Abstract
Moderate to severe chronic graft-versus-host disease (cGVHD) is associated with high morbidity, hospital dependency and poor quality of life. In this study, we analyzed a well-defined consecutive series of 98 patients with acute myelogenous leukemia/myelodysplastic syndrome (AML/MDS) who received allogeneic stem cell transplantation with non-myeloablative (NMA) conditioning to determine risk factors associated with the severity of cGVHD. cGVHD was defined according to the 2005 National Institute of Health consensus criteria. Transfusions before transplantation, presence of HLA antibodies, composition of the graft (CD3+, CD19+, CD34+ cells), sibling or matched unrelated donor, female donor to male recipient, CMV serology and the development of acute GVHD (aGVHD), were considered potential risk factors. Multivariate Cox regression analysis identified the number of CD19+ 106/kg (HR 2.79; 95% CI 1.35–5.74), CD3+ 106/kg (HR 2.18; 95% CI 1.04–4.59) infused cells and the presence of patient HLA antibodies before transplantation (HR 2.34; CI 1.11–4.95) as significant risk factors for the development of moderate to severe cGVHD. In summary, we identified in a small, but well-defined cohort, 3 risk factors associated with the severity of cGVHD that should be validated in a larger multi-center study.
Electronic supplementary material
The online version of this article (10.1007/s13577-019-00297-7) contains supplementary material, which is available to authorized users.
Keywords: Risk factors, Moderate to severe, Chronic graft-versus-host disease, Non-myeloablative, PBSCT
Introduction
AlloSCT is a standard treatment for patients with AML/MDS with high risk of relapse [1–3]. Around 30–70% of transplanted patients who are treated with non-myeloablative conditioning and receive a T-repleted graft collected from peripheral blood develop chronic graft-versus-host disease [4–6]. Moderate to severe cGVHD is associated with high morbidity, hospital dependency and poor quality of life as compared to mild cGVHD [6, 7]. Patients who develop moderate to severe cGVHD are treated with high-dose systemic steroids for months and often years, whereas mild cGVHD can be treated with topical steroids [8, 9]. Long-term steroid treatment impairs immune function and can, therefore, increase the risk of opportunistic infections. Other steroid therapy-related complications include osteoporosis, avascular necrosis, glucose intolerance, cataract, muscle atrophy, hypertension, and disturbance of mood and sleep [10].
Until now, multiple studies have identified risk factors associated with the development of cGVHD [5, 11, 12], but only a few studies have been carried out to identify risk factors associated with the severity of cGVHD [6, 13]. Known risk factors associated with the development of cGVHD independently of the severity of cGVHD are: female donor to male recipient, HLA mismatch, peripheral blood as a source of stem cells, high numbers of infused T cells, recipient age, positive CMV serology and antecedent acute GVHD [5, 11, 12, 14]. Risk factors which are published to be associated with the severity of cGVHD are: transplantation from an immuned female donor to a male recipient, antecedent of aGVHD, CML and NMA conditioning, but most of these factors were identified in the context of bone marrow used as graft source instead of peripheral blood.
Because moderate to severe cGVHD is associated with high morbidity and reduced quality of life and most of the stem cell transplants nowadays use peripheral blood as graft source, it is relevant to identify risk factors that are associated with the development of moderate to severe cGVHD in patients who undergo peripheral blood AlloSCT.
Materials and methods
Setting and data collection
This retrospective single center cohort study was conducted at the University Medical Center Groningen (UMCG), the Netherlands. The UMCG is a JACIE accredited tertiary academic hospital that performs all the allogeneic stem cell transplantations in the North of the Netherlands. We identified a homogeneous, consecutive cohort of patients, treated from July 2003 to September 2015 in our center. Patient data were collected from the UMCG transplantation database “ProMise”, and from the UMCG digital patient database. Diagnosis and grading of cGVHD (mild, moderate or severe) were done according to clinical manifestations and the global severity score based on the consensus criteria of the National Institutes of Health (NIH) 2005 [15]. Classification of cGVHD was conducted by the treating physician.
