Abstract
Introduction
The PIONEER trial programme showed that, after 52 weeks, the novel oral glucagon-like peptide-1 (GLP-1) analogue semaglutide 14 mg was associated with significantly greater reductions in glycated haemoglobin (HbA1c) versus a sodium-glucose cotransporter-2 inhibitor (empagliflozin 25 mg), a dipeptidyl peptidase-4 inhibitor (sitagliptin 100 mg) and an injectable GLP-1 analogue (liraglutide 1.8 mg). The aim of the present analysis was to assess the long-term cost-effectiveness of oral semaglutide 14 mg versus each of these comparators in the UK setting.
Methods
Analyses were performed from a healthcare payer perspective using the IQVIA CORE Diabetes Model, in which outcomes were projected over patient lifetimes (50 years). Baseline cohort characteristics and treatment effects were based on 52-week data from the PIONEER 2, 3 and 4 randomised controlled trials, comparing oral semaglutide with empagliflozin, sitagliptin and liraglutide, respectively. Treatment switching occurred when HbA1c exceeded 7.5% (58 mmol/mol). Utilities, treatment costs and costs of diabetes-related complications (in pounds sterling [GBP]) were taken from published sources. The acquisition cost of oral semaglutide was assumed to match that of once-weekly semaglutide.
Results
Oral semaglutide was associated with improvements in quality-adjusted life expectancy of 0.09 quality-adjusted life years (QALYs) versus empagliflozin, 0.20 QALYs versus sitagliptin and 0.07 QALYs versus liraglutide. Direct costs over a patient’s lifetime were GBP 971 and GBP 963 higher with oral semaglutide than with empagliflozin and sitagliptin, respectively, but GBP 1551 lower versus liraglutide. Oral semaglutide was associated with a reduced incidence of diabetes-related complications versus all comparators. Therefore, oral semaglutide 14 mg was associated with incremental cost-effectiveness ratios of GBP 11,006 and 4930 per QALY gained versus empagliflozin 25 mg and sitagliptin 100 mg, respectively, and was more effective and less costly (dominant) versus liraglutide 1.8 mg.
Conclusion
Oral semaglutide was cost-effective versus empagliflozin and sitagliptin, and dominant versus liraglutide, for the treatment of type 2 diabetes in the UK.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-00736-6) contains supplementary material, which is available to authorized users.
Keywords: Cost effectiveness, Costs and cost analysis, Diabetes mellitus, Empagliflozin, GLP-1 receptor agonist, Liraglutide, Oral semaglutide, Sitagliptin, United Kingdom
Key Summary Points
| Why carry out this study? |
| Type 2 diabetes is associated with a substantial and increasing clinical and economic burden in the UK, which translates into a crucial need for cost-effective therapies that improve patient outcomes while minimising costs for the healthcare payer. |
| The recent PIONEER clinical trial programme showed that oral semaglutide, the first oral medication in its class, was associated with improved glycaemic control and reductions in body weight versus empagliflozin, sitagliptin and liraglutide, factors that have been associated with a reduced risk of long-term diabetes-related complications. |
| The present analysis used a clinically-relevant treatment approach to assess the long-term cost-effectiveness of oral semaglutide 14 mg versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg for the treatment of people with type 2 diabetes from a healthcare payer perspective in the UK. |
| What was learned from the study? |
| Oral semaglutide 14 mg was projected to improve both life expectancy and quality-adjusted life expectancy versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg. |
| Direct costs over patient lifetimes were estimated to be higher with oral semaglutide versus empagliflozin and sitagliptin, but lower versus liraglutide, with costs associated with the treatment of diabetes-related complications lower with oral semaglutide in all comparisons. |
| Oral semaglutide 14 mg was therefore considered to be a cost-effective treatment option versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg for the treatment of type 2 diabetes in the UK. |
Introduction
Diabetes accounts for a substantial health and economic burden in the UK, with over 4.7 million people living with the disease and over 12.3 million people at an increased risk of developing the disease in 2018 [1]. Diabetes-related healthcare expenditure (expressed in pounds sterling [GBP]) was estimated to be over GBP 10 billion in 2018, accounting for 10% of the entire National Health Service (NHS) budget [1]. An estimated 80% of diabetes-related expenditure in the UK is associated with the treatment of long-term complications, with a more than twofold increase in risk of myocardial infarction, heart failure and stroke in people with type 2 diabetes versus those without the disease [1]. Interventions that are cost-effective, offering clinical benefits while providing value for money, are becoming vital as healthcare payers’ budgets come under increasing pressure.
