Abstract
Spinal cord injury (SCI) results in severe damage, which causes functional alterations together with loss of autonomic functions, sensations, and muscle functioning. This injury leads to apoptosis of neurons and oligodendrocytes, which further leads to dysfunction of the spinal cord due to axonal degeneration and demyelination. Taurine is non-proteogenic and an essential amino acid, which plays a major role in the growth and development of brain cells. Ascorbic acid, also known as vitamin C, is found in various foods and is known to prevent scurvy. In this study, we have investigated the therapeutic effect of ascorbic acid and taurine against SCI-induced rats. The rats were divided into the following groups: sham, control, 100 mg/kg of taurine, 100 mg/kg of ascorbic acid, and 100 mg/kg of taurine + 100 mg/kg of ascorbic acid. Treatment was continued daily for 45 consecutive days. The combined treatment of taurine and ascorbic acid decreased caspase-3, bax, pro-NGF, and p53 mRNA expression by more than 30% compared to individual treatments. The combined treatment of taurine and ascorbic acid reduced caspase-3 and p53 expression by 33.7% and 44%, respectively, compared to individual treatments. The combined treatment of taurine and ascorbic acid decreased mRNA expression of interleukin-6 (IL-6), cyclooxygenase-2, tumor necrosis factor-alpha (TNF-α), and inducible nitric oxide synthase (iNOS) compared to the individual treatments of taurine and ascorbic acid. The combined treatment of taurine and ascorbic acid also significantly recovered altered antioxidant markers, and induced lipid peroxidation to near normal levels. In summary, apoptotic, inflammatory and oxidative stress markers were significantly decreased in SCI-induced rats treated with taurine and ascorbic acid.
Keywords: Taurine, Ascorbic acid, Spinal cord injury, Apoptosis, Inflammation
Introduction
Spinal cord injury (SCI) results in severe damage, which causes functional alterations along with loss of autonomic functions, sensations, and muscle functioning (Krishna et al. 2013). Previous studies have reported that SCI leads to apoptosis of neurons and oligodendrocytes, which further leads to dysfunction of the spinal cord due to axonal degeneration and demyelination (Minakov et al. 2018). Additional studies have reported that SCI induces apoptosis and a higher rate of inflammation and oxidative stress in neurons and oligodendrocytes (Bao and Liu 2002). The proinflammatory cytokines and reactive oxygen species (ROS) are released from microglia during inflammatory processes and neurodegeneration (Min et al. 2004). Hussein et al. (2018) reported that interleukins (ILs) are key cytokines that play important roles in immunological reactions. Thus, an effective therapeutic agent is needed against inflammation and oxidative stress.
Taurine is a non-proteogenic and essential amino acid, which plays a major role in the growth and development of brain cells (Schuller-Levis and Park 2003; Wang et al. 2016). Previous studies have reported the active role of taurine in osmoregulation, neurotransmission, calcium homeostasis, and prevention of seizures (Leon et al. 2009; Tsuboyama-Kasaoka et al. 2006; Olive 2002). An additional study has reported the antioxidant potential of taurine against oxidative stress (Zhang et al. 2004). Yanagita et al. (2008) reported the reduction of apolipoprotein B100 and lipid secretion in liver cancer cells, and Nakajima et al. (2010) reported the therapeutic potential of taurine against inflammatory responses in SCI. Ascorbic acid, also known as vitamin C, is found in various foods and is known to prevent scurvy (Yan et al. 2014). Yan et al. (2012) have reported the supplementation of high dose of vitamin C improves the function recovery of SCI. Wang et al. (2015) reported the therapeutic effects of ascorbic acid against SCI-induced rats, and Katoh et al. (1996) reported the protective effect of dietary ascorbic acid against spinal cord compression injury in a rat model. Thus, the present study evaluated the synergistic effects of ascorbic acid and taurine in SCI-induced male albino rats.
Materials and methods
Rats
Male albino Wistar strain rats (180–210 g, 3–4 months old) were obtained from the Animal center of the Academy of Military medical sciences, China. All of the rats were kept in standard rat polypropylene cages (435 × 290 × 150 mm; six rats in each cage) and maintained under standard lighting condition (12 h light/dark) periods with a relative humidity of 60 ± 5% and temperature of 25 ± 0.5 °C with food and water provided ad libitum. Animal experiments were approved by the ethical committee (201308819) of Tianjin Hospital, Tianjin and China.