Treatment protocol
Patients who were included in the study received their AlloSCT between July 16th 2003 and September 4th 2015. Inclusion criteria were: (1) adult patients with AML or MDS who underwent peripheral blood AlloSCT with NMA conditioning, (2) who had received their first AlloSCT without subsequent lymphocyte infusion therapy, (3) had survived at least + 90 days, (4) did not relapse within + 100 days and 5) who developed cGVHD within + 400 days. The last criterion was chosen to identify risk factors that influence the incidence of moderate to severe cGVHD at an early stage and to reduce long-term treatment effects that could bias the outcome of this study. Clinical characteristics of the patients are summarized in Table 1. We defined a control group with the cohort of patients who did not develop cGVHD or who developed only mild cGVHD, versus the cohort of patients who developed moderate to severe cGVHD (Table 2).
Table 1.
Patient and disease characteristics
| No cGVHD and mild cGVHD (n = 60) | Moderate to severe cGVHD (n = 38) | |
|---|---|---|
| Patient sex | ||
| F/m | 30/30 | 14/24 |
| Donor sex | ||
| F/m | 23/37 | 24/14 |
| Patient age | ||
| < 50 | 15/60 (25%) | 9/38 (24%) |
| ≥ 50 | 45/60 (75%) | 29/38 (76%) |
| Donor age | ||
| < 50 | 33/60 (55%) | 23/38 (60%) |
| ≥ 50 | 27/60 (45%) | 15/38 (40%) |
| Donor/patient transplant | ||
| ♂♂ | 19/60 (32%) | 10/38 (26%) |
| ♂♀ | 18/60 (30%) | 4/38 (10%) |
| ♀♀ | 12/60 (20%) | 10/38 (26%) |
| ♀♂ | 11/60 (18%) | 14/38 (38%) |
| Transfusions | ||
| < 76 | 49/58 (84%) | 34/36 (94%) |
| ≥ 76 | 9/58 (16%) | 2/36 (6%) |
| Recipient HLA antibodies positivea | 6/60 (10%) | 11/38 (29%) |
| CMV positive donor/recipient | 52/60 (87%) | 34/38 (89%) |
| CMV positive donor | 32/60 (53%) | 20/38 (53%) |
| CMV positive recipient | 48/60 (80%) | 24/38 (63%) |
| Risk at diagnosis | ||
| Low risk | 1/60 (2%) | 1/37 (3%) |
| Moderate risk | 28/60 (47%) | 22/37 (59%) |
| Poor risk/very poor risk | 31/60 (51%) | 14/37 (38%) |
| Conditioning | ||
| Flu/TBI | 52/60 (92%) | 31/38 (82%) |
| Other | 8/60 (8%) | 7/38 (18%) |
| Remission prior to transplantation | ||
| Complete remission | 53/60 (88%) | 32/38 (84%) |
| No remission | 5/60 (8%) | 5/38 (13%) |
| Persisting aplasia after chemotherapy | 2/60 (4%) | 1/38 (3%) |
| Type of transplant | ||
| Sibling | 34/60 (57%) | 19/38 (50%) |
| MUD | 26/60 (43%) | 19/38 (50%) |
| HLA match | ||
| 10/10 MUD | 33/60 (55%) | 17/38 (45%) |
| 9/10 MUD | 1/60 (2%) | 2/38 (5%) |
| Sibling | 26/60 (43%) | 19/38 (50%) |
| DPB1 | ||
| Match | 40/60 (66%) | 23/38 (60%) |
| Permissive | 10/60 (17%) | 8/38 (21%) |
| Non-permissive | 10/60 (17%) | 7/38 (19%) |
| GVHD prophylaxis | ||
| CsA/MMF | 56/60 (94%) | 32/38 (8%) |
| Other | 4/60 (7%) | 6/38 (16%) |
| Infused cells | Median (range) | Median (range) |
| CD3+ 106/kg | 246.8 (80.0–573.0) | 303.6 (140.2–642.6) |
| CD19+ 106/kg | 55.6 (12.0–131.3) | 70.4 (18.0–333.5) |
| CD34+ 106/kg | 6.6 (2.2–18.5) | 6.4 (2.6–13.5) |
GVHD graft-versus-host disease, HLA human leukocyte antigen, CMV cytomegalovirus, Flu fludarabine, TBI total body irradiation, MUD matched unrelated donor, CsA cyclosporine, MMF mycophenolate mofetil
aHLA antibodies found prior to transplantation in the serum
Table 2.
