Abstract
Introduction
Clinical outcomes after the implantation of allogenic human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) in osteoarthritic knees have been rarely reported. Our study aimed to investigate clinical outcomes of osteoarthritic patients who underwent hUCB-MSC implantation.
Methods
In this case series (level of evidence: 4), from January 2014 to December 2015, 128 patients with full-thickness cartilage lesions (International Cartilage Repair Society grade 4 and Kellgren–Lawrence grade ≤3) who underwent hUCB-MSC implantation were retrospectively evaluated with a minimum of 2-year follow-up. After removing the sclerotic subchondral bone with an arthroscopic burr, 4-mm-diameter holes were created at 2-mm intervals, and hyaluronic acid and hUCB-MSCs were subsequently mixed and implanted in the holes and other articular defect sites.
Clinical outcomes were evaluated preoperatively, 1 year postoperatively, and 2 years postoperatively (minimum) using visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and International Knee Documentation Committee (IKDC) scores. To assess clinical outcomes, patients were divided into two or three groups according to the lesion size, lesion location, number of lesions, body mass index, and age; statistical analyses were performed using these data.
Results
The mean (±standard deviation) VAS, WOMAC, and IKDC scores at 1 and 2 years after surgery including hUCB-MSC implantation improved significantly compared to the preoperative scores (P < 0.001). There were significant differences in the lesion location (P < 0.05). Medial femoral condyle lesions resulted in worse outcomes compared with lateral femoral condyle and trochlea lesions. No adverse reactions or postoperative complications were noted.
Conclusions
Implantation of hUCB-MSCs is effective for treating knee osteoarthritis based on a follow-up lasting a minimum of 2 years.
Keywords: Mesenchymal stem cells, Knee osteoarthritis, Human umbilical cord blood, Allogenic
Abbreviations: AT-MSCs, adipose tissue-derived MSCs; ACI, autologous chondrocyte implantation; BMI, body mass index; BM-MSCs, bone marrow-derived MSCs; hUCB-MSCs, human umbilical cord blood-derived mesenchymal stem cells; HA, hyaluronic acid; IKDC, International Knee Documentation Committee; KL, Kellgren–Lawrence; LFC, lateral femoral condyle; MFC, medial femoral condyle; MRI, magnetic resonance imaging; OA, osteoarthritis; OAT, osteochondral autologous transplantation; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index
1. Introduction
Osteoarthritis (OA) of the knee is a common problem characterized by joint pain, swelling, stiffness, and disability [1,2]. The main problem associated with OA is articular cartilage defect, which has a limited capacity for repair. Because of the lack of effective cartilage repair, it is important to treat cartilage defects early to slow the progression of OA [3].
Autologous chondrocyte implantation (ACI) and osteochondral autologous transplantation (OAT) are effective therapies for articular cartilage regeneration. However, ACI has some limitations, such as difficult application during two-step surgery, difficulty obtaining a suitable amount of chondrocytes, a slow rate of cartilage cell proliferation, and donor-site pathology [4]. OAT is an attractive option for repairing cartilage defects because it is the only procedure that involves real hyaline cartilage [5]; however, it is limited by its donor-site morbidity, a gap or unevenness, and fibrocartilage formation between the cartilage plug and native cartilage [6].
Recently, mesenchymal stem cells (MSCs) were identified as a new option in the field of cartilage regeneration, and some authors have reported that patients with knee OA treated with MSC implantation showed clinical improvements [3, [7], [8], [9], [10], [11], [12]].
Few studies on the mechanism of allogenic hUCB-MSCs in humans have been performed [13]. Wang et al. [14] showed that, similar to bone marrow-derived MSCs (BM-MSCs), allogenic hUCB-MSCs have morphological phenotype characteristics including the expression of various phenotypic markers, multilineage differentiation capacity, immunogenicity, and immunoregulatory potency in vitro. Furthermore, Park et al. reported a symptomatic, large osteochondral defect of the knee joint that was repaired using a composite of hUCB-MSCs and hyaluronic acid (HA). Their case report, which included 5-year follow-up, suggested that pain and function were significantly improved and that a composite of hUCB-MSCs and HA hydrogel is safe for treating large osteochondral defects of the knee joint. Nevertheless, clinical results after hUCB-MSC implantation for knee OA have been rarely reported [11, 15].
Allogenic hUCB-MSCs have some advantages over other autologous adult stem cells such as BM-MSCs and adipose tissue-derived MSCs (AT-MSCs). First, compared with BM-MSC implantation, hUCB-MSC implantation is not an invasive procedure and has low donor-site morbidity [16, 17]. Second, hUCB-MSCs can be easily collected and have a high expansion capacity that is greater than that of BM-MSCs [18, 19]. Third, hUCB-MSCs have low immunogenicity in vitro and in vivo [20], and there is no immunological response, even during xeno-transplantation [[18], [19]]. Fourth, bone formation occurs after BM-MSC implantation, and decreased cartilage repair has been shown with AT-MSC implantation. However, in the study of Park et al. [[18], [19]], hUCB-MSC implantation in 15 Sprague–Dawley rats resulted in superior cartilage repair without bone formation or degenerative changes in the cartilage for up to 8 weeks. Whether the exact mechanism for cartilage repair with hUCB-MSCs is due to direct differentiation or paracrine effects remains unknown [21, 22]. Nonetheless, we believe that they are more useful than other MSC sources for cartilage regeneration because of their advantages and thus hypothesized that hUCB-MSC implantation is safe and effective for treating OA. Hence, this retrospective study aimed to evaluate the clinical results of hUCB-MSC implantation as treatment for knee OA.
