | ||||||
| TG | Waist circumference | 0.030 | 0.006 | 0.319 | 4.731 | 0.000 |
| Cigarette smoking | 0.193 | 0.050 | 0.151 | 3.836 | 0.000 | |
| Diastolic blood pressure | 0.007 | 0.003 | 0.089 | 2.291 | 0.022 | |
| Glucose | 0.124 | 0.028 | 0.165 | 4.394 | 0.000 | |
| Weight | -0.013 | 0.006 | -0.141 | -2.062 | 0.040 | |
| TC | Height | -0.012 | 0.004 | -0.103 | -2.740 | 0.006 |
| Glucose | 0.213 | 0.030 | 0.269 | 7.127 | 0.000 | |
| HDL-C | Waist circumference | -0.013 | 0.002 | -0.227 | -5.947 | 0.000 |
| Gender | 0.124 | 0.049 | 0.118 | 2.558 | 0.011 | |
| Alcohol consumption | 0.121 | 0.035 | 0.160 | 3.417 | 0.001 | |
| Cigarette smoking | -0.112 | 0.037 | -0.142 | -3.039 | 0.002 | |
| LDL-C | Glucose | 0.061 | 0.015 | 0.155 | 3.922 | 0.000 |
| Systolic blood pressure | 0.003 | 0.001 | 0.112 | 2.848 | 0.005 | |
| Height | -0.004 | 0.002 | -0.079 | -2.066 | 0.039 | |
| ApoA1 | Body mass index | -0.012 | 0.002 | -0.186 | -4.936 | 0.000 |
| Alcohol consumption | 0.075 | 0.013 | 0.253 | 5.809 | 0.000 | |
| Gender | 0.064 | 0.018 | 0.155 | 3.571 | 0.000 | |
| Glucose | -0.015 | 0.007 | -0.080 | -2.128 | 0.034 | |
| ApoB | Waist circumference | 0.006 | 0.001 | 0.220 | 5.536 | 0.000 |
| Systolic blood pressure | 0.001 | 0.000 | 0.115 | 2.966 | 0.003 | |
| Height | -0.007 | 0.002 | -0.208 | -4.201 | 0.000 | |
| Gender | -0.077 | 0.024 | -0.152 | -3.133 | 0.002 | |
| ApoA1/ApoB | Waist circumference | -0.007 | 0.003 | -0.156 | -2.016 | 0.044 |
| Body mass index | 0.093 | 0.043 | 0.780 | 2.176 | 0.030 | |
| Glucose | -0.037 | 0.013 | -0.109 | -2.911 | 0.004 | |
| Systolic blood pressure | -0.002 | 0.001 | -0.092 | -2.388 | 0.017 | |
| Alcohol consumption | 0.076 | 0.023 | 0.136 | 3.225 | 0.001 | |
| Gender | 0.160 | 0.040 | 0.206 | 3.994 | 0.000 | |
| Height | 0.040 | 0.012 | 0.817 | 3.186 | 0.002 | |
| Weight | -0.045 | 0.017 | -1.113 | -2.573 | 0.010 | |
| Jing | ||||||
| TG | Waist circumference | 0.047 | 0.007 | 0.463 | 6.529 | 0.000 |
| Cigarette smoking | 0.303 | 0.059 | 0.214 | 5.114 | 0.000 | |
| Height | -0.029 | 0.006 | -0.251 | -5.003 | 0.000 | |
| Diastolic blood pressure | 0.010 | 0.003 | 0.117 | 3.111 | 0.002 | |
| Body mass index | -0.040 | 0.019 | -0.143 | -2.122 | 0.034 | |
| Gender | -0.184 | 0.089 | -0.100 | -2.058 | 0.040 | |
| TC | Age | 0.015 | 0.003 | 0.205 | 4.818 | 0.000 |
| Genotype | 0.204 | 0.084 | 0.094 | 2.422 | 0.016 | |
| Diastolic blood pressure | 0.009 | 0.004 | 0.098 | 2.464 | 0.014 | |
| Pulse pressure | -0.006 | 0.002 | -0.121 | -2.843 | 0.005 | |
| Height | -0.022 | 0.006 | -0.182 | -3.512 | 0.000 | |
| Cigarette smoking | 0.149 | 0.061 | 0.100 | 2.461 | 0.014 | |
| Weight | 0.010 | 0.005 | 0.106 | 2.135 | 0.033 | |
| HDL-C | Waist circumference | -0.016 | 0.002 | -0.327 | -8.753 | 0.000 |
| Gender | 0.148 | 0.036 | 0.162 | 4.068 | 0.000 | |
