Abstract
Introduction
Basal-bolus (BB) regimens are generally used to intensify basal insulin therapy in patients with type 2 diabetes (T2D) not meeting glycemic targets. However, drawbacks include multiple injection burden and risk of weight gain and hypoglycemia. A once-daily titratable fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide (iGlarLixi) may provide a simple, well-tolerated, and efficacious alternative. We compared these treatments in a post hoc propensity score matched analysis using randomized trial data.
Methods
From the LixiLan-L study, 195 patients who had been randomized to iGlarLixi were matched for age, sex, race, T2D duration, baseline body mass index, glycated hemoglobin (HbA1c), fasting plasma glucose, insulin dose, and metformin use to 195 patients who had been randomized to a BB regimen in the GetGoal Duo-2 trial.
Results
At study end, estimated treatment differences for reduction in HbA1c and weight change, and ratio of hypoglycemia events per patient-year (BB vs iGlarLixi) were − 0.28% (standard error 0.08, P = 0.0002), − 1.32 kg (standard error 0.30, P < 0.0001), and 2.85 (P < 0.0001), respectively, all favoring iGlarLixi over BB. Also, proportions of patients reaching individual and composite goals (HbA1c < 7% [< 53 mmol/mol], no weight gain, and no hypoglycemia) were higher in the iGlarLixi compared with the BB treatment group. Gastrointestinal side effects were more common with iGlarLixi.
Conclusions
In patients with T2D inadequately controlled on basal insulin, iGlarLixi offers an effective alternative to BB regimen for reducing HbA1c, without increased risk of hypoglycemia and weight gain.
Trial Registration
ClinicalTrials.gov: NCT02058160 (LixiLan-L trial); NCT01768559 (GetGoal Duo-2 trial).
Plain Language Summary
Plain language summary available for this article.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-00735-7) contains supplementary material, which is available to authorized users.
Keywords: Hypoglycemia, iGlarLixi, Insulin therapy, Type 2 diabetes, Weight control
Key Summary Points
| Why carry out this study? |
| Initial therapy with basal insulin alone may be inadequate to reach optimal glycemic control of type 2 diabetes, and many patients may require additional mealtime bolus-insulin dosing (i.e., a basal-bolus regimen) |
| Basal-bolus regimens are associated with weight gain, a high risk of hypoglycemia, complexity of regimen, and a high injection burden; information is limited on head-to-head comparisons of fixed-ratio combinations of basal insulin plus a glucagon-like peptide 1 receptor agonist (GLP-1RA) versus a basal-bolus or basal-plus regimen |
| In this propensity score matching analysis using data from two randomized controlled trials, we assessed the efficacy and safety of iGlarLixi, the fixed-ratio combination of insulin glargine 100 U/mL and the GLP-1RA lixisenatide, compared with that of basal-bolus insulin in patients with type 2 diabetes uncontrolled on basal insulin |
| What was learned from the study? |
| In this analysis, treatment with iGlarLixi was associated with a significantly greater reduction in glycated hemoglobin (HbA1c) and fewer episodes of overall and nocturnal hypoglycemia events compared with basal bolus regimen |
| In the absence of head-to-head trials, this study provides evidence that treatment intensification with iGlarLixi may be more efficacious and well tolerated than switching to basal bolus for patients with type 2 diabetes uncontrolled on basal insulin |
Plain Language Summary
Basal insulin is often the first medicine that people with type 2 diabetes take, but for many patients it is not enough to get their blood sugar to normal levels. The next step may be basal-bolus insulin, but this involves frequent injections and carries risks of weight gain and hypoglycemia. A combination of basal insulin with another class of drug (a glucagon-like peptide 1 receptor agonist, GLP-1RA) may be a simple and well-tolerated option. However, there are not many trials that have compared this combination to basal-bolus insulin head to head. We used data from two studies and compared the outcomes of 195 patients who took the combination iGlarLixi, which consists of the GLP-1RA lixisenatide with basal insulin, from one trial with the outcomes of 195 patients who took basal-bolus insulin in the other trial, after matching them on the basis of key characteristics. Patients in the iGlarLixi group had greater decreases in blood sugar and weight, and had lower rates of hypoglycemia events per patient per year. Gastrointestinal side effects were more common with iGlarLixi. These results suggest that iGlarLixi may offer an effective alternative to basal-bolus insulin for patients with type 2 diabetes who do not meet their blood sugar goals with basal insulin alone.
Introduction
Many patients with type 2 diabetes will eventually require insulin therapy because of the progressive nature of the disease. Basal insulin alone is the most convenient initial regimen; however, in some patients this therapy may be inadequate to reach optimal glycemic control. According to randomized controlled trial data, 40–70% of patients achieve a glycated hemoglobin (HbA1c) target of < 7% (< 53 mmol/mol) with basal insulin [1, 2]. Furthermore, data from real-world studies is more disappointing. A recent study using a large claims database that included 39,074 patients reported that only 27% of patients were at a target HbA1c < 7% (< 53 mmol/mol) after 3 months of treatment with basal insulin [3]. Thus, many patients may require additional mealtime bolus insulin dosing (i.e., a basal-bolus regimen). However, basal-bolus regimens are associated with weight gain, a high risk of hypoglycemia, complexity of regimen, and a high injection burden. Such drawbacks are factors in “clinical inertia”, or the failure to intensify therapy from basal insulin alone when indicated. Healthcare professionals have identified hypoglycemia, failure to titrate in the absence of symptoms, and low patient motivation as barriers; patients have also expressed concerns about weight gain, the perception of worsening disease, frustration at not reaching goals, and fear of hypoglycemia [4].
iGlarLixi, a once-daily (QD) titratable fixed-ratio combination of insulin glargine 100 U/mL and the glucagon-like peptide 1 receptor agonist (GLP-1RA) lixisenatide, may offer a simple alternative to basal-bolus regimens.