2. Methods
2.1. Patient selection
This study retrospectively evaluated 128 out of 143 patients with knee OA who underwent hUCB-MSC implantation from January 2014 to December 2015 at Gangnam JS Hospital (Seoul, Korea). Inclusion criteria were age older than 40 years, full-thickness cartilage lesion measuring at least 2 cm2, femoro-tibial angle (varus or valgus) < 8° in the mechanical axis, and Kellgren–Lawrence (KL) grade between 1 and 3 (CARTISTEM®; hUCB-MSCs were approved for use for KL grades 1–3 only). Exclusion criteria were grade 3 or less as graded by the International Cartilage Repair Society, KL grade 4, ligament injuries (such as injuries of the anterior cruciate ligament and posterior cruciate ligament), previous cartilage surgery, metabolic arthritis, and joint infection. All patients provided written informed consent. This study was reviewed and approved by the public institutional review board of the Ministry of Health and Welfare and was performed in accordance with the principles of the Declaration of Helsinki.
2.2. Preparation of therapeutic mesenchymal stem cells
In this study, we used CARTISTEM®, which is a medicinal product for the treatment of knee OA. CARTISTEM® with hUCB-MSCs for cartilage repair was produced by MEDIPOST (Seongnam, Gyeonggi-do, South Korea), and its therapeutic use for cartilage repair was approved by the Korea Food and Drug Administration in January 2012 [[21], [22]]. This product contains 1.5 mL of cord blood-derived MSCs (7.5 × 106) and 4% HA.
According to MEDIPOST, CARTISTEM® is made as follows: UCB was collected from maternal umbilical veins during delivery with informed consent and stored at a cord blood bank. hUCB-MSCs were isolated from hUCB and expanded by repeated subcultures [[21], [22]].
Before implantation, hUCB-MSCs and HA were mixed according to the manufacturer's instructions during surgery. The therapeutic dosage of CARTISTEM® was 500 μL/cm2 for a defect area with a cell concentration of 0.5 × 107 cells/mL; the defect area was evaluated using magnetic resonance imaging (MRI) before surgery.
2.3. Surgical technique and postoperative management
The patient was placed in the supine position on the operating table. Standard anteromedial and anterolateral portals were created. If meniscus problems were found during arthroscopy, then meniscectomy or meniscal repair was performed as needed. Arthrotomy was performed 5 mm medial to the patella; a 4-cm long longitudinal arthrotomy was performed. If there was a lateral-side cartilage lesion, arthrotomy was performed 5 mm lateral to the patella. Damaged cartilage was completely removed, and a ruler was used to measure the height and width of the lesion.
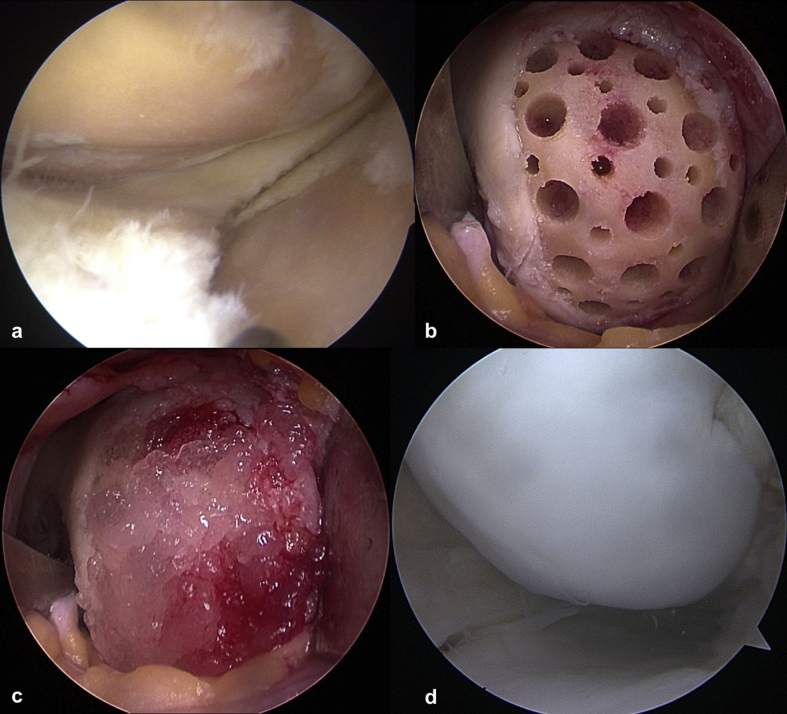
After removing the sclerotic subchondral bone with an arthroscopic burr, main holes were created using a drill bit (MEDIPOST) at 2-mm intervals with a depth and circumference of 4 mm, and smaller holes with a diameter of 2 mm were created between the main holes. Subsequently, HA and hUCB-MSCs were mixed and implanted in the holes and trimmed to the height of the surrounding articular surface (Fig. 1). If the lesion was in the tibial plateau and drilling was not possible, microfracture was performed. We closed the wound at 5 min after implantation. Then, a knee brace was applied.