| Alcohol consumption | 0.144 | 0.036 | 0.159 | 4.001 | 0.000 | |
| Genotype | -0.084 | 0.038 | -0.082 | -2.218 | 0.027 | |
| LDL-C | Age | 0.004 | 0.001 | 0.128 | 3.258 | 0.001 |
| Diastolic blood pressure | 0.004 | 0.002 | 0.101 | 2.563 | 0.011 | |
| ApoA1 | Weight | -0.005 | 0.001 | -0.204 | -5.253 | 0.000 |
| ApoB | Waist circumference | 0.005 | 0.001 | 0.179 | 4.603 | 0.000 |
| Age | 0.002 | 0.001 | 0.105 | 2.693 | 0.007 | |
| ApoA1/ApoB | Waist circumference | -0.007 | 0.003 | -0.155 | -2.381 | 0.018 |
| Genotype | 0.085 | 0.034 | 0.096 | 2.513 | 0.012 | |
| Age | -0.003 | 0.001 | -0.091 | -2.386 | 0.017 | |
| Body mass index | -0.017 | 0.008 | -0.139 | -2.114 | 0.035 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B.
Table 6.
Relationship between serum lipid parameters and relative factors in the males and females of the Han and Jing populations
| Lipid | Risk factor | B | Std.error | Beta | t | P |
|---|---|---|---|---|---|---|
| Jing/male | ||||||
| TC | Diastolic blood pressure | 0.025 | 0.006 | 0.300 | 4.008 | 0.000 |
| Age | 0.016 | 0.004 | 0.260 | 3.705 | 0.000 | |
| Cigarette smoking | 0.167 | 0.064 | 0.171 | 2.620 | 0.009 | |
| Systolic blood pressure | -0.009 | 0.004 | -0.190 | -2.418 | 0.016 | |
| TG | Waist circumference | 0.042 | 0.007 | 0.396 | 6.136 | 0.000 |
| Cigarette smoking | 0.252 | 0.072 | 0.214 | 3.482 | 0.001 | |
| Height | -0.033 | 0.010 | -0.214 | -3.301 | 0.001 | |
| Diastolic blood pressure | 0.014 | 0.006 | 0.139 | 2.321 | 0.021 | |
| Age | -0.010 | 0.005 | -0.135 | -2.111 | 0.036 | |
| LDL-C | Diastolic blood pressure | 0.006 | 0.002 | 0.154 | 2.435 | 0.016 |
| Glucose | -0.131 | 0.061 | -0.136 | -2.143 | 0.033 | |
| HDL-C | Waist circumference | -0.017 | 0.003 | -0.385 | -6.583 | 0.000 |
| Alcohol consumption | 0.147 | 0.034 | 0.249 | 4.275 | 0.000 | |
| Glucose | -0.125 | 0.060 | -0.121 | -2.085 | 0.038 | |
| ApoA1 | Waist circumference | -0.006 | 0.001 | -0.285 | -4.609 | 0.000 |
| Alcohol consumption | 0.038 | 0.018 | 0.131 | 2.116 | 0.035 | |
| ApoB | Body mass index | 0.018 | 0.005 | 0.234 | 3.737 | 0.000 |
| ApoA1/ApoB | Weight | -0.013 | 0.002 | -0.338 | -5.573 | 0.000 |
| Genotype | 0.115 | 0.056 | 0.125 | 2.061 | 0.040 | |
| Jing/female | ||||||
| TG | Waist circumference | 0.028 | 0.005 | 0.291 | 5.992 | 0.000 |
| Cigarette smoking | 0.913 | 0.299 | 0.146 | 3.049 | 0.002 | |
| Height | -0.028 | 0.007 | -0.207 | -4.230 | 0.000 | |
| TC | Age | 0.018 | 0.004 | 0.227 | 4.628 | 0.000 |
| Genotype | 0.276 | 0.111 | 0.122 | 2.476 | 0.014 | |
| LDL-C | Age | 0.008 | 0.002 | 0.207 | 4.164 | 0.000 |
| HDL-C | Waist circumference | -0.017 | 0.003 | -0.309 | -6.414 | 0.000 |
| ApoA1 | Body mass index | -0.011 | 0.004 | -0.152 | -3.029 | 0.003 |
| ApoB | Body mass index | 0.011 | 0.004 | 0.146 | 2.953 | 0.003 |
| Age | 0.003 | 0.001 | 0.162 | 3.273 | 0.001 | |