cGVHD distribution
| No cGVHD and mild cGVHD (n = 60) | Moderate to severe cGVHD (n = 38) | |
|---|---|---|
| Acute GVHD | ||
| Grade I | 5/60 (8%) | 4/38 (10%) |
| Grade II–IV | 18/60 (30%) | 8/38 (20%) |
| Chronic GVHD | ||
| Mild | 27/60 (45%) | – |
| Moderate | – | 27/38 (71%) |
| Severe | – | 11/38 (29%) |
| Cause of death | ||
| Transplantation-related mortality | 6/60 (10%) | 2/38 (5%) |
| Relapse | 15/60 (25%) | 2/38 (5%) |
| Other | 2/60 (3%) | – |
Chemotherapy regimens before transplantation consisted of induction with idarubicin (12 mg/m2 iv) and cytarabine (200 mg/m2 iv) with or without G-CSF (5 ug/kg sc), followed by consolidation with amsacrine (120 mg/m2 iv) and cytarabine (1000 mg/m2 iv).
Non-myeloablative conditioning consisted of fludarabine (30 mg/m2 iv days -4 until -2) and total body irradiation (2 Gy, day -1) in 85% of patients (appendix Table 1). Prophylaxis for GVHD consisted of cyclosporine (CsA) started on day -3 (twice daily 5 mg/kg) and mycophenolate mofetil (MMF) started on day 0 (twice daily 15 mg/kg oral) [16]. MMF was stopped at day + 28 or gradually tapered after day + 40 depending on the type of donor (SIB or MUD, respectively) and CsA was gradually tapered after day + 100 in the absence of GVHD. Acute GVHD grade II was treated as first line with systemic prednisolone 1–2 mg/kg/day and therapeutic doses of CsA. Chronic GVHD was treated as first line with local therapy as mild (0.1% triamcinolone cream, tacrolimus cream, dexamethasone 0.01% suspension) or with prolonged schedule of systemic prednisolone (1 mg/kg/day) progressively tapered over 1 year, in combination with a calcineurin inhibitor in case of moderate to severe cGVHD.
All patients were transplanted with a 10/10 matched SIB or MUD, except 4 patients. One of these 4 patients was transplanted with a 9/10 HLA-A mismatched SIB (patient homozygous on A locus) and three patients were transplanted with a 9/10 mismatched MUD (2 A mismatched and 1 DQB1 mismatched). None of the patients transplanted with a mismatched donor had HLA antibodies against the mismatched locus. Matching was performed using standard high-resolution typing of HLA-A, B, C, DRB1, and DQB1. All patients were typed for HLA-DPB1 and the DPB1 T Cell epitope algorithm was used to determine permissiveness of the combinations [17].
The number of cells in the graft (CD3+, CD19+, CD34+) was determined using fluorescence-activated cell sorting. Patient serum samples collected before transplantation were examined on HLA class I and class II IgG antibody reactivity using complement-dependent cytotoxicity and/or bead array (Lifescreen de luxe or LSA, Immucor). HLA antibody positivity was determined according to the manufacturer.
Risk factors
Three categories of risk factors were analyzed for association with the development of moderate to severe cGVHD: (1) pre-transplantation factors: recipient: age, number of transfusions before transplantation, presence of HLA IgG antibodies and HLA-DPB1; (2) donor-related factors: age, composition of the graft (CD3+, CD19+, CD34+ cells), type of donor, female donor to male recipient and positive CMV serology; (3) post-transplantation factor: development of aGVHD.
Statistical analysis
To describe patients and their disease, patients were stratified on the main outcome: no to mild cGVHD versus moderate to severe cGVHD. Time started at the date of transplantation and ended when moderate or severe cGVHD occurred. Death or relapse was a censoring event unless moderate or severe cGVHD was present before death or relapse. A cutoff Q75 was selected to quantify the effect of the donor-infused cells on the development of moderate to severe cGVHD. Univariate Cox regression analyses were performed to estimate time to the development of moderate to severe cGVHD. In this way, hazard ratios (HRs) and 95% confidence intervals (95% Cis) were provided. In the multivariable stepped forward Cox Regression analyses, all independent variables that contributed statistically significantly to the univariate analysis (P < 0.20) were entered. To correct for any confounding effect from SIB or MUD transplantation on the outcome, the type of transplant source was added to the multivariate Cox regression model. Risk factors with a P value < 0.05 were considered statistically significant. Comparing clinical characteristics between patients according to the identified risk factors were performed using the independent t test and Fisher’s exact test. Relapse and treatment-related mortality were calculated using Kaplan–Meier and comparisons were done by log-rank test. Analyses were performed in SPSS 22.