In this analysis, we assessed the efficacy and safety of iGlarLixi (using data from the LixiLan-L trial) [5] compared with that of basal-bolus insulin (using data from the GetGoal Duo-2 trial) [6] in patients with type 2 diabetes uncontrolled on basal insulin.
Methods
Study Design and Participants
The full design and description of both LixiLan-L (NCT02058160) and GetGoal Duo-2 (NCT01768559) have been described previously and are discussed briefly here in the electronic supplementary material (Fig. S1) [5, 6]. The trials were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines for good clinical practice, and all applicable laws and regulations. All protocols were reviewed and approved by independent ethics committees and institutional review boards. All patients gave their informed consent prior to their inclusion in the study.
The two studies have some common features as well as some differences. The common features included that the populations of both trials were adult patients with type 2 diabetes of at least 1-year duration at screening, uncontrolled on basal insulin with or without oral antidiabetes drugs for at least 6 months. In addition, in both GetGoal Duo-2 and LixiLan-L, insulin glargine titration was adjusted weekly to maintain a fasting daily self-monitored plasma glucose of 80–100 mg/dL (4.4–5.6 mmol/L) while avoiding hypoglycemia; however, the initial doses were stabilized for 2 and 4 weeks, respectively [5, 6]. Differences between the studies included the dose cap, the run-in periods, and the trial durations. In the LixiLan-L trial, the maximum iGlarLixi QD dose was capped at 60 U of insulin glargine plus 20 µg of lixisenatide (an insulin glargine dose > 60 U was permitted in GetGoal Duo-2). While both studies comprised a run-in period during which insulin glargine was introduced and/or further titrated (and oral antidiabetes drugs other than metformin were stopped), the run-in period for GetGoal Duo-2 was 12 weeks, and the run-in period for LixiLan-L was 6 weeks. Finally, in LixiLan-L, patients received 30 weeks of randomized treatment, while in GetGoal Duo-2, patients received 26 weeks of randomized treatment. Primary/co-primary endpoints over the respective treatment periods were change in HbA1c for LixiLan-L, and change in HbA1c and body weight for GetGoal Duo-2.
Endpoints
As indicated in the methods, the treatment duration of the two studies differed by 4 weeks; to avoid confounding from the longer exposure in the LixiLan-L trial, we took a conservative approach and assessed HbA1c and weight changes at 24 weeks (instead of 30) for iGlarLixi (LixiLan-L) and 26 weeks for basal bolus (GetGoal Duo-2). However, for safety and insulin dose we chose a longer treatment exposure for iGlarLixi (LixiLan-L) to avoid favoring iGlarLixi over basal bolus for these endpoints. Documented symptomatic hypoglycemia event rates were determined over 30 weeks for iGlarLixi (LixiLan-L) and 26 weeks for basal bolus (GetGoal Duo-2). Definitions of hypoglycemia were similar between trials [5, 6]. For the purposes of this analysis, hypoglycemia is defined as symptomatic hypoglycemia with a documented plasma glucose measurement of < 54 mg/dL (< 3.0 mmol/L) indicating clinically important hypoglycemia [7]. This definition of hypoglycemia hereafter will be referred to as hypoglycemia. Additional safety outcomes over the course of each study included gastrointestinal adverse events (AEs). Basal doses (for both arms) and bolus doses (for the basal-bolus regimen) were summarized by treatment group at the end of the study.
Two-hour postprandial plasma glucose (PPG) was assessed at the end of 30 weeks for iGlarLixi and 26 weeks for basal bolus, as PPG measurements post-baseline were available at only these time points.
The proportion of patients achieving HbA1c < 7% (< 53 mmol/mol), having no weight gain, and having no hypoglycemia (< 54 mg/dL [< 3.0 mmol/L]), individually and as composites, was also assessed at 24 weeks for iGlarLixi and 26 weeks for basal bolus.
Statistical Methods
A one-to-one propensity score matching (PSM) technique was used to select patients receiving iGlarLixi in LixiLan-L [5] or basal bolus in GetGoal Duo-2 [6] (as previously described [8]) on the basis of age, sex, race, and diabetes duration, and baseline body mass index (BMI), HbA1c, fasting plasma glucose, insulin glargine dose, and metformin use. Efficacy analyses were evaluated using the modified intent-to-treat population, comprising all randomized patients with a baseline assessment and at least one post-baseline assessment of any efficacy variable. The exception was PPG, which for GetGoal Duo-2 was assessed in the subgroup of patients who received an injection of study medication before breakfast. The safety population (used for rates of hypoglycemia and other AEs) was defined as all randomized patients who received at least one dose of study medication.
Changes from baseline in HbA1c and weight were analyzed using a mixed-effects model with repeated measures (MMRM), with treatment groups and randomization strata (HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] at screening and metformin use) as fixed effects, and visit, baseline by visit interaction, and treatment by visit interaction as covariates.
Hypoglycemia event rates and nocturnal hypoglycemia event rates were compared using Poisson regression, with treatment as a fixed factor and log value of patient-years of exposure as an offset variable.
Two-hour PPG was compared with analysis of covariance, using the last observation carried forward, with treatment group, randomization strata of HbA1c (< 8.0%, ≥ 8.0% [< 64, ≥ 64 mmol/mol]) at screening, and metformin use as fixed effects, and baseline value as a covariate.