Fig. 1.
(a) A 58-year-old woman had a lesion on the medial femoral condyle and a focal lesion on the medial tibial plateau of the left knee. (b) The damaged cartilage was completely removed, and the sclerotic subchondral bone was removed. Multiple holes with a diameter of 4 mm and 2 mm were created on the medial femoral condyle. (c) After a mixture of hyaluronic acid and human umbilical cord blood-derived mesenchymal stem cells was implanted in the holes and trimmed to the height of surrounding articular surface on the medial femoral condyle. (d) Second-look arthroscopy at 15 months after surgery. This was performed during opposite knee surgery.
Patients wore a brace and used a crutch to perform non-weight-bearing walking for 8 weeks after surgery. If the lesion was an isolated trochlea lesion, partial weight-bearing was allowed with full extension with a locking brace. On day 4 postoperatively, patients started range-of-motion exercises with a continuous passive motion machine and progressive quadriceps-strengthening exercises.
2.4. Outcome evaluation
The visual analog scale (VAS) scores, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores, and International Knee Documentation Committee (IKDC) scores were evaluated preoperatively and 1 and 2 years postoperatively. Patients were divided into groups according to characteristics including age (younger group, younger than 60 years; older group, 60 years or older), body mass index (BMI; not obese group, BMI <25; obese group, BMI ≥25), number of lesions (single-lesion group, two-lesion group, three-lesion group), lesion location (medial femoral condyle [MFC] lesion group, lateral femoral condyle [LFC] lesion group, and trochlea lesion group), and lesion size of the single-lesion group (small group, <4 cm2; large group, ≥4 cm2). Then, the influences of these characteristics on clinical outcomes were evaluated. We also investigated complications such as infections and allergic reactions after surgery.
Radiological evaluation included the following: (1) morphological MRI, which was performed in patients who agreed to undergo MRI to assess the status of cartilage repair at 3–6 months and >1 year after surgery, and (2) assessments using the modified Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scoring system.
2.5. Statistical analysis
In this study, the independent variables were age, BMI, number of lesions, lesion location, and lesion size; dependent variables were IKDC, VAS, and WOMAC scores. All values were described as mean ± standard deviation and range. The Wilcoxon signed-rank test was performed to compare the IKDC, VAS, and WOMAC scores preoperatively, 1 year postoperatively, and at the time of the final evaluation postoperatively for all patients. The Mann–Whitney test and Kruskal–Wallis test were used to compare two or three independent variables. After the Kruskal–Wallis test was performed, a post hoc nonparametric test was performed after Bonferroni adjustment of the significance level and a Mann–Whitney U test was performed. All statistical analyses were performed using IBM SPSS statistics version 23.0 (IBM Corp., Armonk, NY); significance was defined as P < 0.05.
3. Results
Fifteen patients were excluded (10 patients younger than 40 years old; 2 patients with small lesions; 1 patient who died of a heart attack; 2 patients who were lost during follow-up) among 143 patients. Therefore, 128 patients were enrolled in this study. There were 86 (67.2%) women and 42 (32.8%) men with a mean age of 56.5 years (±7.9 years; range, 40–78 years).
There were 109 patients (85.2%) with a meniscal problem; 53 (41.4%) of these patients underwent meniscectomy and 56 (43.8%) underwent meniscal repair. Furthermore, 96 (75%) patients with a lesion on the tibial plateau underwent microfracture.
The mean follow-up period was 36.1 ± 6.4 (range, 25–47) months. The mean BMI was 24.6 ± 3.6 (range, 17–45.8) kg/m2. The mean sizes of the cartilage lesions of the single-lesion, two-lesion, and three-lesion groups were 4.5 ± 1.3 cm2 (range, 2.6–8.3 cm2; 67 patients), 7.3 ± 2.9 cm2 (sum of two-lesion site; range, 3.6–20.1 cm2; 49 patients), and 9.8 ± 3.6 cm2 (sum of three-lesion site; range, 4.6–18.6 cm2; 12 patients), respectively. The mean sizes of the cartilage lesions of the MFC, LFC, and trochlea groups were 4.3 ± 1.2 cm2 (range, 3.2–7.1 cm2; 38 patients), 5.2 ± 1.0 cm2 (range, 3.2–5.9 cm2; 6 patients), and 4.6 ± 1.6 cm2 (range, 2.6–8.3 cm2; 22 patients), respectively (Table 1).
Table 1.