Results
A total of 100 consecutive patients fulfilled the inclusion criteria for this study. Two patients were excluded from this cohort due to missing data (Fig. 1). The final study cohort comprised 98 patients, 85 with AML and 13 with MDS. Median patient age at the time of transplantation was 57 years (range 24–74) and median donor age was 45 years (range 19–71). Clinical characteristics of the patients are summarized in Table 1.
Fig. 1.
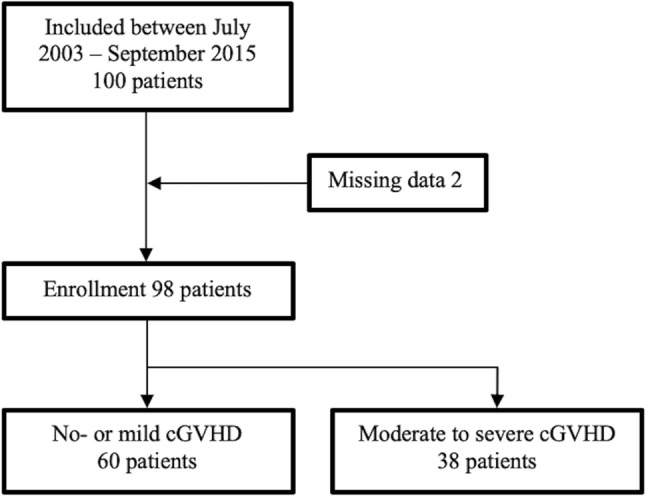
Cohort selection and patient distribution according to no or mild versus moderate to severe cGVHD
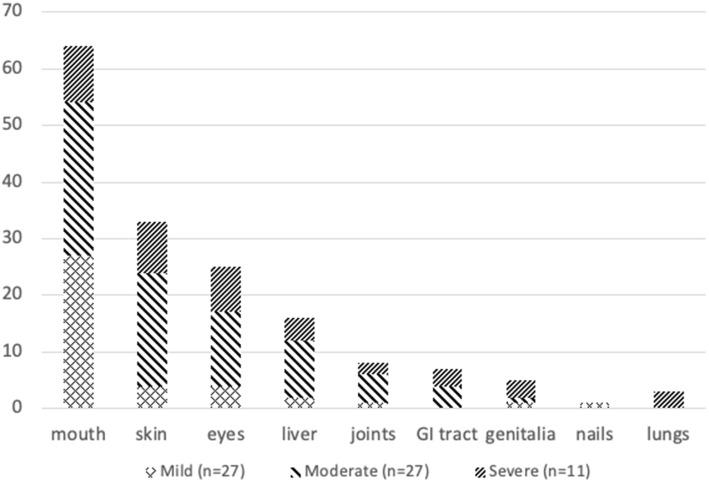
In our cohort, 65/98 (67%) patients developed cGVHD, 27 patients developed mild cGVHD and 38 patients developed moderate to severe cGVHD. The median time from transplantation to onset of mild cGVHD was 208 days (26–398). Onset of mild cGVHD was: de novo (no prior aGVHD) in 17 (63%), quiescent (prior aGVHD, but not currently active) in 3 (11%) patients, and progressive (progression from aGVHD to cGVHD) in 7 (26%) patients. The most frequent organ involvement in patients who developed mild cGVHD was mouth (100%), followed by eyes (15%) and skin (15%). Overall cGVHD severity distribution is described in Table 2. The median time from transplantation to onset of moderate to severe cGVHD was 208 days (54–380). Onset of moderate to severe cGVHD was: de novo in 26 (68%) patients, quiescent in 2 (5%) patients, and progressive in 10 (27%) patients. The most frequent organ involvement in patients who developed moderate to severe cGVHD was mouth in 37 (97%) patients, followed by skin in 29 (76%) and eyes in 21 (55%) patients. Other affected organs were liver in 37%, joints in 18%, gastrointestinal tract (GI) in 18%, genital/urologic tract in 10%, and lungs in 8% of the patients. The median number of organs involved in moderate to severe cGVHD was 3 per patient. The organ distribution according to severity of cGVHD in both groups is shown in Fig. 2.
Fig. 2.