The proportion of patients achieving HbA1c < 7% (< 53 mmol/mol), no weight gain, and no hypoglycemia, assessed individually and as composites, was compared using a Cochran–Mantel–Haenszel test for the weighted difference between treatment groups in all categories (randomization strata of HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] and metformin use). Average daily insulin dose was compared with an MMRM, with treatment groups and randomization strata (HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] at screening and metformin use) as fixed effects, and visit (week 2, week 6, week 12, and week 30/26), baseline by visit interaction, and treatment by visit interaction as covariates.
A sensitivity analysis was also performed on selected efficacy endpoints that included patients matched for the same variables as the primary analysis, except for insulin dose: age, sex, race, diabetes duration, BMI, HbA1c, fasting plasma glucose, and metformin use. Additional sensitivity analyses were performed on selected efficacy endpoints that matched patients receiving iGlarLixi separately to QD and three times daily (TID) basal bolus on age, sex, race, and diabetes duration, and baseline BMI, HbA1c, fasting plasma glucose, insulin glargine dose, and metformin use.
All P values are reported as nominal P values without multiplicity adjustment.
Results
Population Characteristics After PSM
Patient demographics and baseline characteristics were similar after PSM, across the iGlarLixi and basal-bolus groups (n = 195 in each group; Table 1); approximately 9 of 10 patients were taking metformin at randomization, and roughly half of patients had HbA1c ≥ 8% (≥ 64 mmol/mol) at randomization. Tables S1 and S2 in the electronic supplementary material show the characteristics of the complete randomized population of each trial for comparison.
Table 1.
Demographics and baseline characteristics after PSM based on age, sex, race, diabetes duration, baseline BMI, HbA1c, FPG, insulin glargine dose, and metformin use
| iGlarLixi (n = 195) |
Basal bolusa (n = 195) |
|
|---|---|---|
| Age, years | 60.1 (9.1) | 60.8 (8.9) |
| Male, % | 48.2 | 45.6 |
| White, % | 93.3 | 90.8 |
| Non-Hispanic, % | 80.5 | 76.9 |
| Duration of type 2 diabetes, years | 12.49 (7.08) | 12.59 (6.68) |
| Baseline BMI, kg/m2 | 30.92 (4.02) | 30.91 (4.55) |
| HbA1c at screening, % | 8.46 (0.68) | 8.42 (0.73) |
| HbA1c at screening, mmol/mol | 69.00 (7.44) | 68.53 (8.03) |
| HbA1c at randomization, % | 7.92 (0.68) | 7.90 (0.66) |
| HbA1c at randomization, mmol/mol | 63.05 (7.41) | 62.86 (7.18) |
| Randomization strata of HbA1c ≥ 8% (≥ 64 mmol/mol), % | 51.3 | 50.3 |
| Baseline FPG, mg/dL | 127.47 (34.17) | 124.28 (36.73) |
| Baseline FPG, mmol/L | 7.08 (1.90) | 6.90 (2.04) |
| Baseline 2-h PPG, mg/dL | 263.30 (64.43) | 251.11 (61.70)b |
| Baseline 2-h PPG, mmol/L | 14.62 (3.58) | 13.94 (3.43)b |
| Randomization strata of metformin use, % | 89.2 | 89.7 |
| Baseline daily insulin dose, U | 38.78 (8.29) | 38.45 (11.06) |
All data are mean (SD) unless stated otherwise. Based on the randomized patient population
Patients were matched based on the nearest neighbor matching within a specified caliper distance. The logit of the propensity scores and caliper widths equal to 0.2 of the pooled SD of the logit of the propensity score were used
BMI body mass index, FPG fasting plasma glucose, HbA1c glycated hemoglobin, iGlarLixi fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, PPG postprandial plasma glucose, PSM propensity score matching, QD once daily, SD standard deviation, TID three times daily
aIn the basal-bolus group, 49% of patients (n = 95) were receiving basal bolus TID, and the rest were receiving basal bolus QD
bn = 69
Outcomes
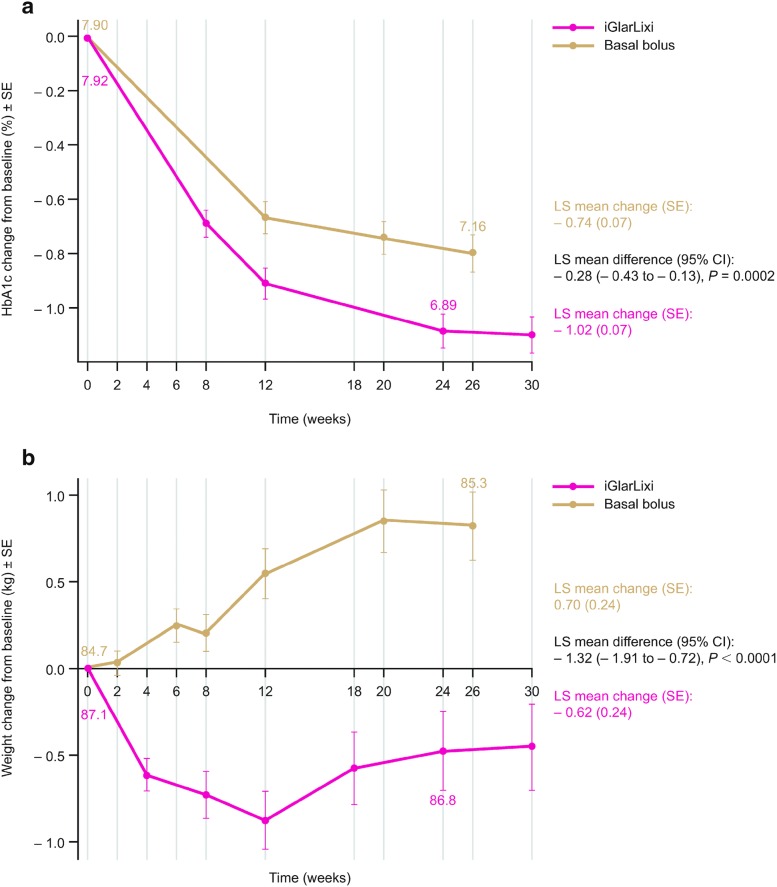
HbA1c changes from baseline with iGlarLixi and basal bolus are shown in Fig. 1a. HbA1c was reduced by a least squares (LS) mean (standard error [SE]) of 1.02% (0.07) in patients in the iGlarLixi group, while it was reduced by 0.74% (0.07) in the basal-bolus group.