| ApoA1/ApoB | Body mass index | -0.024 | 0.006 | -0.211 | -4.305 | 0.000 |
| Age | -0.003 | 0.001 | -0.111 | -2.236 | 0.026 | |
| Glucose | -0.108 | 0.050 | -0.106 | -2.151 | 0.032 | |
| Han/male | ||||||
| TG | Waist circumference | 0.041 | 0.013 | 0.374 | 3.257 | 0.001 |
| Cigarette smoking | 0.190 | 0.064 | 0.185 | 2.972 | 0.003 | |
| Diastolic blood pressure | 0.015 | 0.005 | 0.177 | 2.915 | 0.004 | |
| Age | -0.014 | 0.005 | -0.182 | -2.765 | 0.006 | |
| Glucose | 0.171 | 0.052 | 0.201 | 3.304 | 0.001 | |
| Weight | -0.026 | 0.013 | -0.243 | -2.068 | 0.040 | |
| TC | Diastolic blood pressure | 0.012 | 0.005 | 0.153 | 2.525 | 0.012 |
| Glucose | 0.242 | 0.048 | 0.305 | 5.035 | 0.000 | |
| LDL-C | Diastolic blood pressure | 0.006 | 0.003 | 0.155 | 2.527 | 0.012 |
| Glucose | 0.103 | 0.025 | 0.255 | 4.161 | 0.000 | |
| HDL-C | Waist circumference | -0.017 | 0.004 | -0.267 | -4.250 | 0.000 |
| Alcohol consumption | 0.107 | 0.038 | 0.187 | 2.825 | 0.005 | |
| Diastolic blood pressure | 0.006 | 0.003 | 0.128 | 2.041 | 0.042 | |
| Cigarette smoking | -0.121 | 0.038 | -0.207 | -3.175 | 0.002 | |
| ApoA1 | Waist circumference | -0.005 | 0.001 | -0.213 | -3.576 | 0.000 |
| Alcohol consumption | 0.078 | 0.013 | 0.350 | 5.865 | 0.000 | |
| ApoB | Waist circumference | 0.006 | 0.002 | 0.219 | 3.522 | 0.001 |
| Systolic blood pressure | 0.002 | 0.001 | 0.173 | 2.782 | 0.006 | |
| ApoA1/ApoB | Waist circumference | -0.013 | 0.003 | -0.279 | -4.559 | 0.000 |
| Alcohol consumption | 0.085 | 0.026 | 0.199 | 3.255 | 0.001 | |
| Systolic blood pressure | -0.003 | 0.001 | -0.138 | -2.238 | 0.026 | |
| Han/female | ||||||
| TG | Waist circumference | 0.021 | 0.004 | 0.246 | 5.174 | 0.000 |
| Glucose | 0.125 | 0.033 | 0.182 | 3.817 | 0.000 | |
| TC | Glucose | 0.206 | 0.038 | 0.261 | 5.410 | 0.000 |
| LDL-C | Glucose | 0.047 | 0.019 | 0.121 | 2.404 | 0.017 |
| Age | 0.003 | 0.002 | 0.091 | 1.736 | 0.083 | |
| Height | -0.007 | 0.004 | -0.109 | -2.128 | 0.034 | |
| HDL-C | Body mass index | -0.031 | 0.007 | -0.209 | -4.307 | 0.000 |
| Genotype | -0.140 | 0.054 | -0.126 | -2.593 | 0.010 | |
| Pulse pressure | -0.003 | 0.002 | -0.111 | -2.293 | 0.022 | |
| ApoA1 | Body mass index | -0.011 | 0.003 | -0.192 | -3.948 | 0.000 |
| Glucose | -0.022 | 0.009 | -0.125 | -2.562 | 0.011 | |
| ApoB | Age | 0.003 | 0.001 | 0.146 | 2.952 | 0.003 |
| Waist circumference | 0.006 | 0.001 | 0.231 | 4.786 | 0.000 | |
| Height | -0.008 | 0.002 | -0.195 | -3.888 | 0.000 | |
| ApoA1/ApoB | Weight | -0.077 | 0.024 | -1.856 | -3.201 | 0.001 |
| Body mass index | 0.147 | 0.056 | 1.332 | 2.619 | 0.009 | |
| Age | -0.004 | 0.001 | -0.142 | -2.945 | 0.003 | |
| Glucose | -0.040 | 0.015 | -0.122 | -2.627 | 0.009 | |
| Height | 0.062 | 0.017 | 1.083 | 3.695 | 0.000 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of apolipoprotein A1 to apolipoprotein B.