Demographic data.
| Patients, n | 128 |
|---|---|
| Age, y | 56.5 ± 7.9 |
| Sex, female/male, n | 86 (67.2%)/42 (32.8%) |
| Follow-up period, mo | 36.1 ± 6.4 |
| Mean BMI, kg/m2 | 24.6 ± 3.6 |
| Meniscal problem, n | 109 |
| Meniscectomy, n | 53 |
| Meniscal repair, n | 56 |
| Microfracture of tibial plateau, n | 96 |
| Lesions, one/two/three, n (cm2) | 67 (4.5 ± 1.3)/49 (7.3 ± 2.9)/12 (9.8 ± 3.6) |
| Lesion site, MFC/LFC/trochlea, n (cm2) | 38 (4.3 ± 1.2)/6 (5.2 ± 1.0)/22 (4.6 ± 1.6) |
BMI, body mass index; LFC, lateral femoral condyle; MFC, medial femoral condyle; mo., months; N, number; y, years.
IKDC, VAS, and WOMAC scores at 1 year and at final follow-up examination after surgery improved significantly compared to the preoperative scores (P<0.001 for all) (Table 2). Scores at the final follow-up after surgery improved significantly compared to those at 1 year after surgery (P<0.001 for all). Preoperative IKDC scores were significantly different (P < 0.05), but other scores at 1 year and the final follow-up after surgery were not significantly different (P>0.05) between the younger group and older group (Table 3).
Table 2.
Preoperative and follow-up clinical scoresa.
| Preoperative | 1-year follow-up | Final follow-up | Pc | Pd | Pe | |
|---|---|---|---|---|---|---|
| VAS scoreb | 7.0 ± 1.6 | 2.5 ± 1.7 | 2.0 ± 2.1 | <0.001 | <0.001 | <0.001 |
| WOMAC scoreb | 39.3 ± 12.2 | 17.2 ± 12.7 | 13.9 ± 14.1 | <0.001 | <0.001 | <0.001 |
| IKDC scoreb | 32.5 ± 8.3 | 55.8 ± 14.3 | 61.2 ± 17.2 | <0.001 | <0.001 | <0.001 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Bold indicates statistical significance (P < 0.001). IKDC, International Knee Documentation Committee; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Wilcoxon signed-rank test.
P value of preoperative score vs. score at 1 year after surgery.
P value of preoperative score vs. score at final follow-up after surgery.
P value of score at 1 year after surgery vs. score at final follow-up after surgery.
Table 3.
Comparisons of clinical scores according to age and body mass indexa.
| Age |
BMI, kg/m2 |
|||||
|---|---|---|---|---|---|---|
| <60 (n = 86) | ≥60 (n = 42) | P | <25 (n = 77) | ≥25 (n = 51) | P | |
| VAS scoreb | ||||||
| Preoperative | 7.0 ± 1.6 | 7.2 ± 1.5 | 0.458 | 7.2 ± 1.6 | 7.0 ± 1.5 | 0.563 |
| 1-year follow-up | 2.5 ± 1.7 | 2.5 ± 1.7 | 0.944 | 2.5 ± 1.7 | 2.6 ± 1.6 | 0.393 |
| Final follow-up | 2.0 ± 2.1 | 1.9 ± 2.0 | 0.732 | 2.0 ± 2.2 | 2.0 ± 1.9 | 0.547 |
| WOMAC scoreb | ||||||
| Preoperative | 38.4 ± 12.4 | 41.2 ± 12.0 | 0.162 | 39.1 ± 12.7 | 39.6 ± 11.8 | 0.604 |
| 1-year follow-up | 17.0 ± 12.7 | 17.7 ± 12.9 | 0.648 | 17.6 ± 13.7 | 16.7 ± 11.2 | 0.872 |
| Final follow-up | 13.6 ± 13.9 | 14.4 ± 14.7 | 0.545 | 14.4 ± 15.4 | 13.1 ± 12.1 | 0.979 |
| IKDC scoreb | ||||||
| Preoperative | 33.9 ± 8.2 | 29.5 ± 7.7 | 0.005 | 32.4 ± 8.3 | 32.5 ± 8.2 | 0.808 |
| 1-year follow-up | 56.7 ± 14.4 | 53.8 ± 13.9 | 0.278 | 55.4 ± 14.5 | 56.4 ± 14.0 | 0.752 |
| Final follow-up | 62.3 ± 17.2 | 59.1 ± 17.2 | 0.344 | 61.6 ± 18.0 | 60.7 ± 16.0 | 0.644 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Bold indicates statistical significance (P < 0.05). BMI, body mass index; IKDC, International Knee Documentation Committee; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Mann-Whiney U test.
All scores at 1 year and at final follow-up after surgery were not significantly different between the not obese group and the obese group (P > 0.05) or among the groups with one lesion, two lesions, and three lesions (P>0.05) (Table 3, Table 4). However, among the groups with MFC, LFC, and trochlea lesions, the VAS and WOMAC scores at 1 year and at final follow-up were significantly different (P > 0.05 for all) (Table 5). Preoperative VAS and WOMAC scores were significantly different between the small lesion group and large lesion group (P = 0.010 and P = 0.019, respectively); however, other scores were not significantly different (P > 0.05) (Table 6). No allergic reaction or infection was associated with hUCB-MSC implantation.