Experimental groups
SCI was induced in rats as previously reported (Krishna et al. 2013). Rats were divided into the following groups: group I, sham; group II, control (SCI); group III, 100 mg/kg of taurine; group IV, 100 mg/kg of ascorbic acid; and group V, 100 mg/kg of taurine + 100 mg/kg of ascorbic acid. Sham and control rats were given saline, and treated rats received 1 mL of taurine or ascorbic acid. Treatment was continued daily for 45 consecutive days.
Collection of blood
Blood was collected at the end of treatment by cardiac puncture with the use of a syringe (5 mL) with a 23-gauge needle. The animals were then anesthetized using xylazine (10 mg/kg body weight) and ketamine hydrochloride (100 mg/kg body weight), and sacrificed by decapitation.
Tissue homogenate preparation
Rats were sacrificed by decapitation following anesthetization with ketamine hydrochloride (100 mg/kg body weight) and xylazine (10 mg/kg body weight). Spinal cord samples (12 mm) were obtained from the operated region of the rats. Spinal cord tissue was sliced into several pieces and homogenized in Tris–HCl buffer (50 mM, pH 7.4) for 10 min at 6000 rpm. The homogenate was then centrifuged, and the supernatant was preserved at 4 °C for experiments.
Apoptosis
p53, caspase-3, bax, bcl-2, and pro-NGF mRNA expression levels were determined as previously described (Bernal et al. 2005). Total RNA was isolated from the spinal cord tissue and transcribed into cDNA. Primers used for the amplification of these genes using real-time PCR are listed in Table 1. Protein expression of caspase-3 (ab4051, Abcam) and p53 (ab131442, Abcam) in the spinal cord tissue was determined according to Ali et al. (2017).
Table 1.
List of primers used in real-time PCR reactions
| S. no. | Gene name | Sense primer | Anti-sense primer |
|---|---|---|---|
| 1 | TNF-α | 5′-CCCAGACCCTCACACTCAGAT-3′ | 5′-TTG TCC CTTGAA GAG AAC CTG-3′ |
| 2 | IL-6 | 5′-AAGTTTCTCTCCGCAAGATAC TTCCAGCCA-3′ | 5′-AGG CAAATTTCCTGGTTATATCCA GTTT-3′ |
| 3 | Cyclooxygenase-2 | 5′-CCA TGT CAA AAC CGT GGTGAATG-3′ | 5′-ATG GGAGTTGGGCAGTCATCAG-3′ |
| 4 | iNOS | 5′-CTCCATGACTCTCAGCACAGAG-3′ | 5′-GCACCGAAGATATCCTCATGAT-3′ |
| 5 | p53 | 5′-TAACAGTTCCTGCATGGGCGGC-3′ | 5′-AGGACAGGCACAAACACGCACC-3′ |
| 6 | Bax | 5′-TGG AGCTGCAGAGGATGATTG-3′ | 5′-GAAGTTGCCGTCAGAAAACATG-3′ |
| 7 | Caspase-3 | 5′-TTAATAAAGGTATCCATGGAGAACACT-3′ | 5′-TTAGTGATAAAAATAGAGTTCTTTTGTGAG-3' |
| 8 | Bcl-2 | 5′-CAC CCC TGG CAT CTT CTC CTT-3′ | 5′-AGC GTC TTC AGA GAC AGC CAG-3′ |
| 9 | pro-NGF | 5′-CTTCAGCATTCCCTTGACAC-3′ | 5′-TGAGCACACACACGCAGGC-3′ |
| 10 | GAPDH | 5′-TCCCTCAAGATTGTCAGCAA-3′ | 5′-AGATCCACAACGGATACATT-3′ |
Inflammation
The nitrite level was determined as a marker of nitric oxide (NO) content as previously described (Shaheen et al. 2016). Serum levels of tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) were determined according to Afshari et al. (2005). IL-6, cyclooxygenase-2, inducible nitric oxide synthase (iNOS), and TNF-α mRNA expression levels were determined according to Bernal et al. (2005). Table 1 lists the primers used for real-time PCR.
Oxidative stress
Catalase, superoxide dismutase (SOD), and glutathione peroxidase (Gpx) activities were determined according to Kaddour et al. (2016). Reduced glutathione (GSH), ROS, and lipid peroxidation were also determined as previously described (Arutyunyan et al. 2016).
Statistical analysis
Experimental results are expressed as mean ± standard deviation. The results were compared using a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. The difference between control and treated samples was considered significant at P < 0.05.