Discussion
In the present study, we showed that the levels of weight, waist circumference, BMI, TG, glucose were higher in Jing than in Han, but the ratio of ApoA1/ApoB, the percentages of subjects consuming alcohol, the levels of ApoA1 and HDL-C were lower in Jing than in Han (P < 0.05-0.001). There were no significant differences in the levels of LDL-C, TC, ApoB between the two ethnic groups. These differences in serum lipid profiles between the two ethnic groups may result from the combined action of genetic including lipid-associated gene variants, environmental factors including diet, alcohol consumption, cigarette smoking, obesity, exercise, hypertension and their interactions [23,27,28]. Jing is the only Chinese minority for coastal fisheries and is the only sea people in China. In this case, it has a very special lifestyle and dietary habits compared with the other landlocked nationalities. Jing nationality is a relatively conservative and isolated minority in China that retains its regional and special customs. Their marriages were family-arranged in the old days when they sing antiphonal songs to look for the other half. After antiphonal singing, if the boy’s into the girl he would kick sand toward her while approaching her. If the girl feel the same she would kick back, which means engagement. Jing stays endogamy, intermarriage with Han or Zhuang people is seldom happened. Jing people don’t get married with someone sharing the same last name, also cross-cousin marriage is strictly forbidden. Therefore, we deduced that some hereditary characteristics and genotypes of specific lipid metabolism-associated genes in this population may be different from those in the Han people.
The genotypic and allelic frequencies of the RBM5 rs2013208 SNP in diverse racial/ethnic groups are significantly different. According to the 1000 genomes project data, the frequency of the rs2013208G allele was 13.59% in Han Chinese from Beijing, 10.48% in Southern Han Chinese, 19.23% in Japanese from Tokyo, 52.75% in British in England and Scotland, 45.96% in Finnish in Finland, 52.34% in Iberian population in Spain. However, in African ancestry, the frequency of the rs2013208G allele was 46.72% in Americans of African ancestry in SW USA, 34.85% of Esan in Nigeria, 41.15% in Gambian in Western Divisions in the Gambia. In the present study, we showed that the frequencies of G alleles were 14.4% in Han and 13.6% in Jing (P > 0.05); respectively. Apparently, the minor allele frequency was lower in Asian than the Western populations. There were no conspicuous differences in the genotypic and allelic frequencies of the rs2013208 SNP between the Jing and Han populations, or between males and females in the both ethnic groups. As compared with the data in the 1000 genomes project data, we found that the frequencies of the G allele in our study populations (14.4% in Han and 13.6% in Jing, P > 0.05) were higher than those in Southern Han Chinese (10.48%), which may be caused by different sample sizes and regions.
Recently, a newly study identifies and annotates 157 loci associated with lipid levels obtained from Joint GWAS and Metabochip Meta-analysis (P < 5 × 10-8) including 62 loci not previously associated with lipid levels in humans which referred to the association between the RBM5 rs2013208 SNP and HDL-C levels [14]. Besides this, rare studies have previously reported the direct effect of the RBM5 rs2013208 SNP on serum lipid levels. Because the effects of newly identified loci were generally smaller than in earlier GWAS, more work is needed to be done to actually confirm the findings. In the present study, the levels of TC and the ratio of ApoA1 to ApoB were different between the AA and AG/GG genotypes in Jing (P < 0.05) but not in Han, the G allele carriers had higher TC levels and ApoA1/ApoB ratio than the G allele non-carriers. The G allele carriers had lower serum HDL-C levels than the G allele non-carriers in both Han and Jing. In the subgroup analyses, the G allele carriers in Han females but not in Han males had lower HDL-C levels than the G allele non-carriers (P < 0.05). The G allele carriers in Jing females but not in Jing males had higher TC levels than the G allele non-carriers (P < 0.001). The G allele carriers in Jing males but not in Jing females had lower ApoA1/ApoB ratio than the G allele non-carriers (P < 0.05). There was no significant difference in the remaining serum lipid parameters between the genotypes in Jing, Han, males, or females (P > 0.05 for all). These results suggest that there may be an ethnic and/or sex specific-association of the RBM5 rs2013208 SNP and serum lipid parameters. As far as we know, our study is the first replication of GWAS signals about the association of RBM5 rs2013208 SNP with serum lipid levels in the Chinese populations. Therefore, further studies with larger sample size are still needed to confirm this association.