Fig. 1.
a HbA1c change from baseline and b weight change from baseline. Data labels represent mean HbA1c (%) or body weight (kg) at the corresponding time point. The changes from baseline in HbA1c and weight were analyzed using an MMRM with treatment groups and randomization strata (HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] at screening and metformin use) as fixed effects, and visit (week 12 and week 24/26), baseline by visit interaction, and treatment by visit interaction as covariates. CI confidence interval, HbA1c glycated hemoglobin, iGlarLixi fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, LS least squares, MMRM mixed-effects model with repeated measures, SE standard error
The estimated LS mean treatment difference in HbA1c decline over the study was − 0.28% (SE 0.08; 95% confidence interval [CI] − 0.43 to − 0.13, P = 0.0002) in favor of iGlarLixi.
Weight changes from baseline with iGlarLixi and basal bolus are shown in Fig. 1b; weight decreased with iGlarLixi by an LS mean (SE) of 0.62 kg (0.24) and increased with basal bolus by an LS mean (SE) of 0.70 kg (0.24) (estimated treatment difference − 1.32 kg [95% CI − 1.91 to − 0.72, P < 0.0001]).
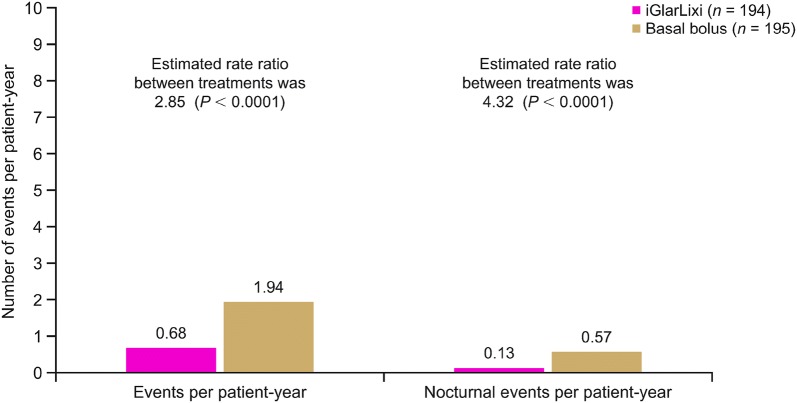
The rate of hypoglycemia events per patient-year (Fig. 2) was higher with basal bolus compared with iGlarLixi; overall, the estimated rate ratio of basal bolus to iGlarLixi was 2.85 (P < 0.0001), while the rate ratio for nocturnal events was 4.32 (P < 0.0001).
Fig. 2.
Hypoglycemia events per patient-year. Hypoglycemia was defined as documented symptomatic hypoglycemia (plasma glucose concentration < 54 mg/dL [< 3 mmol/L]). Patient-years of exposure were calculated as time from first to last injection plus 1 day. P values were estimated from Poisson regression with treatment as a fixed factor and log value of patient-years of exposure as an offset variable. iGlarLixi fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide
LS mean (SE) change from baseline to week 30/26 for 2-h PPG (analyzed in 179 and 46 patients in the iGlarLixi and basal-bolus groups, respectively) was − 5.37 mmol/L (0.38) for iGlarLixi at week 30 versus − 1.98 mmol/L (0.59) for basal bolus at week 26 (LS mean [SE] difference − 3.39 [0.55], P < 0.0001) (Fig. S2).