Improvements in glycated haemoglobin (HbA1c) and blood pressure have long been associated with reductions in long-term diabetes-related complications, as demonstrated in the landmark United Kingdom Prospective Diabetes Study (UKPDS), and more recent evidence has suggested that reductions in other parameters, such as body weight, can provide further benefits [2–5]. Clinical guidelines published by the National Institute for Health and Care Excellence (NICE) in the UK recommend an individualised approach for the treatment of each patient that incorporates personal preferences and comorbidities [6]. Moreover, the most recent guidelines released by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) recommend a more holistic approach to diabetes treatment, rather than a sole focus on glycaemic control [7]. In particular, updated recommendations for people with type 2 diabetes with established cardiovascular disease or for those who are overweight or obese or have a high risk of hypoglycaemia now consider the effects of therapies on cardiovascular disease, body weight and hypoglycaemia risk alongside reductions in HbA1c [7].
Glucagon-like peptide-1 (GLP-1) receptor agonists represent a highly efficacious class of interventions for the treatment of type 2 diabetes, with the injectable GLP-1 analogue once-weekly semaglutide shown to be both efficacious and cost-effective versus a variety of therapies [8–11]. However, until recently, GLP-1 receptor agonists have only been available in injectable formulations, which may have been a barrier to patient use compared with other modern treatment options such as sodium-glucose cotransporter-2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors, which are administered orally. Indeed, in the UK, injectable GLP-1 receptor agonists are only recommended as an intensification step for patients with inadequate glycaemic control following a triple therapy combination of metformin plus a DPP-4 inhibitor with a sulfonylurea, pioglitazone or an SGLT2 inhibitor, for whom insulin therapy would have significant occupational implications or weight loss would benefit other obesity-related comorbidities [6]. Given the efficacy benefits GLP-1 receptor agonists appear to offer, earlier intensification to such medications could overcome the documented and substantial therapeutic inertia in people with type 2 diabetes [8–12].
Oral semaglutide is a novel formulation of the GLP-1 analogue semaglutide developed for once-daily oral administration, in which the absorption enhancer sodium N-(8-[2-hydroxybenzoyl]amino) caprylate facilitates absorption across the gastric mucosa. The efficacy and safety of oral semaglutide has been assessed in the PIONEER clinical trial programme, with once-daily oral semaglutide 14 mg compared with once-daily SGLT2 inhibitor empagliflozin 25 mg in PIONEER 2, with once-daily DPP-4 inhibitor sitagliptin 100 mg in PIONEER 3 and with once-daily injectable GLP-1 receptor agonist liraglutide 1.8 mg in PIONEER 4 [13–15].
The aim of the present analysis was to assess the long-term cost-effectiveness of oral semaglutide 14 mg versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg in the UK setting, based on the results of the PIONEER 2, 3 and 4 studies, respectively.
Methods
Modelling Approach
Long-term projections of clinical and cost outcomes were performed from a healthcare payer perspective using the IQVIA CORE Diabetes Model (version 9.0), a proprietary, validated, internet-based, interactive computer model developed to determine the long-term health outcomes and economic consequences of implementing interventions in the treatment of type 1 and type 2 diabetes mellitus (accessible at http://www.core-diabetes.com) [16, 17]. The architecture, assumptions, features and capabilities of the model have been previously published [16]. Validation studies of the model have been published both in 2004 and more recently in 2014 [17, 18].
Model outputs include time to onset and cumulative incidence of complications, life expectancy, quality-adjusted life expectancy (QALE; expressed in quality-adjusted life years [QALYs]), direct costs and, where required, incremental cost-effectiveness ratios (ICERs), which describe the cost per additional unit of effectiveness gained for the intervention versus the comparator. In comparisons where an intervention is associated with cost savings while providing greater clinical benefits, no calculation of an ICER is required and the intervention is considered to be dominant versus the comparator.
Analyses were performed over patient lifetimes (up to 50 years), as recommended in the guidelines for the cost-effectiveness assessment of interventions for type 2 diabetes, to ensure all relevant diabetes-related complications and their impact on clinical and cost outcomes were captured [19]. The UKPDS 68 risk equations were applied to predict model outcomes. Background mortality was captured based on UK-specific life tables published by the World Health Organisation (Electronic Supplementary Material [ESM] Table S1) [20]. Health-state utilities and event disutilities were based on published sources (ESM Table S2) [21–27].