In the present study, multiple linear regression analysis showed that serum lipid parameters were associated with age, gender, alcohol consumption, cigarette smoking, BMI, fasting blood glucose levels and blood pressure in both Jing and Han, or males and females in both ethnic groups. These data suggest that the environmental factors also play an important role in determining lipid profiles in our study populations. Jing is the only Chinese minority for coastal fisheries, meanwhile is the only sea people in China. In this case, it has a very special lifestyle and dietary habits compared with the other landlocked nationalities. Although rice and corn are the staple foods in both ethnic groups, the people of Jing nationality like to eat seafood like fish, shrimp, crabs, shellfish and sandworm. Fishery is the major source of income for Jing population and fish is appeared most frequently dish on their tables. A kind of fish sauce called nuoc-mam is also popular on Jing people’s dinner table, which contains 17 amino acids (8 essential amino acids included of course). It has been reported that consuming fish or fish oil containing the n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) is associated with decreased cardiovascular death [29]. In a previous meta-analysis of 11 prospective cohort studies (encompassing 222 364 persons with an average of 11.8 y of follow-up) indicated that each 20-g/d increase in fish intake was associated with a 7% lower risk of coronary heart disease mortality [30]. Jing people also prefer sweet food such as sweet glutinous rice porridge, mung bean syrup, because they believe sweet food is a symbol for happiness. This preference of sugariness may be lead to the higher glucose level in Jing than in Han people.
In the present study, we also found that the levels of weight, waist circumference, and BMI were higher and the percentages of subjects consuming alcohol were lower in Jing than in Han. A recent study in Japan showed that high BMI (> 26) was associated with higher systolic blood pressure (SBP), LDL-C, fasting blood glucose (FBG), and TG in both sexes. An increase ≥ 1.1 BMI units in 5 years was associated with increased diastolic blood pressure (DBP), LDL-C, TG, HbA1c, and FBG and decreased HDL-C. In contrast, decreased BMI was associated with decreased blood pressure and LDL-C and increased HDL-C in both sexes, and decreased TG in men and FBG in women [31]. Their researches showed that maintaining a desirable weight or losing weight may help prevent hypertension and metabolic syndrome (MS), even in non-obese individuals. Alcohol intake has a significant influence on the human serum lipid metabolism. Many studies showed that moderate alcohol intake has been associated with reduced cardiovascular events which were attributed in great part to the increase in HDL-C caused by alcohol consumption [32,33]. In contrast, heavy alcohol consumption was robustly positively associated with serum TG, LDL-C levels and blood pressure [32,34,35]. In addition, our previous studies also documented that BMI and alcohol consumption may interact with certain lipid-related gene variants to modify the serum lipid levels in Bai Ku Yao and Han ethnic groups [36]. Consequently, the joint effects of different dietary habits, lifestyles, and environmental factors probably further modify the association of genetic variations and serum lipid levels in our study populations.
Limitations
There are some potential limitations in our study. First, this is the first time to report the sex-specific association of the RBM5 rs2013208 SNP and no previous evidence to support our findings and the number of subjects in our study is moderate, the statistical power is relatively reliable. Thus, further studies with larger samples are needed to replicate our findings in other populations. Second, we only measured serum TC, TG, HDL-C, LDL-C, ApoA1, ApoB levels, and the ratio of ApoA1 to ApoB, but the subclasses lipoproteins such as HDL2, HDL3, small dense LDL, and large buoyant LDL were not detected their associations with rs2013208 SNP. Third, we were not able to alleviate the effect of diet during the statistical analysis since the diet intake was self-reported and difficult to classify.