Results
The effect of taurine and ascorbic acid on apoptotic markers
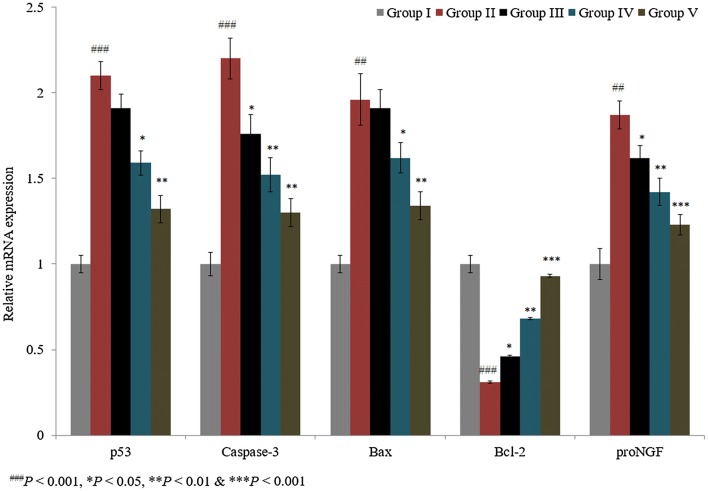
We showed the synergistic effect of ascorbic acid and taurine in a spinal cord injury-induced rat model. Caspase-3, p53, bax, bcl-2, and pro-NGF mRNA expression levels were quantified using RT-PCR. p53, caspase-3, bax, and pro-NGF mRNA expression levels were substantially increased by 110%, 120%, 96%, and 87%, respectively, in control rats, whereas bcl-2 mRNA expression was decreased by 69% in control rats (Fig. 1; P < 0.05). The individual treatments of taurine and ascorbic acid reduced mRNA levels of p53 by 9% and 24.3% in groups III and IV, respectively. However, the combined treatment of taurine and ascorbic acid decreased p53 mRNA by 37.1% in group V (Fig. 1; P < 0.05).
Fig. 1.
Synergistic protective effect of taurine and ascorbic acid on p53, caspase-3, bax, bcl-2, and pro-NGF mRNA expression. The combined treatment of taurine and ascorbic acid recovered the altered mRNA expression levels. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control
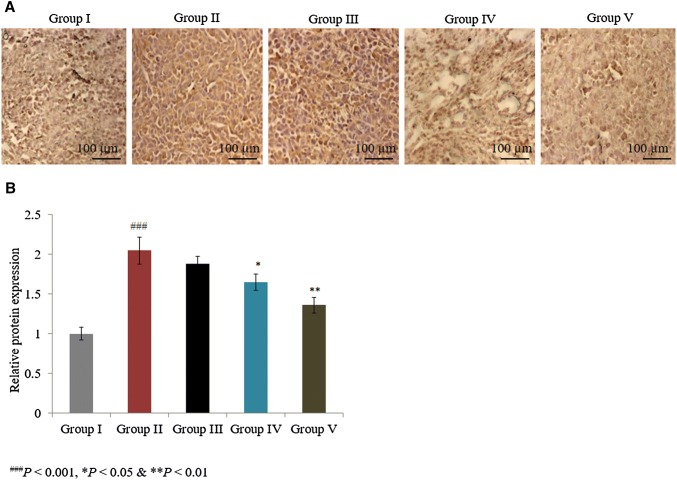
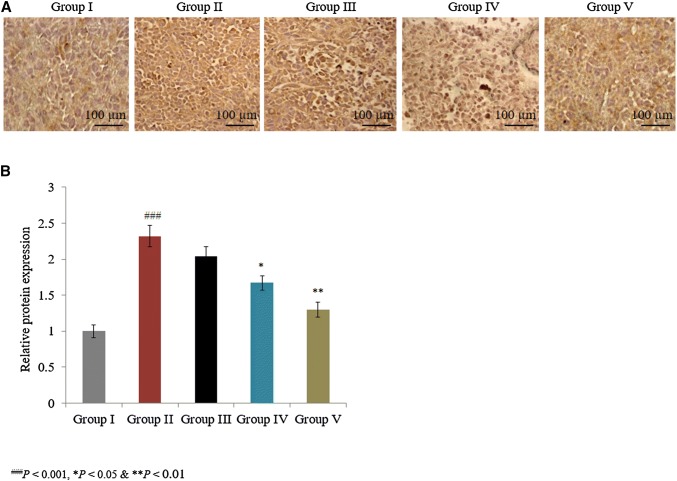
In a similar manner, the combined treatment of taurine and ascorbic acid decreased caspase-3, bax, and pro-NGF mRNA expression by more than 30% in group V (Fig. 1; P < 0.05). The individual treatments of taurine and ascorbic acid increased the mRNA expression of bcl-2 by 48% and 119.4% in groups III and IV, respectively, and the combined treatment of taurine and ascorbic acid significantly increased bcl-2 mRNA expression by 200% in group V (Fig. 1; P < 0.05). Immunohistochemical analysis revealed that caspase-3 and p53 protein expression was significantly increased by 105% and 132%, respectively, in control rats (Figs. 2, 3; P < 0.05). The individual treatments of taurine and ascorbic acid reduced caspase-3 protein expression by 8.3% and 19.5% in groups III and IV, respectively, whereas p53 expression was reduced by 12.1% and 28%, respectively (Figs. 2, 3). However, the combined treatment of taurine and ascorbic acid significantly reduced caspase-3 and p53 expression by 33.7% and 44%, respectively, in group V (Figs. 2, 3; P < 0.05).