Table 4.
Comparisons of clinical scores according to the number of lesionsa.
| Single lesion (n = 67) | Two lesions (n = 49) | Three lesions (n = 12) | P | |
|---|---|---|---|---|
| VAS scoreb | ||||
| Preoperative | 6.8 ± 1.6 | 7.3 ± 1.5 | 7.6 ± 1.7 | 0.151 |
| 1-year follow-up | 2.4 ± 1.8 | 2.6 ± 1.5 | 3.0 ± 2.0 | 0.508 |
| Final follow-up | 1.9 ± 2.1 | 2.1 ± 2.0 | 2.4 ± 2.0 | 0.428 |
| WOMAC scoreb | ||||
| Preoperative | 40.2 ± 13.4 | 38.6 ± 10.3 | 37.3 ± 13.8 | 0.848 |
| 1-year follow-up | 18.6 ± 14.8 | 15.0 ± 8.6 | 18.6 ± 13.8 | 0.723 |
| Final follow-up | 14.6 ± 16.0 | 12.6 ± 11.2 | 15.1 ± 13.9 | 0.957 |
| IKDC scoreb | ||||
| Preoperative | 33.7 ± 8.7 | 31.1 ± 7.6 | 31.3 ± 8.0 | 0.295 |
| 1-year follow-up | 55.2 ± 15.0 | 56.7 ± 12.3 | 55.3 ± 18.2 | 0.915 |
| Final follow-up | 62.0 ± 18.1 | 60.5 ± 2.2 | 59.6 ± 5.4 | 0.695 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Bold indicates statistical significance (P < 0.05). IKDC, International Knee Documentation Committee; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Kruskal-Wallis test.
Table 5.
Comparisons of clinical scores according to lesion sitea.
| MFC (n = 38) | LFC (n = 6) | Trochlea (n = 22) | P | |
|---|---|---|---|---|
| VAS scoreb | ||||
| Preoperative | 6.8 ± 1.7 | 7.5 ± 1.9 | 6.6 ± 1.6 | 0.565 |
| 1-year follow-up | 2.7 ± 1.8 | 2.8 ± 2.1 | 1.6 ± 1.4 | 0.048c |
| Final follow-up | 2.4 ± 2.3 | 2.0 ± 2.4 | 0.8 ± 1.2 | 0.019c |
| WOMAC scoreb | ||||
| Preoperative | 41.6 ± 13.0 | 41.5 ± 9.7 | 37.45 ± 15.3 | 0.437 |
| 1-year follow-up | 22.0 ± 16.3 | 20.2 ± 11.6 | 11.8 ± 10.3 | 0.019 |
| Final follow-up | 18.9 ± 18.6 | 13.0 ± 13.8 | 7.2 ± 7.4 | 0.044 |
| IKDC scoreb | ||||
| Preoperative | 33.9 ± 8.3 | 28.0 ± 11.2 | 35.0 ± 8.4 | 0.412 |
| 1-year follow-up | 52.1 ± 15.6 | 54.7 ± 15.7 | 61.5 ± 12.2 | 0.055 |
| Final follow-up | 58.0 ± 19.6 | 63.4 ± 22.0 | 69.3 ± 11.7 | 0.061 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Bold indicates statistical significance (P < 0.05). IKDC, International Knee Documentation Committee; LFC, lateral femoral condyle; MFC, medial femoral condyle; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Kruskal-Wallis test. cMFC < trochlea, MFC = LFC, LFC = trochlea (post hoc nonparametric test used with Bonferroni adjustment of the significance level with the Mann–Whitney U test).
Table 6.
Comparisons of clinical scores according to lesion sizea.
| Lesion size, cm2 |
|||
|---|---|---|---|
| Small, <4 cm2 (n = 34) | Large, ≥4 cm2 (n = 32) | P | |
| VAS score b | |||
| Preoperative | 6.4 ± 1.6 | 7.3 ± 1.5 | 0.010 |
| 1-year follow-up | 2.0 ± 1.7 | 2.7 ± 1.8 | 0.115 |
| Final follow-up | 1.6 ± 2.0 | 2.1 ± 2.2 | 0.322 |
| WOMAC score b | |||
| Preoperative | 38.1 ± 13.7 | 42.5 ± 13.7 | 0.119 |
| 1-year follow-up | 15.9 ± 14.4 | 21.0 ± 15.0 | 0.085 |
| Final follow-up | 12.5 ± 14.4 | 16.6 ± 17.7 | 0.247 |
| IKDC score b | |||
| Preoperative | 36.6 ± 8.3 | 30.8 ± 8.3 | 0.019 |
| 1-year follow-up | 58.3 ± 16.0 | 52.5 ± 13.4 | 0.058 |
| Final follow-up | 63.9 ± 16.9 | 60.5 ± 19.4 | 0.476 |
Values are expressed as mean ± standard deviation unless otherwise indicated. Bold indicates statistical significance (P < 0.05). IKDC, International Knee Documentation Committee; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Mann-Whiney U test.