Conclusions
Our study showed that the association of the RBM5 rs2013208 SNP and serum lipid levels was different between the Jing and Han populations, and between males and females in the both ethnic groups. These findings suggest that there may be an ethnic- and/or sex-specific association between the RBM5 rs2013208 SNP and serum lipid levels in different populations.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No: 81160111).
Disclosure of conflict of interest
None.
References
- 1.Deaton C, Froelicher ES, Wu LH, Ho C, Shishani K, Jaarsma T. The global burden of cardiovascular disease. Eur J Cardiovasc Nurs. 2011;10(Suppl 2):S5–13. doi: 10.1016/S1474-5151(11)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in sub-Saharan Africa: the global burden of diseases, injuries and risk factors 2010 study. Prog Cardiovasc Dis. 2013;56:234–239. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB, Lange LA, Almoguera B, Appelman YE, Barnard J, Baumert J, Beitelshees AL, Bhangale TR, Chen YD, Gaunt TR, Gong Y, Hopewell JC, Johnson T, Kleber ME, Langaee TY, Li M, Li YR, Liu K, McDonough CW, Meijs MF, Middelberg RP, Musunuru K, Nelson CP, O’Connell JR, Padmanabhan S, Pankow JS, Pankratz N, Rafelt S, Rajagopalan R, Romaine SP, Schork NJ, Shaffer J, Shen H, Smith EN, Tischfield SE, van der Most PJ, van Vliet-Ostaptchouk JV, Verweij N, Volcik KA, Zhang L, Bailey KR, Bailey KM, Bauer F, Boer JM, Braund PS, Burt A, Burton PR, Buxbaum SG, Chen W, Cooper-Dehoff RM, Cupples LA, deJong JS, Delles C, Duggan D, Fornage M, Furlong CE, Glazer N, Gums JG, Hastie C, Holmes MV, Illig T, Kirkland SA, Kivimaki M, Klein R, Klein BE, Kooperberg C, Kottke-Marchant K, Kumari M, LaCroix AZ, Mallela L, Murugesan G, Ordovas J, Ouwehand WH, Post WS, Saxena R, Scharnagl H, Schreiner PJ, Shah T, Shields DC, Shimbo D, Srinivasan SR, Stolk RP, Swerdlow DI, Taylor HA Jr, Topol EJ, Toskala E, van Pelt JL, van Setten J, Yusuf S, Whittaker JC, Zwinderman AH, Anand SS, Balmforth AJ, Berenson GS, Bezzina CR, Boehm BO, Boerwinkle E, Casas JP, Caulfield MJ, Clarke R, Connell JM, Cruickshanks KJ, Davidson KW, Day IN, de Bakker PI, Doevendans PA, Dominiczak AF, Hall AS, Hartman CA, Hengstenberg C, Hillege HL, Hofker MH, Humphries SE, Jarvik GP, Johnson JA, Kaess BM, Kathiresan S, Koenig W, Lawlor DA, Marz W, Melander O, Mitchell BD, Montgomery GW, Munroe PB, Murray SS, Newhouse SJ, Onland-Moret NC, Poulter N, Psaty B, Redline S, Rich SS, Rotter JI, Schunkert H, Sever P, Shuldiner AR, Silverstein RL, Stanton A, Thorand B, Trip MD, Tsai MY, van der Harst P, van der Schoot E, van der Schouw YT, Verschuren WM, Watkins H, Wilde AA, Wolffenbuttel BH, Whitfield JB, Hovingh GK, Ballantyne CM, Wijmenga C, Reilly MP, Martin NG, Wilson JG, Rader DJ, Samani NJ, Reiner AP, Hegele RA, Kastelein JJ, Hingorani AD, Talmud PJ, Hakonarson H, Elbers CC, Keating BJ, Drenos F. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet. 2012;91:823–838. doi: 10.1016/j.ajhg.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand SS. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. Law MR, Wald NJ, Rudnicka AR. BMJ 2003; 326: 1407-408. Vasc Med. 2003;8:289–290. doi: 10.1191/1358863x03vm518xx. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Neaton JD, Cohen JD, Cutler J, Eberly L, Grandits G, Kuller LH, Ockene J, Prineas R. Multiple risk factor intervention trial revisited: a new perspective based on nonfatal and fatal composite endpoints, coronary and cardiovascular, during the trial. J Am Heart Assoc. 2012;1:e003640. doi: 10.1161/JAHA.112.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corella D, Ordovas JM. Single nucleotide polymorphisms that influence lipid metabolism: interaction with dietary factors. Annu Rev Nutr. 2005;25:341–390. doi: 10.1146/annurev.nutr.25.050304.092656. [DOI] [PubMed] [Google Scholar]