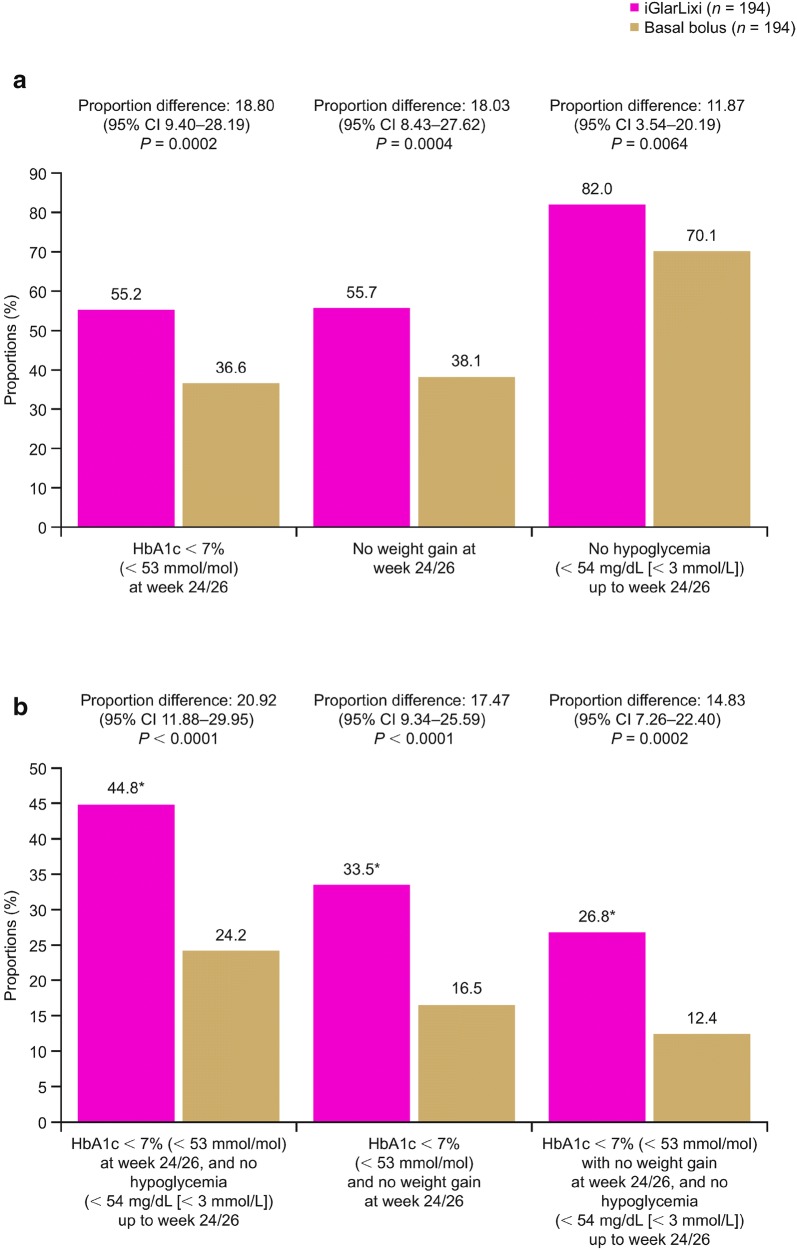
iGlarLixi was associated with significantly (P < 0.01 for all) greater proportions of patients achieving both individual (HbA1c < 7% [< 53 mmol/mol], no weight gain, and no hypoglycemia) and composite endpoints compared with basal bolus (Fig. 3a, b). In the iGlarLixi group, at week 24, 26.8% of patients achieved the HbA1c target of < 7% (< 53 mmol/mol) with no weight gain, and no hypoglycemia up to week 24, versus 12.4% of patients in the basal-bolus group at week 26 (proportion difference 14.83; 95% CI 7.26–22.40, P = 0.0002).
Fig. 3.
Proportions of patients reaching a individual and b composite endpoints. Hypoglycemia was defined as documented symptomatic hypoglycemia (plasma glucose concentration < 54 mg/dL [< 3 mmol/L]). *P < 0.01 from Cochran–Mantel–Haenszel test for the weighted difference between treatment groups in all categories (randomization strata of HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] and metformin use). CI confidence interval, HbA1c glycated hemoglobin, iGlarLixi fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide
Gastrointestinal AEs (i.e., diarrhea, nausea, and vomiting) were more frequent among patients receiving iGlarLixi (Table S3). Overall, 17.5% of patients in the iGlarLixi group experienced a gastrointestinal AE, compared with 8.2% of patients in the basal-bolus group. There were no discontinuations due to gastrointestinal AEs in either group.
As shown in Table 2, the LS mean (SE) change from baseline in daily insulin dose was significantly lower in the iGlarLixi group compared with the basal-bolus group (− 5 U (1), P = 0.0003). The total mean (standard deviation [SD]) basal insulin dose at the time of the final study dose taken (including those who did not complete the study) was 48 U (12) in the iGlarLixi arm versus 38 U (14) in the basal-bolus arm. The average (SD) bolus insulin dose in the basal-bolus group, as of the last study dose taken, was 14 U (11).
Table 2.
Average daily insulin dose
| iGlarLixi (n = 194) | Basal bolus (n = 194) | |
|---|---|---|
| Baseline, mean (SD), U | 39 (8) | 38 (11) |
| Week 30/26, mean (SD), Ua | 49 (11) | 53 (23) |
| LS mean (SE) change from baseline, Ua | 9 (1) | 14 (1) |
| LS mean treatment difference ± SE, Ua | 5 ± 1 | |
| P valuea | 0.0003 | |
| Basal insulin dose at end of study, mean (SD), Ub | 48 (12) | 38 (14) |
| Bolus insulin dose at end of study, mean (SD), Ub | – | 14 (11) |
Unless otherwise noted, results are based on the modified intent-to-treat population (all subjects with baseline and at least one post-baseline measurement)
iGlarLixi fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide, LS least squares, MMRM mixed-effects model with repeated measures, SD standard deviation, SE standard error
aObserved cases are used for this analysis (n = 179 and n = 157 at week 30/26 for iGlarLixi and basal bolus, respectively). Based on MMRM with treatment groups and randomization strata (HbA1c [< 8.0%, ≥ 8.0% (< 64, ≥ 64 mmol/mol)] at screening and metformin use) as fixed effects, and visit (week 2, week 6, week 12, and week 30/26), baseline by visit interaction, and treatment by visit interaction as covariates
bBased on final study dose taken (n = 192 and n = 192 for iGlarLixi and basal bolus, respectively)
In the sensitivity analysis that did not include insulin dose as one of the matching variables (n = 329 for both iGlarLixi and basal bolus) (Table S4), the mean (SD) basal insulin dose was 35 U (9) for iGlarLixi and 63 U (29) for basal bolus at baseline; at week 30/26, the total insulin dose increased to 47 U (13) and 77 U (36), respectively. The LS mean (SE) change in total insulin dose from baseline to week 30/26 was 11 U (1) versus 13 U (1), for a 3 U (2) greater increase in the basal-bolus group (P = 0.0710).