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Clinical Data
Baseline cohort characteristics and treatment effects were sourced from the PIONEER 2, 3 and 4 trials for comparisons of oral semaglutide 14 mg with empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg, respectively (ESM Table S3; Table 1). PIONEER 2 enrolled people with type 2 diabetes with HbA1c values between 7.0 and 10.5% (53–91 mmol/mol) who were receiving metformin; PIONEER 3 enrolled people with type 2 diabetes with HbA1c values between 7.0 and 10.5% who were receiving metformin with or without a sulfonylurea; and PIONEER 4 enrolled people with type 2 diabetes with HbA1c values between 7.0 and 9.5% (53–80 mmol/mol) who were receiving metformin with or without an SGLT2 inhibitor. The PIONEER trial programme used two estimands, namely the treatment policy estimand and the trial product estimand, to address two different efficacy questions. The treatment policy estimand reflected the intention-to-treat principle by including all study participants randomly assigned to each treatment, using data regardless of discontinuation of study medications and/or use of additional anti-diabetic medications during the trial [28, 29]. In contrast, the trial product estimand assessed treatment effects under the assumption that patients received the study drug for the duration of the trial and did not receive any additional anti-diabetic medications, aiming to reflect the effects of the study medications without the confounding effects of rescue medication or any other changes in glucose-lowering medication [28]. To match the annual cycle length of the model, and to avoid the confounding impact of additional anti-diabetic medications on clinical and cost outcomes, the analyses were performed using the 52-week data evaluated by the trial product estimand. The impact of using data evaluated by the treatment policy estimand was explored in a sensitivity analysis.
Table 1.
Treatment effects and adverse event rates sourced from the PIONEER 2, 3 and 4 trials that were applied in the analyses
| Parameter | PIONEER 2 | PIONEER 3 | PIONEER 4 | |||
|---|---|---|---|---|---|---|
| Oral semaglutide 14 mg | Empagliflozin 25 mg | Oral semaglutide 14 mg | Sitagliptin 100 mg | Oral semaglutide 14 mg | Liraglutide 1.8 mg | |
| Physiological parameters applied in the first year of the analysis, mean (SE) | ||||||
| HbA1c (%) | − 1.30 (0.05)* | − 0.79 (0.05) | − 1.25 (0.05)* | − 0.52 (0.05) | − 1.19 (0.06)* | − 0.92 (0.06) |
| Systolic blood pressure (mmHg) | − 4.85 (0.65) | − 4.34 (0.63) | − 3.13 (0.63)* | − 0.82 (0.61) | − 3.36 (0.75) | − 2.86 (0.74) |
| Diastolic blood pressure (mmHg) | − 2.27 (0.45) | − 2.67 (0.44) | − 1.07 (0.39) | − 0.92 (0.38) | − 1.10 (0.45) | − 1.05 (0.44) |
| Total cholesterol (mg/dL)a | − 5.08 (1.62)* | 4.74 (1.57) | − 3.66 (1.50)* | 1.02 (0.57) | − 5.47 (2.07) | − 5.36 (2.05) |
| HDL cholesterol (mg/dL)a | 0.73 (0.35)* | 3.11 (0.34) | 0.54 (0.34) | 0.20 (0.35) | 1.17 (0.41) | 0.23 (0.41) |
| BMI (kg/m2) | − 1.73 (0.10)* | − 1.37 (0.09) | − 1.36 (0.07)* | − 0.32 (0.07) | − 1.82 (0.11)* | − 1.11 (0.11) |
| Hypoglycaemic event rates applied while patients received treatment | ||||||
| Non-severe hypoglycaemic event rate (events per 100 patient-years)b | 2.25 | 1.90 | 12.12 | 11.99 | 0.71 | 3.16 |
| Severe hypoglycaemic event rate (events per 100 patient-years)b | 0.25 | 0.24 | 0.24 | 0.90 | 0.00 | 0.00 |
| Proportion of non-severe hypoglycaemic events that are nocturnalb | 0.11 | 0.13 | 0.14 | 0.13 | 0.00 | 0.11 |
| Proportion of severe hypoglycaemic events that are nocturnalb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
*Statistically significant difference at the 95% confidence level
Hypoglycaemic events were assessed as safety endpoints at 57 weeks in PIONEER 2 and 4 and 52 weeks in PIONEER 3 [13–15]. All data, unless otherwise indicated, were evaluated by the trial product estimand at 52 weeks [13–15]
BMI Body mass index, HbA1c glycated haemoglobin, HDL high-density lipoprotein, SE standard error
aEstimated with an arithmetic mean
bData on file (not previously published)
Treatment Switching and Long-Term Parameter Progression
Following application of the treatment effects in the first year of the analysis, HbA1c was modelled to follow the UKPDS progression equation, and patients were assumed to receive oral semaglutide or comparator treatment until HbA1c exceeded 7.5% (58 mmol/mol), which is the threshold for treatment intensification defined in the NICE guidelines [6]. At this stage, treatment with oral semaglutide or the comparator was discontinued, and patients were assumed to intensify treatment to basal insulin, with a reduction in HbA1c based on an insulin-naïve population derived from the “Core” multivariate equations estimated by Willis et al. [30]. HbA1c was subsequently modelled to follow the UKPDS progression equation for the remainder of patient lifetimes. This approach was chosen to mirror the HbA1c progression used by NICE for evaluating SGLT2 inhibitors as monotherapy in the UK and to reflect common clinical practice in which, due to the progressive nature of type 2 diabetes, glycaemic control cannot be maintained indefinitely by the addition of one medication [7, 31]. Variations in the thresholds for treatment switching and further treatment intensification to basal–bolus insulin were explored in sensitivity analyses.