Distribution of cGVHD severity according to organ involvement
Pre-transplantation factors associated with cGVHD
In the univariate analysis, we identified the following variables as risk factors for the development of moderate to severe cGVHD: presence of HLA antibodies in the patient before transplantation, graft composition with CD3+ cells ≥ 325 106/kg or CD19+ cells ≥ 82 106/kg. Multivariate Cox regression analysis confirmed HLA antibodies (P = 0.03, HR = 2.34, CI 95% 1.11–4.95), CD3+ cells in the graft ≥ 325 106/kg (P = 0.04, HR 2.18, CI 95% 1.04–4.59) and CD19+ cells in the graft ≥ 82 106/kg (P < 0.01, HR = 2.79, CI 95% 1.35–5.74) as independent risk factors associated with the development of moderate to severe cGVHD (Table 3). Other factors including type of donor were not significantly associated with the severity of cGVHD in this cohort.
Table 3.
Variables related to the development of moderate to severe cGVHD
| Risk factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Patient age | ||||||
| < 50 years | 1 | |||||
| ≥ 50 years | 1.18 | 0.55–2.50 | 0.662 | |||
| Donor age | ||||||
| < 50 years | 1 | |||||
| ≥ 50 years | 0.874 | 0.45–1.67 | 0.686 | |||
| Blood transfusions | ||||||
| < 76 | 1 | |||||
| ≥ 76 | 0.38 | 0.09–1.61 | 0.192 | |||
| HLA antibodies in the recipienta | ||||||
| HLA antibodies negative | 1 | |||||
| HLA antibodies positive | 2.49 | 1.22–5.08 | 0.012 | 2.34 | 1.11–4.95 | 0.026 |
| Donor/recipient CMV status | ||||||
| Matched | 1 | |||||
| Mismatched | 1.04 | 0.37–2.94 | 0.935 | |||
| Donor/recipient gender | ||||||
| Matched | 1 | |||||
| Female to male | 1.80 | 0.93–3.48 | 0.081 | |||
| Type of donor | ||||||
| SIB | 1 | |||||
| MUD | 1.36 | 0.72–2.59 | 0.342 | |||
| DPB1 | ||||||
| Match | 1 | |||||
| Permissive | 1.13 | 0.51–2.53 | 0.768 | |||
| Non-permissive | 1.40 | 0.60–3.28 | 0.441 | |||
| Permissive/non-permissive | 1.24 | 0.65–2.38 | 0.519 | |||
| Infused cells in the recipient: | ||||||
| CD3 + 106/kg < 325 | 1 | |||||
| CD3 + 106/kg ≥ 325 | 3.06 | 1.56–6.00 | 0.001 | 2.18 | 1.04–4.59 | 0.040 |
| CD19 + 106/kg < 82 | 1 | |||||
| CD19 + 106/kg≥ 82 | 3.77 | 1.93–7.37 | 0.000 | 2.79 | 1.35–5.74 | 0.005 |
| CD34 + 106/kg < 8.6 | 1 | |||||
| CD34 + 106/kg ≥ 8.6 | 1.47 | 0.73–2.97 | 0.281 | |||
| GVHD | ||||||
| No acute GVHD | 1 | |||||
| Acute GVHD | 0.84 | 0.42–1.66 | 0.612 | |||
Total of infused cells boundaries were determined based on Q75 for all cells
HLA human leukocyte antigen, CMV cytomegalovirus, GVHD graft-versus-host disease
aHLA antibodies found prior to transplantation in the serum
Bold values indicate that statistical significance P < 0.05)
Comparing the clinical characteristics between patients according to the identified risk factors, there were no differences among groups. In the group of patients with positive vs negative pre-transplant HLA antibodies (17 vs 81 patients), median patient age was 54 (35–68) vs 56 years (24–74) (t(96) = 1,17, P = 0.24), median donor age was 40 (20–66) vs 48 years (19–71) (t(96) = 1.88, P = 0.06), 76% vs 86% received fludarabine/TBI as conditioning (P = 0.24) and 90% in both groups received CsA/MMF as immunosuppression (P = 0.54). Nevertheless, 11 (65%) vs 27 (33%) patients developed moderate to severe cGVHD. The HLA antibodies detected in these 17 patients were directed against HLA class I in 6 patients, against class HLA class II in 2 patients and against HLA class I and II in 9 patients. None of the patients with HLA antibodies had donor-specific antibodies.