- 7.Burnett JR. Lipids, lipoproteins, atherosclerosis and cardiovascular disease. Clin Biochem Rev. 2004;25:2. [PMC free article] [PubMed] [Google Scholar]
- 8.Ordovas JM, Shen AH. Genetics, the environment, and lipid abnormalities. Curr Cardiol Rep. 2002;4:508–513. doi: 10.1007/s11886-002-0115-4. [DOI] [PubMed] [Google Scholar]
- 9.Yip AG, Ma Q, Wilcox M, Panhuysen CI, Farrell J, Farrer LA, Wyszynski DF. Search for genetic factors predisposing to atherogenic dyslipidemia. BMC Genet. 2003;4(Suppl 1):S100. doi: 10.1186/1471-2156-4-S1-S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller DA, de Faire U, Pedersen NL, Dahlen G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med. 1993;328:1150–1156. doi: 10.1056/NEJM199304223281603. [DOI] [PubMed] [Google Scholar]
- 11.Perusse L, Rice T, Despres JP, Bergeron J, Province MA, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Familial resemblance of plasma lipids, lipoproteins and postheparin lipoprotein and hepatic lipases in the heritage family study. Arterioscler Thromb Vasc Biol. 1997;17:3263–3269. doi: 10.1161/01.atv.17.11.3263. [DOI] [PubMed] [Google Scholar]
- 12.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barter PJ, Rye KA. Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J Lipid Res. 2012;53:1755–1766. doi: 10.1194/jlr.R024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Global Lipids Genetics Consortium. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF, Bonnycastle LL, Jackson AU, Crawford G, Surti A, Guiducci C, Burtt NP, Parish S, Clarke R, Zelenika D, Kubalanza KA, Morken MA, Scott LJ, Stringham HM, Galan P, Swift AJ, Kuusisto J, Bergman RN, Sundvall J, Laakso M, Ferrucci L, Scheet P, Sanna S, Uda M, Yang Q, Lunetta KL, Dupuis J, de Bakker PI, O’Donnell CJ, Chambers JC, Kooner JS, Hercberg S, Meneton P, Lakatta EG, Scuteri A, Schlessinger D, Tuomilehto J, Collins FS, Groop L, Altshuler D, Collins R, Lathrop GM, Melander O, Salomaa V, Peltonen L, Orho-Melander M, Ordovas JM, Boehnke M, Abecasis GR, Mohlke KL, Cupples LA. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JH, Liu ZH, Lv FJ, Fu YG, Fan XL, Li SY, Lu JM, Liu XY, Xu AL. Molecular analyses of HLADRB1, -DPB1, and -DQB1 in Jing ethnic minority of Southwest China. Hum Immunol. 2003;64:830–834. doi: 10.1016/s0198-8859(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 17.Sun JQ, Yin RX, Shi GY, Shen SW, Chen X, Bin Y, Huang F, Wang W, Lin WX, Pan SL. Association of the ARL15 rs6450176 SNP and serum lipid levels in the Jing and Han populations. Int J Clin Exp Pathol. 2015;8:12977–12994. [PMC free article] [PubMed] [Google Scholar]
- 18.An epidemiological study of cardiovascular and cardiopulmonary disease risk factors in four populations in the People’s Republic of China. Baseline report from the P.R.C.-U.S.A. collaborative study. People’s Republic of China--United States cardiovascular and cardiopulmonary epidemiology research group. Circulation. 1992;85:1083–1096. doi: 10.1161/01.cir.85.3.1083. [DOI] [PubMed] [Google Scholar]
- 19.Aung LH, Yin RX, Wu JZ, Wu DF, Wang W, Li H. Association between the MLX interacting protein-like, BUD13 homolog and zinc finger protein 259 gene polymorphisms and serum lipid levels. Sci Rep. 2014;4:5565. doi: 10.1038/srep05565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aung LH, Yin RX, Wu DF, Li Q, Yan TT, Wang YM, Li H, Wei DX, Shi YL, Yang DZ. Association of the TRIB1 tribbles homolog 1 gene rs17321515 A > G polymorphism and serum lipid levels in the Mulao and Han populations. Lipids Health Dis. 2011;10:230. doi: 10.1186/1476-511X-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin RX, Aung LH, Long XJ, Yan TT, Cao XL, Huang F, Wu JZ, Yang DZ, Lin WX, Pan SL. Interactions of several genetic polymorphisms and alcohol consumption on blood pressure levels. Biofactors. 2015;41:339–351. doi: 10.1002/biof.1234. [DOI] [PubMed] [Google Scholar]