Fig. 2.
Synergistic protective potential of taurine and ascorbic acid on the protein expression of caspase-3. The combined treatment of taurine and ascorbic acid recovered the altered protein expression. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control. Scale bar 100 μm, N = 6
Fig. 3.
Synergistic protective potential of taurine and ascorbic acid on the protein expression of p53. The combined treatment of taurine and ascorbic acid recovered the altered protein expression. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control. Scale bar 100 μm, N = 6
The effect of taurine and ascorbic acid on inflammatory markers
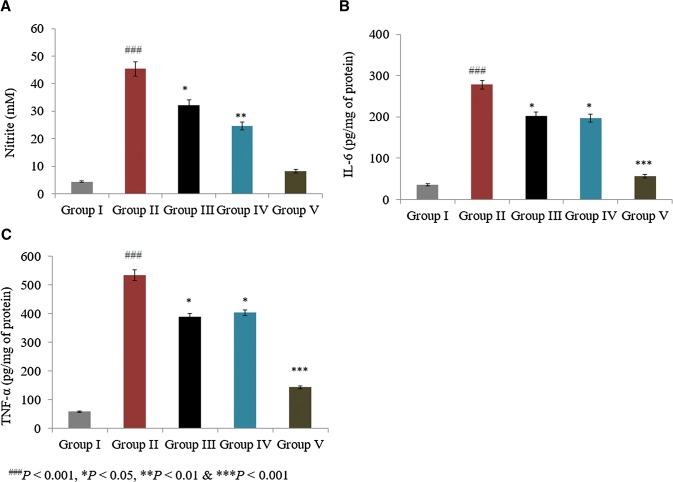
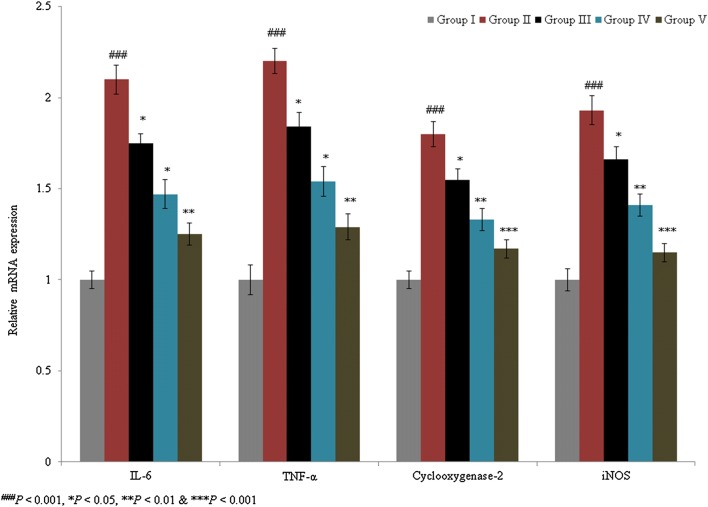
Serum nitrite level was determined as a marker of NO levels. Serum NO content was 3.7 mM in sham rats, which was significantly increased by more than 900% in control rats. The individual treatments of taurine and ascorbic acid reduced NO levels by 28.9% and 45.5%, respectively, in groups III and IV. However, the combined treatment of taurine and ascorbic acid decreased the NO level by 81.9% in group V (Fig. 4a; P < 0.05). In a similar manner, the combined treatment of taurine and ascorbic acid significantly reduced IL-6 and TNF-α expression by more than 30% in group V (Fig. 4b, c; P < 0.05). IL-6, cyclooxygenase-2, TNF-α, and iNOS mRNA levels were substantially increased by 110%, 80%, 120%, and 93%, respectively, in control rats. The combined treatment of taurine and ascorbic acid decreased IL-6, cyclooxygenase-2, TNF-α, and iNOS mRNA levels when compared with the individual treatments of taurine and ascorbic acid (Fig. 5; P < 0.05).