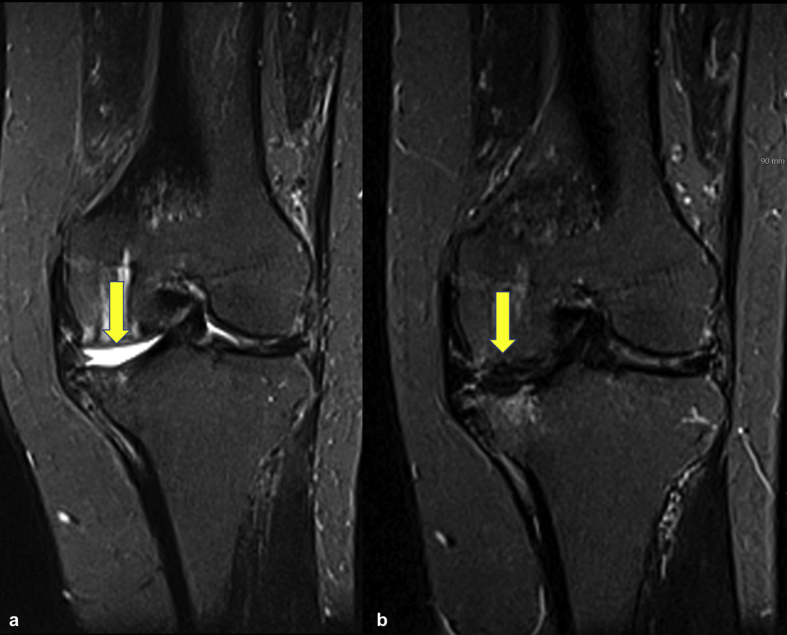
In this study, the data of 34 out of 128 patients were available for the analysis of MOCART score. The first MRI was performed at 3–6 months after surgery (average, 3.8 months), whereas the second MRI was carried out after 12 months (average, 21.2 months). The average modified MOCART score was 30.58 for the first MRI and 55.44 for the second MRI (Fig. 2).
Fig. 2.
(a) Short tau inversion recovery MRI of a 55-year-old female patient showing International Cartilage Repair Society grade IV defect (arrow) on the medial femoral condyle (b), which is completely filled with repair tissue (arrow) at 13 months after surgery.
4. Discussion
To our knowledge, this is the first study to report allogenic hUCB-MSC implantation used for a large sample comprising 128 patients with OA and to analyze the characteristics of patients during follow-up that lasted a minimum of 2 years. In our study, outcomes were significantly improved clinically and statistically, and the final follow-up results were significantly better than the 1-year follow-up results.
Although we do not know the exact mechanism that led to improvements in outcomes over time, we believe that allogenic hUCB-MSC implantation is an effective treatment for OA. Similar to our study, other studies have reported improvements over time. Gobbi et al. [7] reported that BM-MSCs and a second-generation matrix were transplanted in patients with large full-thickness chondral defects, resulting in VAS scores, Knee injury and Osteoarthritis Outcome scores, IKDC scores, Tegner scores, and Lysholm scores that improved over the course of follow-up lasting a minimum of 3 years. Park et al. [11] implanted allogenic hUCB-MSCs and hyaluronate hydrogel (same allogenic hUCB-MSCs as those used in our study) in patients with OA; they reported that pain and function were improved and that significant deterioration did not occur over 7 years of follow-up.
In some in vitro or animal studies, it has been reported that older age negatively affects autologous MSCs such as BM-MSCs and AT-MSCs [[23], [24], [25], [26], [27]]. Moreover, in a clinical study, Kim et al. [9] investigated clinical outcomes, such as IKDC scores, Tegner activity scores, and patients’ overall satisfaction, and statistically analyzed factors influencing clinical outcomes after AT-MSC implantation for patients with knee OA (49 patients; 55 knees). Their study showed that patient age was an important prognostic factor, especially age older than 60 years (P < 0.05). In addition, they suggested that lesion size of 6.0 cm2 is the upper limit for obtaining positive outcomes; in another study, they reported that large lesion size (≥5.4 cm2) and high BMI (≥27.5 kg/m2) were significant predictors of poor clinical and arthroscopic outcomes. In a systematic review of ACI, Pareek et al. [28] reported that older age and increased lesion size (>4.5 cm2) significantly correlated with an increased risk of reoperation and failure.
Obesity is another factor associated with knee OA. Wu et al. [29] extracted BM-MSCs, subcutaneous adipose tissue, and infrapatellar fat pad tissue, and MSCs from obese mice showed decreased chondrogenic potential. They suggested that obesity could change the characteristics of stem cells. Their results indicated that chondrogenic ability was decreased in the obese group.
In our study, we analyzed patients' characteristics, such as age, lesion size, and obesity, to determine whether these factors affected clinical outcomes. Interestingly, there were no significant differences in clinical outcomes based on age, lesion size, and obesity (P > 0.05, respectively). Although we cannot accurately explain this result, we believe that BM-MSCs or AT-MSCs are likely to be affected by age, lesion size, or obesity because those MSCs are directly extracted from a patient's body; however, hUCB-MSCs are allogenic and are extracted from placenta. Therefore, hUCB-MSCs do not seem to be affected by age, lesion size, and obesity.