Comparing the group of patients who received grafts with ≥ 325 vs < 325 106/kg CD3 + cells (24 vs 73 patients), median patient age was 58 (44–69) vs 55 years (24–74) (t(95) = − 1.16, P = 0.24), median donor age was 44 (20–69) vs 45 years (19–71) (t(33) = 0.28, P = 0.78), 75% vs 88% received fludarabine/TBI as conditioning (P = 0.19) and 83% vs 91% received CsA/MMF as immunosuppression (P = 0.26). Nevertheless, 15 (63%) vs 22 (30%) patients developed moderate to severe cGVHD.
Comparing the group of patients who received grafts with ≥ 82 vs < 82 106/kg CD19 + cells (24 vs 71 patients), median patient age was 59 (44–69) vs 56 years (24–74) (t(93) = − 1.41, P = 0.16), median donor age was 47 (20–67) vs 45 years (19–71) (t(93) = 0.071, P = 0.48), 79% vs 86% received fludarabine/TBI as conditioning (P = 0.31) and 96% vs 87% received CsA/MMF as immunosuppression (P = 0.22). Nevertheless, 16 (67%) vs 20 (28%) patients developed moderate to severe cGVHD.
Comparing treatment-related mortality (TRM) 3 years after AlloSCT between patients in the control group versus the group with moderate to severe cGVHD, we saw no significant difference (15% vs 5%, P = 0.20). Transplantation-related causes of death are described in Table 4.
Table 4.
Transplantation-related causes of mortality
| No cGVHD | Mild cGVHD | Moderate to severe cGVHD | |
|---|---|---|---|
| Sepsis | 1 | 1 | 1 |
| Progressive encephalopathy | 1 | – | – |
| Multiple viral/bacterial infections | 1 | – | – |
| Treatment-resistant aGVHD | 1 | – | – |
| Multi-organ failure | 1 | – | 1 |
Comparing the occurrence of relapse rate (RR) 3 years after AlloSCT, we found a significantly higher cumulative RR in patients in the control group versus patients with moderate to severe cGVHD (30% vs 7%, P < 0.01). Six (10%) patients in the control group relapsed within 6 months with no acute or mild cGVHD, 3/60 (6%) patients relapsed within 6 months with previous aGVHD, 1/60 (2%) relapsed within 6 months with previous acute and mild cGVHD, the other (8%) patients relapsed after 6 months. In the moderate to severe group, two patients relapsed, after 11 months and 3 years with moderate cGVHD. In our study, the group “no cGVHD and mild cGVHD” has a significantly higher relapse rate after 3 months and within 1 year after ALLOSCT compared with the group who developed moderate to severe cGVHD. The high relapse rate in this group explains the higher mortality rate in the group “no cGVHD and mild cGVHD” versus “moderate to severe cGVHD” (appendix Table 2). If we analyzed which factors could explain this difference, we know that the distribution of patients according to AML/MDS risk status by diagnosis and according to remission status before ALLOSCT (low—and intermediate risk versus poor—and very poor risk) did not differ between these 2 groups. Moreover, the conditioning regimen, immunosuppression regimen and the type of donor did not differ between these 2 groups. Thus, the factors related with a high percentage of relapses in the group with no-GVHD may be related to early immunological factors (as activation of donor T cells, migration of donor immune cells into target organs and thymic injury) which avoid tolerance and therefore recognize residual leukemic cells avoiding relapses in patients who develop moderate to severe cGVHD [18].
Discussion
In this well-defined unselected series of patients diagnosed with AML and/or MDS who consecutively received a peripheral blood T-cell repleted NMA AlloSCT, moderate or severe chronic graft-versus-host disease was associated with high morbidity, hospital dependency and poor quality of life as compared to mild cGVHD [6, 7]. The goal of this study was to identify risk factors associated with severity, i.e., with the development of moderate to severe cGVHD in this population.
We identified 3 independent risk factors associated with the development of moderate to severe cGVHD: HLA antibodies in patient’s serum before transplantation, CD19+ ≥ 82 106/kg cells and/or CD3+ ≥ 325 106/kg cells in the graft.