Fig. 4.
Synergistic protective effect of taurine and ascorbic acid on the levels of nitric oxide (NO), interleukin (IL)-6, and tumor necrosis factor (TNF)-α. The combined treatment of taurine and ascorbic acid recovered the altered protein expression levels. Serum levels of NO (a), IL-6 (b), and TNF-α (c). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control. Scale bar 100 μm, N = 6
Fig. 5.
Synergistic protective effects of taurine and ascorbic acid on interleukin (IL-6), cyclooxygenase-2, tumor necrosis factor (TNF-α), and inducible nitric oxide synthase (iNOS) mRNA expression. The combined treatment of taurine and ascorbic acid recovered the altered mRNA expression levels. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control
The effect of taurine and ascorbic acid on oxidative markers
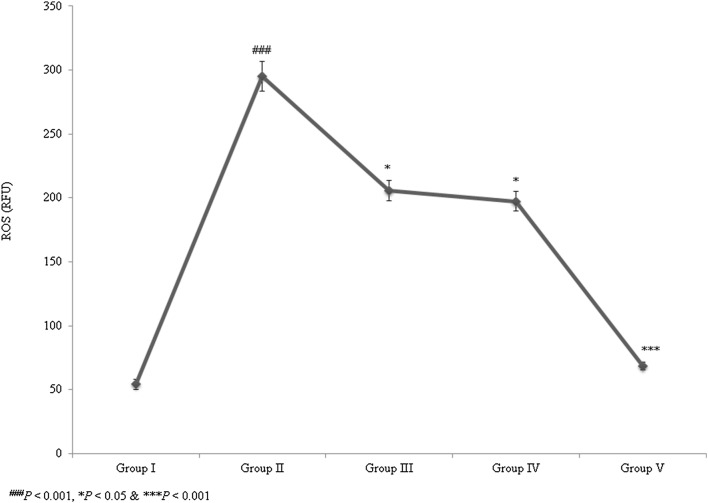
The ROS level was substantially increased by 444.2% in control rats. The individual treatments of taurine and ascorbic acid reduced ROS levels by 30.4% and 33.1%, respectively, in groups III and IV. However, the combined treatment of taurine and ascorbic acid significantly reduced ROS levels by 76.9% in group V (Fig. 6; P < 0.05). SOD, catalase, and Gpx activities, as well as GSH content, were significantly decreased in control rats. However, the combined treatment of taurine and ascorbic acid significantly recovered these altered oxidative markers to normal levels (Table 1; P < 0.05). Lipid peroxidation was significantly reduced to near normal levels following combined treatment of taurine and ascorbic acid.
Fig. 6.
Synergistic protective effect of taurine and ascorbic acid on reactive oxygen species (ROS) levels. The combined treatment of taurine and ascorbic acid reduced ROS levels. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; ###P < 0.001 vs. sham control
Discussion
In the present study, we showed the synergistic effect of taurine and ascorbic acid in the SCI-induced rat model. Studies have reported that SCI leads to apoptosis of neurons and oligodendrocytes, which further leads to dysfunction of the spinal cord due to axonal degeneration and demyelination (Minakov et al. 2018). Studies have reported that SCI induces apoptosis and a higher rate of inflammation and oxidative stress in neurons and oligodendrocytes (Bao and Liu 2002). The proinflammatory cytokines and reactive oxygen species (ROS) are released from microglia during inflammatory processes and neurodegeneration. Hussein et al. (2018) have reported that ILs are key cytokines that play vital roles in immunological reactions. In the present study, expression levels of caspase-3, bax, bcl-2, p53, and pro-NGF were significantly reduced in SCI-induced rats treated with taurine and ascorbic acid, which confirmed the protective role of taurine and ascorbic acid against SCI (Table 2).