In the same sensitivity analysis, iGlarLixi was associated with a greater reduction in HbA1c and greater proportions of patients reaching individual and composite endpoints; safety results were also similar to those in the primary analysis. Consistently, in the sensitivity analysis that matched patients taking iGlarLixi separately to patients taking QD or TID basal-bolus regimens (demographics and baseline characteristics are shown in Table S5), change in HbA1c and weight was in favor of iGlarLixi regardless of the basal-bolus regimen (QD or TID). Similarly, greater proportions of patients in the iGlarLixi group achieved individual and composite endpoints compared with either QD basal bolus or TID basal bolus (Table S6). Similar trends in antihyperglycemic efficacy were observed with iGlarLixi when compared with each basal-bolus dose regimen and when iGlarLixi was compared with both basal-bolus arms pooled together.
Discussion
In this post hoc PSM analysis, treatment with iGlarLixi was associated with a significantly greater reduction in HbA1c and fewer episodes of overall and nocturnal hypoglycemia events compared with basal bolus therapy. Furthermore, patients on iGlarLixi lost weight, while the basal-bolus group increased in body weight. iGlarLixi was also associated with a greater proportion of patients achieving both individual treatment targets (such as HbA1c < 7% [< 53 mmol/mol]) and composite endpoints. These treatment effects with iGlarLixi were achievable with only one injection daily versus the multiple injections necessary for basal-bolus insulin, in addition to requiring fewer self-monitored plasma glucose measurements and having the need to titrate only one insulin dose.
Numerous randomized controlled trials have confirmed the ability of combined basal insulin and GLP-1RA (BI + GLP-1RA), whether as “free” or fixed-ratio combinations, to provide greater reductions in HbA1c and/or body weight when compared with up-titration of basal insulin. A recent meta-analysis by Maiorino et al. found advantages of 0.53% in HbA1c change and 1.9 kg in body weight change for BI + GLP-1RA combinations versus basal insulin, suggesting that BI + GLP-1RA combinations could provide efficacious alternatives to up-titration of basal insulin [9]. Furthermore, BI + GLP-1RA combinations have also been compared with basal-bolus regimens. A head-to-head comparison of the GLP-1RA exenatide twice daily or TID bolus insulin, each added to titrated prandial insulin in patients failing basal insulin (the 4B study), found that exenatide demonstrated non-inferior efficacy to insulin lispro in reducing HbA1c, while weight decreased with exenatide and increased with insulin lispro, and exenatide was associated with fewer nocturnal hypoglycemic events [10]. Consistent with the GetGoal Duo-2 study included in the present analysis, the 4B study suggests that after failure of basal insulin in type 2 diabetes, the add-on of prandial GLP-1RA is as effective as prandial insulin in lowering HbA1c, with added benefits of reducing body weight and risk for hypoglycemia [11]. By contrast, information is limited on head-to-head comparisons of fixed-ratio combinations of BI + GLP-1RA versus a basal-bolus or basal-plus regimen.
The differences between treatment groups taking BI + GLP-1RA (whether in “free” or fixed-ratio combinations) versus basal-bolus or basal-plus regimens in HbA1c and weight change in another meta-analysis were compatible with those observed in the current PSM analysis (HbA1c − 0.11 vs − 0.28%; body weight − 4.1 vs − 1.32 kg) [12].
The 32% relative risk reduction in hypoglycemia shows an advantage for the combination treatment, as does the difference in events per patient-year in our PSM analysis (2.85 rate ratio for basal-bolus vs iGlarLixi) [12]. The results for hypoglycemia and weight change observed in this post hoc PSM research are also consistent with those seen in the randomized head-to-head comparison of insulin degludec/liraglutide fixed-ratio combination versus basal bolus (the DUAL VII trial) [13]. However, the present study showed significant benefits on glycemic control with the fixed-ratio combination versus basal-bolus insulin, with significantly larger reductions in HbA1c from baseline and significantly greater proportions of patients achieving a target of HbA1c < 7% with iGlarLixi compared with those taking basal-bolus. In contrast, non-inferiority was found regarding the glycemic effect of IDegLira compared to basal-bolus therapy in DUAL VII. Furthermore, similar proportions in both groups achieved glycemic target. Although there were differences in the study outcomes, the populations were comparable in terms of T2D duration (12.5–12.6 years in this study versus 13.2–13.3 years in DUAL VII), baseline BMI (30.9 kg/m2 in this study versus 31.7 kg/m2 in DUAL VII) and baseline HbA1c (7.9% in this study versus 8.2% in DUAL VII). It is possible that differences between trials in the total insulin dose and/or proportion of insulin doses given as bolus could have affected the likelihood of achieving glycemic control in the BB group. It is important to note that these and other methodological differences make it difficult to compare the results of the current post hoc analysis and the DUAL VII randomized study.
The composite outcome of achievement of the glycemic goal while avoiding weight gain and hypoglycemia, which has been used in previous research [14], offers a single clinically relevant measurement that is more comprehensive than individual assessments.