Our results on the correlation of HLA antibodies with the severity of cGVHD are in accordance with a previously reported study [19]. Pan et al. described a correlation between the presence in patients’ serum of antibodies against HLA before or in the 1st month after transplantation and cGVHD. They studied a cohort of patients diagnosed with hematological malignancies who received a BM/PBSCT with myeloablative conditioning. They found a higher rate of extensive cGVHD in the group with HLA antibodies in comparison to the group of patients without HLA antibodies prior to transplantation [19]. In our study, the HLA antibody status before transplantation was associated with the development of moderate to severe cGVHD. We know that pre-formed HLA antibodies prior to AlloSCT can be unaffected by standard transplantation conditioning regimens [20]. Broad sensitization against many HLA antigens can occur when the immune system is only exposed to a single non-self HLA antigen, resulting in HLA antibodies that can react to more than one antigen (cross-reactivity) [21]. In a patient receiving allogeneic cells followed by a changing very active immune system, HLA antibody cross-reactivity to non-self or auto-antigens could stimulate auto-inflammatory reactions. Having identified HLA antibodies in patients before AlloSCT as risk factor for moderate to severe cGVHD, we hypothesize that the presence of HLA antibodies may indicate a state of high immune reactivity in patients, increasing the probability of triggering a more severe cGVHD.
We also identified the amount of infused CD19+ cells as a risk factor for the development of moderate to severe cGVHD. Recent studies demonstrated that B cells play an important role in the complex immuno-pathophysiology of cGVHD having an effector function, generating alloantibodies and producing transforming growth factor beta [22–25]. Patients with cGVHD have high levels of B-cell-activating factor, present with increased survival of alloreactive- and auto-reactive B cells, and subvert the development of B-cell tolerance by attenuating B-cell receptor-triggered apoptosis of newly created polyreactive B cells [23, 24]. Moreover, a subtype of B cells (CD19+ CD21 low) has been found as an expanded population with features of exhaustion in patients with cGVHD and they have been correlated with severity of cGVHD [26]. These articles support the role of CD19+ cells as a parameter of severity in patients with cGVHD.
The correlation between the number of T cells in the graft with the incidence of moderate to severe cGVHD is in accordance with previously published studies [14, 27, 28]. These reports show a lower incidence of cGVHD in patients transplanted with BM as source of stem cells as compared to PB stem cells. Typically, PBSC grafts obtained using G-CSF for PBSC mobilization contain more T cells than BM grafts, resulting in a higher risk to develop GVHD [14, 27–30].
Our study has limitations that can explain why other expected risk factors as CMV serostatus and recipient gender were not found associated with the development of moderate to severe cGVHD [6, 31]. In our cohort, we did not focus on the development of global cGVHD, but we focussed on severity. A role of CMV serostatus as a risk factor for the development of moderate to severe cGVHD was not found. This could be explained by the fact that almost all donor/recipients in our cohort had a positive CMV serostatus and therefore had no discriminating value. For female donor to male recipient transplantations, there was a trend towards a higher chance for moderate to severe cGVHD, but this did not reach statistical significance. We also did not find a role of previous aGVHD as a predictor for the development of moderate to severe cGVHD, as opposed to other studies [5, 6]. A possible explanation may be early RR and TRM that occurred in the control group, resulting in a relatively high mortality rate within 6 months after transplantation. Nevertheless, the association of the identified risk factors with severity of cGVHD has biological support and opens up the opportunity to be validated in a multi-center study.