The hUCB-MSCs that we used in this study are an “off-the-shelf” product. Because UCB-MSCs have a high capacity for expanding and high cryopreservation ability, they can be used “off the shelf” [14]. This feature is an advantage because a certain amount of cells can be transplanted according to the patient's lesion size.
We believe that the amount of stem cells according to the lesion size is important for cartilage regeneration. Jo et al. [8, 30] reported that AT-MSCs were injected in 18 patients with knee OA divided into low-dose (1.0 × 107 cells), mid-dose (5.0 × 107), and high-dose (1.0 × 108) groups; they showed that pain and function were improved by hyaline-like articular cartilage regeneration in the high-dose group. MEDIPOST, which manufactures CARTISTEM®, recommends increasing the dose according to the lesion size of the patient.
In our study, we used a mixture of HA and allogenic hUCB-MSCs supplied by MEDIPOST. Regarding the effects of mixed HA and allogenic hUCB-MSCs, Park et al. [[8], [30]] showed that the implantation of allogenic hUCB-MSCs with 4% HA in cartilage defects led to improvements in cartilage repair compared with 4% HA only. The results of these animal studies revealed that implantation of allogenic hUCB-MSCs with 4% HA is a possible alternative treatment for repairing cartilage defect.
In addition, our study compared the results of lesion location, lesion numbers, and other patient characteristics. There was no significant difference among groups with one lesion, two lesions, and three lesions (P > 0.05), but there was a significant difference among the groups with MFC, LFC, and trochlea lesions (P < 0.05). The WOMAC and VAS scores of the trochlea lesion group were better than those of the MFC lesion group at 1-year follow-up and final follow-up.
It is difficult to explain why the MFC lesion group had worse outcomes than the trochlea lesion group, but it was probably due to the adduction moment acting on the medial side of the knee. Our study included only patients with knee alignment <8°; however, many studies reported that the knee adduction moment increased the medial compartment loading of the knee joint approximately 2.5 times more than the lateral compartment, which was associated with knee joint OA [[31], [32], [33], [34]]. Therefore, if there is an alignment deformity during allogenic hUCB-MSC implantation, we recommend conducting high tibial osteotomy to shift the axial load effectively.
All patients did not agree to undergo postoperative check-up because of the high cost of MRI. Nevertheless, the MRI data of 34 patients indicated an average modified MOCART score of 55.44, which was similar to that of other successful cartilage repair techniques [35].
There were some limitations to this study. First, our study was a retrospective case series without control groups. However, there were differences in indications for allogenic hUCB-MSC implantation and for microfracture or ACI. Microfracture is not appropriate for patients with severe OA or large lesions, and ACI is not suitable for patients with severe OA or large lesions. In Korea, insurance is used only for those younger than 50 years old. However, allogenic hUCB-MSCs were approved for patients with OA, except for those with K-L grade 4. In the future, the use of other stem cells such as BM-MSCs or AT-MSC should be compared to identify the precise effects of allogenic hUCB-MSCs. Although our study did not have a control group, it included a large number of patients, and various analyses were performed. According to the method of Park et al. [11], who first reported allogenic hUCB-MSC implantation, we created multiple holes in the lesion before allogenic hUCB-MSC implantation. However, we do not know how this process affected the results. This study showed only clinical outcomes such as IKDC, WOMAC, and VAS scores; second-look arthroscopy or MRI could not be performed because we did not have any reason to use these invasive or expensive procedures to check the cartilage regeneration status.
5. Conclusions
In our study, we demonstrated improvements in pain and function of patients with knee OA at least 2 years after implantation of allogenic hUCB-MSCs. We believe that the clinical outcomes were not affected by patient characteristics such as age, obesity, and lesion size because allogenic hUCB-MSCs were used. In addition, when performing implantation for MFC lesions, it is desirable to consider the knee alignment of the patient. If necessary, high tibial osteotomy should be performed.
Declarations of interest
None.
Funding
There was no external funding source for this study.
Ethical approval
This study was reviewed and approved by the institutional review board of the Korea Ministry of Health and Welfare (P01-201807-21-006). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
All data included in the manuscript are available upon request.