- 22.Ramazauskiene V, Petkeviciene J, Klumbiene J, Kriaucioniene V, Sakyte E. Diet and serum lipids: changes over socio-economic transition period in Lithuanian rural population. BMC Public Health. 2011;11:447. doi: 10.1186/1471-2458-11-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruixing Y, Yuming C, Shangling P, Fengping H, Tangwei L, Dezhai Y, Jinzhen W, Limei Y, Weixiong L, Rongshan L, Jiandong H. Effects of demographic, dietary and other lifestyle factors on the prevalence of hyperlipidemia in Guangxi Hei Yi Zhuang and Han populations. Eur J Cardiovasc Prev Rehabil. 2006;13:977–984. doi: 10.1097/01.hjr.0000239476.79428.25. [DOI] [PubMed] [Google Scholar]
- 24.Ishii M. [The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure, and 1999 world health organization-international society of hypertension guidelines for the management of hypertension] . Nihon Rinsho. 2000;58(Suppl 1):267–275. [PubMed] [Google Scholar]
- 25.Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T. 1999 world health organization-international society of hypertension guidelines for the management of hypertension. Guidelines sub-committee of the world health organization. Clin Exp Hypertens. 1999;21:1009–1060. doi: 10.3109/10641969909061028. [DOI] [PubMed] [Google Scholar]
- 26.Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 27.Bruce KD, Cagampang FR. Epigenetic priming of the metabolic syndrome. Toxicol Mech Methods. 2011;21:353–361. doi: 10.3109/15376516.2011.559370. [DOI] [PubMed] [Google Scholar]
- 28.Ruixing Y, Yiyang L, Meng L, Kela L, Xingjiang L, Lin Z, Wanying L, Jinzhen W, Dezhai Y, Weixiong L. Interactions of the apolipoprotein C-III 3238C > G polymorphism and alcohol consumption on serum triglyceride levels. Lipids Health Dis. 2010;9:86. doi: 10.1186/1476-511X-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the cardiovascular health study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 30.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 31.Mandai N, Akazawa K, Hara N, Ide Y, Ide K, Dazai U, Chishaki A, Chishaki H. Body weight reduction results in favorable changes in blood pressure, serum lipids, and blood sugar in middle-aged japanese persons: a 5-year interval observational study of 26,824 cases. Glob J Health Sci. 2015;7:159–170. doi: 10.5539/gjhs.v7n5p159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among U. S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55:1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koppes LL, Twisk JW, Van Mechelen W, Snel J, Kemper HC. Cross-sectional and longitudinal relationships between alcohol consumption and lipids, blood pressure and body weight indices. J Stud Alcohol. 2005;66:713–721. doi: 10.15288/jsa.2005.66.713. [DOI] [PubMed] [Google Scholar]
- 34.Onat A, Hergenc G, Dursunoglu D, Ordu S, Can G, Bulur S, Yuksel H. Associations of alcohol consumption with blood pressure, lipoproteins, and subclinical inflammation among Turks. Alcohol. 2008;42:593–601. doi: 10.1016/j.alcohol.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 36.Justesen JM, Allin KH, Sandholt CH, Borglykke A, Krarup NT, Grarup N, Linneberg A, Jorgensen T, Hansen T, Pedersen O. Interactions of lipid genetic risk scores with estimates of metabolic health in a Danish population. Circ Cardiovasc Genet. 2015;8:465–472. doi: 10.1161/CIRCGENETICS.114.000637. [DOI] [PubMed] [Google Scholar]