Table 2.
Therapeutic effect of taurine and ascorbic acid on MDA, GSH, SOD, catalase and Gpx in spinal cord injury-induced male albino rats
| Oxidative markers | Group I (Sham) | Group II (control) | Group III (100 mg/kg of taurine) | Group IV (100 mg/kg of ascorbic acid) | Group V (100 mg/kg of taurine + 100 mg/kg of ascorbic acid) |
|---|---|---|---|---|---|
| GSH (mg/g) | 81.4 ± 3.15 | 30.2 ± 1.2### | 45.2 ± 2.2* | 48.4 ± 2.5* | 75.9 ± 3.8*** |
| SOD (U/mg) | 8.2 ± 0.2 | 3.3 ± 0.1### | 4.9 ± 0.2* | 5.2 ± 0.2* | 7.8 ± 0.3*** |
| Catalase (U/g) | 14.2 ± 1 | 4.1 ± 0.1### | 7.8 ± 0.2* | 8.6 ± 0.3* | 12.7 ± 0.7*** |
| Gpx (mg/protein) | 0.98 ± 0.001 | 0.31 ± 0.005## | 0.52 ± 0.003* | 0.67 ± 0.005* | 0.84 ± 0.01*** |
| MDA (nmol/g) | 20.5 ± 1.1 | 77 ± 3### | 51.6 ± 2* | 49.5 ± 2.5* | 28.4 ± 1.2*** |
##P < 0.01, ###P < 0.001, *P < 0.05, ***P < 0.001
Studies have reported that treatment with sufficient amount of antioxidants was effective against various neurodegenerative diseases (Liu et al. 2002). Studies have also reported the functional role of taurine in osmoregulation, neurotransmission, calcium homeostasis, and the prevention of seizures (Leon et al. 2009; Tsuboyama-Kasaoka et al. 2006; Olive 2002), and other studies have reported the antioxidant potential of taurine against oxidative stress (Zhang et al. 2004). Yanagita et al. (2008) reported the reduction of apolipoprotein B100 and lipid secretion in liver cancer cells, and Nakajima et al. (2010) reported the therapeutic potential of taurine against inflammatory responses in SCI. Yan et al. (2012) have reported the supplementation of a high dose of vitamin C improves the function recovery of SCI. Wang et al. (2015) further reported the therapeutic effects of ascorbic acid against SCI-induced rats, and Katoh et al. (1996) reported the protective potential of dietary ascorbic acid on spinal cord compression injury in a rat model. Another study reported the downregulation of p53 and caspase-3 with a concomitant elevation in the ratio of bax/bcl-2o (Yu et al. 2016). Our results are consistent with these findings; decreased levels of oxidative, apoptotic, and inflammatory markers were observed in rats with a SCI.
Conclusion
Apoptotic, inflammatory, and oxidative stress markers were significantly decreased in spinal cord injury-induced rats treated with taurine and ascorbic acid.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
References
- Afshari JT, Ghomian N, Shameli A, Shakeri MT, Fahmidehkar MA, Mahajer E, Khoshnavaz R, Emadzadeh M. Determination of interleukin-6 and tumor necrosis factor-alpha concentrations in Iranian-Khorasanian patients with preeclampsia. BMC Pregnancy Childbirth. 2005;5:14. doi: 10.1186/1471-2393-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Abd Al Haleem EN, Khaleel SA, Sallam AS. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol Rep. 2017;69(2):268–275. doi: 10.1016/j.pharep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Arutyunyan TV, Korystova AF, Kublik LN, Levitman MKh, Shaposhnikova VV, Korystov YN. Taxifolin and fucoidin abolish the irradiation-induced increase in the production of reactive oxygen species in rat aorta. Bull Exp Biol Med. 2016;160:635–638. doi: 10.1007/s10517-016-3236-2. [DOI] [PubMed] [Google Scholar]
- Bao F, Liu D. Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience. 2002;115:839–849. doi: 10.1016/S0306-4522(02)00506-7. [DOI] [PubMed] [Google Scholar]
- Bernal F, Hartung HP, Kieseier BC. Tissue mRNA expression in rat of newly described matrix metalloproteinases. Biol Res. 2005;38(2–3):267–271. doi: 10.4067/s0716-97602005000200016. [DOI] [PubMed] [Google Scholar]
- Hussein J, El-Banna M, Razik TA, El-Naggar ME. Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications. Int J Biol Macromol. 2018;107(Pt A):748–754. doi: 10.1016/j.ijbiomac.2017.09.056. [DOI] [PubMed] [Google Scholar]