Author contributions
Jun-Seob Song: conception and design. Ki-Taek Hong and Na-Min Kim: manuscript writing. Jae-Yub Jung and Han-Soo Park: collection of data. Sang Heon Lee and Yoon Joo Cho: data analysis. Seok Jung Kim: developing the concept. All authors critically reviewed and approved the final version of the manuscript.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster P., Schulz M., Mayer P., Schlumberger M., Immendoerfer M., Richter J. Open-wedge high tibial osteotomy and combined abrasion/microfracture in severe medial osteoarthritis and varus malalignment: 5-year results and arthroscopic findings after 2 years. Arthroscopy. 2015;31:1279–1288. doi: 10.1016/j.arthro.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Koh Y.G., Choi Y.J., Kwon O.R., Kim Y.S. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42:1628–1637. doi: 10.1177/0363546514529641. [DOI] [PubMed] [Google Scholar]
- 4.Nejadnik H., Hui J.H., Feng Choong E.P., Tai B.C., Lee E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 5.Minzlaff P., Feucht M.J., Saier T., Schuster T., Braun S., Imhoff A.B. Osteochondral autologous transfer combined with valgus high tibial osteotomy: long-term results and survivorship analysis. Am J Sports Med. 2013;41:2325–2332. doi: 10.1177/0363546513496624. [DOI] [PubMed] [Google Scholar]
- 6.Buda R., Vannini F., Cavallo M., Grigolo B., Cenacchi A., Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 7.Gobbi A., Karnatzikos G., Sankineani S.R. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–657. doi: 10.1177/0363546513518007. [DOI] [PubMed] [Google Scholar]
- 8.Jo C.H., Lee Y.G., Shin W.H., Kim H., Chai J.W., Jeong E.C. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.S., Choi Y.J., Koh Y.G. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med. 2015;43:2293–2301. doi: 10.1177/0363546515588317. [DOI] [PubMed] [Google Scholar]
- 10.Feng Hua. Is stem cell therapy the future of orthopedics? Knee Surg Relat Res. 2018;30:177–178. doi: 10.5792/ksrr.18.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y.B., Ha C.W., Lee C.H., Yoon Y.C., Park Y.G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Y., Feng G., Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39:5683–5689. doi: 10.1007/s11033-011-1376-z. [DOI] [PubMed] [Google Scholar]
- 13.Mobasheri A., Kalamegam G., Musumeci G., Batt M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Wang M., Yang Y., Yang D., Luo F., Liang W., Guo S. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park Y.B., Ha C.W., Lee C.H., Park Y.G. Restoration of a large osteochondral defect of the knee using a composite of umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel: a case report with a 5-year follow-up. BMC Muscoskelet Disord. 2017;18:59. doi: 10.1186/s12891-017-1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J., Dennis J.E., Solchaga L.A., Awadallah A.S., Goldberg V.M., Caplan A.I. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7:363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 17.Park Y.B., Song M., Lee C.H., Kim J.A., Ha C.W. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33:1580–1586. doi: 10.1002/jor.22950. [DOI] [PubMed] [Google Scholar]
- 18.Yang S.E., Ha C.W., Jung M.H., Jin H.J., Lee M., Song H. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 19.Schipplein O.D., Andriacchi T.P. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 20.Erices A., Conget P., Minguell J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee M., Jeong S.Y., Ha J., Kim M., Jin H.J., Kwon S.J. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983–989. doi: 10.1016/j.bbrc.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Tredget E.E., Wu P.Y.G., Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong S.Y., Kim D.H., Ha J., Jin H.J., Kwon S.J., Chang J.W. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–2148. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 24.Baker N., Boyette L.B., Tuan R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone. 2015;70:37–47. doi: 10.1016/j.bone.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Chang H.X., Yang L., Li Z., Chen G., Dai G. Age-related biological characterization of mesenchymal progenitor cells in human articular cartilage. Orthopedics. 2011;34:e382–e388. doi: 10.3928/01477447-20110627-06. [DOI] [PubMed] [Google Scholar]
- 26.Choudhery M.S., Badowski M., Muise A., Pierce J., Harris D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller S.M., Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 28.Wu L.W., Wang Y.L., Christensen J.M., Khalifian S., Schneeberger S., Raimondi G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30:122–127. doi: 10.1016/j.trim.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Pareek A., Carey J.L., Reardon P.J., Peterson L., Stuart M.J., Krych A.J. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7:298–308. doi: 10.1177/1947603516630786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C.L., Diekman B.O., Jain D., Guilak F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes (Lond). 2013;37:1079–1087. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo C.H., Chai J.W., Jeong E.C., Oh S., Shin J.S., Shim H. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45:2774–2783. doi: 10.1177/0363546517716641. [DOI] [PubMed] [Google Scholar]
- 32.Baliunas A.J., Hurwitz D.E., Ryals A.B., Karrar A., Case J.P., Block J.A. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthr Cartil. 2002;10:573–579. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- 33.Nha Kyung Wook, Shin Young-Soo, Kwon Hyuk Min, Sim Jae Ang, Na Young Gon. Navigated versus conventional technique in high tibial osteotomy: a meta-analysis focusing on weight bearing effect. Knee Surg Relat Res. 2019;31:81–102. doi: 10.5792/ksrr.17.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma L., Hurwitz D.E., Thonar E.J., Sum J.A., Lenz M.E., Dunlop D.D. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Anderson D.E., Williams R.J., DeBerardino T.M., Taylor D.C., Ma C.B., Kane M.S. Magnetic resonance imaging characterization and clinical outcomes after NeoCart surgical therapy as a primary reparative treatment for knee cartilage injuries. Am J Sports Med. 2017;45:875–883. doi: 10.1177/0363546516677255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in the manuscript are available upon request.