In conclusion, we identified HLA antibodies in patients and number of CD19 + and/or CD3 + cells in the graft to be associated with the development of moderate to severe cGVHD in patients with AML or MDS who received a NMA AlloPBSCT. Our results should be confirmed in a larger multi-center cohort of patients to confirm clinical significance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
All patients included in the study had signed the informed consent to eventually use the data for scientific goals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission—a review from the acute leukemia working party of the EBMT. Haematologica. 2015;100:859–869. doi: 10.3324/haematol.2015.123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Baldomero H, Passweg J, Urbano-Ispizua A. Increasing use of reduced intensity conditioning transplants: report of the 2001 EBMT activity survey. Bone Marrow Transplant. 2002;30:813–831. doi: 10.1038/sj.bmt.1703819. [DOI] [PubMed] [Google Scholar]
- 3.Maris M, Niederwieser D, Sandmaier BM, Storer B, Stuart M, Maloney D, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 4.Flowers MED, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615. doi: 10.1182/blood-2014-08-551994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation results from a single-center observational study. Biol Blood Marrow Transplant. 2016;22:1781–1791. doi: 10.1016/j.bbmt.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Remberger M, Kumlien G, Aschan J, Barkholt L, Hentschke P, Ljungman P, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:674–682. doi: 10.1053/bbmt.2002.v8.abbmt080674. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Kim HT, Ho VT, Cutler C, Alyea EP, Soiffer RJ, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38:305–310. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- 8.Vogelsang GB. How I treat chronic graft-versus-host disease. Blood. 2001;97:1196–1201. doi: 10.1182/blood.V97.5.1196. [DOI] [PubMed] [Google Scholar]
- 9.Koc S, Leisenring W, Flowers MED, Anasetti C, Joachim Deeg H, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.V100.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SR, Sizemore CA, Ridgeway M, Zhang X, Smith J, Brown S, et al. Corticosteroid-free primary treatment of chronic extensive graft-versus-host disease incorporating rituximab. Biol Blood Marrow Transplant. 2015;21:1576–1582. doi: 10.1016/j.bbmt.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 12.Flowers MED, Inamoto Y, Carpenter PA, Lee SJ, Kiem H, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afram G, Simón JAP, Remberger M, Caballero-Velázquez T, Martino R, Piñana JL, et al. Reduced intensity conditioning increases risk of severe cGVHD: identification of risk factors for cGVHD in a multicenter setting. Med Oncol. 2018;35:79. doi: 10.1007/s12032-018-1127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–767. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 response criteria working group report. Biol Blood Marrow Transplant. 2015;21:984–999. doi: 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 17.Crivello P, Zito L, Sizzano F, Zino E, Maiers M, Mulder A, et al. The impact of amino acid variability on alloreactivity defines a functional distance predictive of permissive HLA-DPB1 mismatches in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:233–241. doi: 10.1016/j.bbmt.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: a task force report from the national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23:211–234. doi: 10.1016/j.bbmt.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Z, Yuan X, Li Y, Wu X, Zhu W, Bao X, et al. Dynamic detection of anti-human leukocyte antigen (HLA) antibodies but not HLA-DP loci mismatches can predict acute graft-versus-host disease and overall survival in HLA 12/12-matched unrelated donor allogeneic hematopoietic stem cell transplantation for hematological malignancies. Biol Blood Marrow Transplant. 2016;22:86–95. doi: 10.1016/j.bbmt.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Koclega A, Markiewicz M, Siekiera U, Dobrowolska A, Sylwia M, Dzierzak-Mietla M, et al. The presence of anti-HLA antibodies before and after allogeneic hematopoietic stem cells transplantation from HLA-mismatched unrelated donors. Bone Marrow Res. 2012;2012:1–7. doi: 10.1155/2012/539825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickey MJ, Valenzuela NM, Reed EF. Alloantibody generation and effector function following sensitization to human leukocyte antigen. Front Immunol. 2016;7:30. doi: 10.3389/fimmu.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tayebi H, Lapierre V, Saas P, Lienard A, Sutton L, Milpied N, et al. Enhanced activation of B cells in a granulocyte colony-stimulating factor-mobilized peripheral blood stem cell graft. Br J Haematol. 2001;114:698–700. doi: 10.1046/j.1365-2141.2001.02965.x. [DOI] [PubMed] [Google Scholar]
- 23.Socié G. Chronic GVHD: B cells come of age. Blood. 2011;117:2086–2087. doi: 10.1182/blood-2010-12-322297. [DOI] [PubMed] [Google Scholar]
- 24.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:16–23. doi: 10.1016/j.bbmt.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyama M, Hill GR. Alloantigen presentation and graft-versus-host disease: fuel for the fire. Blood. 2016;127:2963–2970. doi: 10.1182/blood-2016-02-697250. [DOI] [PubMed] [Google Scholar]
- 26.Khoder A, Alsuliman A, Basar R, Sobieski C, Kondo K, Alousi AM, et al. Evidence for B cell exhaustion in chronic graft-versus-host disease. Front Immunol. 2017;8:1937. doi: 10.3389/fimmu.2017.01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Soeiete Francaise de Greffe de Moelle. J Clin Oncol. 2000;18:537–546. doi: 10.1200/JCO.2000.18.3.537. [DOI] [PubMed] [Google Scholar]
- 29.Eapen M, Logan BR, Confer DL, Haagenson M, Wagner JE, Weisdorf DJ, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13:1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald KPA, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129:13–21. doi: 10.1182/blood-2016-06-686618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–1700. doi: 10.1182/blood.V98.6.1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.