As previously reported in GetGoal Duo-2, patients receiving lixisenatide (plus insulin glargine) were twice as likely to achieve the triple composite outcome of HbA1c < 7% (< 53 mmol/mol) without weight gain or documented symptomatic hypoglycemia, versus those receiving basal bolus [6]. Lixisenatide plus insulin glargine yielded 13.0% more patients achieving the composite endpoint versus a bolus regimen QD, and 11.4% more versus a bolus regimen TID. The difference in the composite endpoint of 14.8% between treatments observed here was achieved with the convenience of a fixed-ratio combination, which allows for reduced injection burden and titration of only one product [6]. The composite endpoint may be a particularly appropriate measure given the multiple effects of lixisenatide, which exerts its effects via delayed gastric emptying and blunting of postprandial glucose excursions in addition to insulin-dependent action, and minimizes the potential for weight gain and hypoglycemia seen with insulin use [15, 16].
Though evaluations of adherence and persistence to iGlarLixi in the real world are pending, previous research has found an improved adherence to fixed-dose formulations versus “loose-dose” regimens in patients with type 2 diabetes beginning combination therapy [17]. A previous PSM post hoc study found that early treatment with iGlarLixi may be more effective and possess better gastrointestinal tolerability than a sequential approach of adding a GLP-1RA in patients with uncontrolled type 2 diabetes initiating or intensifying basal insulin therapy [8], and this could possibly translate into greater adherence to medication with a fixed-ratio combination versus the stepwise approach. In this study, gastrointestinal AEs were more frequent with iGlarLixi than with a basal-bolus regimen (nausea was seen in 10.8% and 2.1% in the respective treatment groups in the primary analysis, and in 10.4% and 1.8%, respectively, in the sensitivity analysis not matched for baseline insulin dose). This drawback that accompanies the superior glycemic effects seen in the iGlarLixi group should in turn be considered in the context of the higher frequency of gastrointestinal effects seen when giving a GLP-1RA without combined titratable insulin [18], and the related possible implications for adherence. However, it is worth noting that gastrointestinal events with iGlarLixi were generally mild to moderate and did not lead to any discontinuations in the PSM population; treatment discontinuation due to gastrointestinal events among patients taking iGlarLixi in the overall LixiLan-L study population was only 1.1% as previously described [5].
The strengths of this research include the fact that these data are from two randomized controlled trials with similar designs and inclusion/exclusion criteria, such as disease duration and inadequate glycemic control despite basal insulin therapy. The PSM methods yielded well-matched populations for comparison. Individual-level data were used, which allows for subgroup analysis and more precise estimations.
This study also has its limitations: this report describes post hoc analyses from two trials with different protocols, not randomized head-to-head clinical trial evidence, and the results should be considered as hypothesis generating. PSM analyses can be subject to unmeasured confounding factors. There is a risk for discordance between PSM outcomes and those obtained from head-to-head randomized controlled trials. In analyses from other therapeutic areas, systematic comparisons of results obtained from randomized controlled trials to those from PSM analyses of observational data found generally consistent results between the two study types, although the magnitude of treatment effect could vary substantially [19, 20]. PSM analyses, even if employing data from randomized trials as in the present case, cannot be regarded as substitutes for head-to-head evaluation. Additionally, in considering the trials included in this analysis, it should be noted that the length of follow-up differed by 4 weeks; it is possible that evaluating endpoints at 30 weeks for both regimens would have provided more time to observe the effect of the fixed-ratio combination on HbA1c. However, as HbA1c data for the basal-bolus arm were only available to 26 weeks, we took a conservative approach and used iGlarLixi results from the 24-week mark, comparing it with the effects of basal bolus over 26 weeks. PPG was not available for each individual. In the basal-bolus group, there were 64 patients with baseline values for PPG and 46 (23.6% of the total basal-bolus group) who had follow-up measurements, limiting the sample size for analysis of this endpoint. As mentioned in a previous PSM analysis including GetGoal Duo-2, the low number of patients from GetGoal Duo-2 with baseline 2-h PPG also prevented the use of this variable as a covariate [8]. However, change from baseline PPG in this analysis was comparable to the original trials: − 5.4 mmol/L for iGlarLixi here versus − 4.7 mmol/L in LixiLan-L, and − 2.0 mmol/L for basal bolus here versus − 1.6 mmol/L and − 1.4 mmol/L in GetGoal Duo-2 [5, 6]. Despite these limitations, in the absence of head-to-head comparisons between iGlarLixi and basal-bolus insulin therapy, a PSM analysis can be valuable, as it provides some probable evidence for the truth of the conclusion by inductive reasoning.
Conclusions
In this post hoc PSM analysis, patients who took iGlarLixi had superior results compared with those taking a basal-bolus regimen, including individual endpoints and composite assessments such as achievement of HbA1c < 7% (< 53 mmol/mol) while remaining free from weight gain and hypoglycemia. In patients with type 2 diabetes uncontrolled on basal insulin, treatment intensification with iGlarLixi may be more efficacious and well tolerated than switching to basal bolus, while offering convenience benefits with potential implications for adherence.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants of the LixiLan-L and GetGoal Duo-2 trials.
Funding
This study and the Rapid Service Fee were funded by Sanofi.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance in the preparation of this article was provided by Rob Coover of Caudex, New York, which was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
FJT and CW contributed to the acquisition of original trial data. AS and PS contributed to conception of the data analysis. ML performed the data analysis. All authors contributed to drafting the manuscript and/or revising it for intellectual content, and provided their final approval of the manuscript.