- Kaddour T, Omar K, Oussama AT, Nouria H, Iméne B, Abdelkader A. Aluminium-induced acute neurotoxicity in rats: treatment with aqueous extract of Arthrophytum (Hammada scoparia) J Acute Dis. 2016;5(6):470–482. doi: 10.1016/j.joad.2016.08.028. [DOI] [Google Scholar]
- Katoh D, Ikata T, Katoh S, Hamada Y, Fukuzawa K. Effect of dietary vitamin C on compression injury of the spinal cord in a rat mutant unable to synthesize ascorbic acid and its correlation with that of vitamin E. Spinal Cord. 1996;34(4):234–238. doi: 10.1038/sc.1996.43. [DOI] [PubMed] [Google Scholar]
- Krishna V, Andrews H, Jin X, Yu J, Varma A, Wen X, Kindy M. A contusion model of severe spinal cord injury in rats. J Vis Exp. 2013;78:50111. doi: 10.3791/50111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY. Protective function of taurine in glutamate-induced apoptosis in cultured neurons. J Neurosci Res. 2009;87(5):1185–94. doi: 10.1002/jnr.21926. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-l-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci USA. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Minakov AN, Chernov AS, Asutin DS, Konovalov NA, Telegin GB. Experimental models of spinal cord injury in laboratory rats. Acta Naturae. 2018;10(3):4–10. doi: 10.32607/20758251-2018-10-3-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Osuka K, Seki Y, Gupta RC, Hara M, Takayasu M, Wakabayashi T. Taurine reduces inflammatory responses after spinal cord injury. J Neurotrauma. 2010;27(2):403–410. doi: 10.1089/neu.2009.1044. [DOI] [PubMed] [Google Scholar]
- Olive MF. Interactions between taurine and ethanol in the central nervous system. Amino Acids. 2002;23(4):345–57. doi: 10.1007/s00726-002-0203-1. [DOI] [PubMed] [Google Scholar]
- Schuller-Levis GB, Park E. Taurine: new implications for an old amino acid. FEMS Microbiol Lett. 2003;226:195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- Shaheen TI, El-Naggar MI, Hussein JS, El-Bana M, Emara E, El-Khayat Z, Fouda MMG, Ebaid H, Hebeish A. Antidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomed Pharmacother. 2016;83:865–875. doi: 10.1016/j.biopha.2016.07.052. [DOI] [PubMed] [Google Scholar]
- Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology. 2006;147(7):3276–84. doi: 10.1210/en.2005-1007. [DOI] [PubMed] [Google Scholar]
- Wang WG, Xiu RJ, Xu ZW, Yin YX, Feng Y, Cao XC, Wang PS. Protective effects of vitamin C against spinal cord injury-induced renal damage through suppression of NF-κB and proinflammatory cytokines. Neurol Sci. 2015;36(4):521–526. doi: 10.1007/s10072-014-1965-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fan W, Cai Y, Wu Q, Mo L, Huang Z, Huang H. Protective effects of taurine in traumatic brain injury via mitochondria and cerebral blood flow. Amino Acids. 2016;48(9):2169–2177. doi: 10.1007/s00726-016-2244-x. [DOI] [PubMed] [Google Scholar]
- Yan M, Yang M, Shao W, Mao XG, Yuan B, Chen YF, Ye ZX, Liang W, Luo ZJ. High-dose ascorbic acid administration improves functional recovery in rats with spinal cord contusion injury. Spinal Cord. 2014;52(11):803–808. doi: 10.1038/sc.2014.135. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Han SY, Hu Y, Nagao K, Kitajima H, Murakami S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis. 2008;7:38. doi: 10.1186/1476-511X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Li X, Cao X. Cardioprotective effects of phenylethanoid glycoside-rich extract from Cistanche deserticola in ischemia–reperfusion-induced myocardial infarction in rats. Ann Vasc Surg. 2016;34:234–242. doi: 10.1016/j.avsg.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang M, Izumi I, Kagamimori S, Sokejima S, Yamagami T, Liu Z, Qi B. Role of taurine supplementation to prevent exercise-induced oxidative stress in healthy young men. Amino Acids. 2004;26(2):203–207. doi: 10.1007/s00726-003-0002-3. [DOI] [PubMed] [Google Scholar]