Prior Presentation
This research was previously presented as poster 786 at the 54th Annual Meeting of the European Association for the Study of Diabetes, October 1–5, 2018, Berlin, Germany.
Disclosures
Ádám G. Tabák has received consultancy and advisory board fees from 77 Elektronika, Lilly Hungária, and Sanofi; and speaker fees from AstraZeneca, Berlin-Chemie, Eli Lilly, Novo Nordisk, and Sanofi. John Anderson has received consultancy and advisory board fees from Abbott, AstraZeneca, Eli Lilly, Janssen, MannKind, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and speaker fees from AstraZeneca, Eli Lilly, Janssen, Novo Nordisk, and Sanofi. Pablo Aschner has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novartis, and Sanofi; and on speaker bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi. Minzhi Liu has received consultancy fees from BDM Consulting, Inc., and Sanofi. Aramesh Saremi was an employee of Sanofi at the time of study completion and is now an employee of Intercept Pharmaceuticals, San Diego, CA, USA. Peter Stella is an employee of Sanofi. Francisco J. Tinahones has received consultancy and advisory board fees from, and has served on speaker bureaus for, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Regeneron Pharmaceuticals, and Sanofi. Carol Wysham has received consultancy and advisory board fees from AstraZeneca, Janssen, and Sanofi; research support from AstraZeneca and Novo Nordisk; and speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi. Juris J. Meier has received advisory board fees from Abbott and Sanofi; is a board member of Novo Nordisk; has received consultancy fees from AstraZeneca, Merck Sharp & Dohme, and Novo Nordisk; and has received speaker fees from Bayer, Eli Lilly, LifeScan, Merck Sharp & Dohme, Novartis, Roche, and Sanofi.
Compliance with Ethics Guidelines
The trials were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines for good clinical practice, and all applicable laws and regulations. All protocols were reviewed and approved by independent ethics committees and institutional review boards. All patients gave their informed consent prior to their inclusion in the study.
Data Availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10303784.
References
- 1.Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target < 7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14:228–233. doi: 10.1111/j.1463-1326.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 2.Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 3.Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract. 2016;121:17–26. doi: 10.1016/j.diabres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: results of a quantitative survey. Diabetes Obes Metab. 2018;20:301–308. doi: 10.1111/dom.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–1980. doi: 10.2337/dc16-1495. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care. 2016;39:1318–1328. doi: 10.2337/dc16-0014. [DOI] [PubMed] [Google Scholar]
- 7.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–157. doi: 10.2337/dc16-2215. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Handelsman Y, Vidal J, et al. Propensity score-matched comparative analyses of simultaneously administered fixed-ratio iGlarLixi (LixiLan) vs sequential administration of insulin glargine and lixisenatide in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2018;20:2821–2829. doi: 10.1111/dom.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiorino MI, Chiodini P, Bellastella G, et al. Free and fixed-ratio combinations of basal insulin and GLP-1 receptor agonists versus basal insulin intensification in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20:2309–2313. doi: 10.1111/dom.13343. [DOI] [PubMed] [Google Scholar]
- 10.Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37:2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 11.Porcellati F, Lucidi P, Bolli GB, Fanelli CG. GLP-1 RAs as compared to prandial insulin after failure of basal insulin in type 2 diabetes: lessons from the 4B and Get-Goal DUO 2 trials. Diabetes Metab. 2015;41:6S16–6S20. doi: 10.1016/S1262-3636(16)30004-0. [DOI] [PubMed] [Google Scholar]
- 12.Maiorino MI, Chiodini P, Bellastella G, Capuano A, Esposito K, Giugliano D. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017;40:614–624. doi: 10.2337/dc16-1957. [DOI] [PubMed] [Google Scholar]
- 13.Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care. 2018;41:1009–1016. doi: 10.2337/dc17-1114. [DOI] [PubMed] [Google Scholar]
- 14.Dungan KM, Raz I, Skrivanek Z, Sealls W, Fahrbach JL. Achieving the composite endpoint of glycated haemoglobin < 7.0%, no weight gain and no hypoglycaemia in the once-weekly dulaglutide AWARD programme. Diabetes Obes Metab. 2016;18:49–55. doi: 10.1111/dom.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabe D, Ambos A, Cariou B, et al. Efficacy of lixisenatide in patients with type 2 diabetes: a post hoc analysis of patients with diverse beta-cell function in the GetGoal-M and GetGoal-S trials. J Diabetes Complications. 2016;30:1385–1392. doi: 10.1016/j.jdiacomp.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins V, Zhang B, Fleurence RL, Krishnarajah G, Graham J. A systematic review of adherence, treatment satisfaction and costs, in fixed-dose combination regimens in type 2 diabetes. Curr Med Res Opin. 2011;27:1157–1168. doi: 10.1185/03007995.2011.570745. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–2035. doi: 10.2337/dc16-0917. [DOI] [PubMed] [Google Scholar]
- 19.Kitsios GD, Dahabreh IJ, Callahan S, Paulus JK, Campagna AC, Dargin JM. Can we trust observational studies using propensity scores in the critical care literature? A systematic comparison with randomized clinical trials. Crit Care Med. 2015;43:1870–1879. doi: 10.1097/CCM.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 20.Dahabreh IJ, Sheldrick RC, Paulus JK, et al. Do observational studies using propensity score methods agree with randomized trials? A systematic comparison of studies on acute coronary syndromes. Eur Heart J. 2012;33:1893–1901. doi: 10.1093/eurheartj/ehs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.