Body mass index (BMI) benefits were assumed to persist while patients received either oral semaglutide or comparator treatment, before reverting to baseline following intensification to basal insulin therapy. Therefore, no difference in BMI was seen between the patient arms following treatment intensification with basal insulin.
Changes in blood pressure and serum lipids were assumed to follow the natural progression algorithms built into the IQVIA CORE Diabetes Model in all arms, based on the UKPDS or Framingham data (as described by Palmer et al. [16]), following application of the treatment effects in the first year of the analysis. Hypoglycaemia rates following treatment intensification were based on published data, with non-severe and severe hypoglycaemic events projected to increase to 4.08 and 0.10 events per patient per year, respectively [32].
Cost Data
Costs were accounted from a UK healthcare payer perspective. Captured direct costs included pharmacy costs, costs associated with diabetes-related complications and patient management costs (ESM Tables S4, S5). The annual acquisition cost of oral semaglutide was assumed to be the same as that of once-weekly semaglutide, based on the similar level of pricing seen between the GLP-1 analogues in the US market. Costs of other included medications and consumables were based on published list prices (sourced in July 2019), while costs of diabetes-related complications were identified through a 2017 literature review and updated or inflated where necessary to the most recent costs available (2018 GBP) using published NHS diagnosis-related groups and the healthcare inflation index published by the Personal Social Services Research Unit [33–42]. No self-monitoring of blood glucose (SMBG) testing costs were associated with oral semaglutide, empagliflozin, sitagliptin or liraglutide, as all these interventions are associated with low rates of hypoglycaemia and, consequently, little to no SMBG testing would be required. No needles were required for the administration of oral semaglutide, empagliflozin or sitagliptin as these medications are administered orally, but one needle per day was required for the administration of liraglutide. Following treatment intensification to basal insulin (assumed to be insulin Abasaglar®, the most widely used biosimilar of insulin glargine in the UK), patients were assumed to require one SMBG test per day and to use one needle per day for the administration of basal insulin.
Sensitivity Analyses
The extrapolation of clinical results by modelling the long-term consequences is associated with uncertainty. Sensitivity analyses were therefore performed on key parameters in the modelling analysis to assess the robustness of the base case findings. Sensitivity analyses conducted for all comparisons included: applying only statistically significant differences between the treatment arms; shortening the time horizon of the analyses to 35, 20 and 10 years (for which it should be noted that some patients were still alive at the end of the modelling period and, therefore, not all costs and consequences were captured); applying discount rates of 0 and 6% in separate analyses; applying the upper and lower limits of the 95% confidence intervals for the estimated treatment differences in HbA1c and BMI in separate analyses; maintaining BMI treatment effects for patient lifetimes; altering the HbA1c threshold for treatment intensification to 7.0% (53 mmol/mol) and 8.0% (64 mmol/mol); applying a second treatment intensification step to basal–bolus insulin at an HbA1c threshold of 7.5% (58 mmol/mol); exploring the effect of applying alternative basal insulin costs (insulin neutral protamine Hagedorn [NPH], Semglee® [Mylan, biosimilar of insulin glargine] and Lantus® [insulin glargine]) following treatment intensification; increasing and decreasing the annual acquisition cost of oral semaglutide by 5% in separate analyses; application of the liraglutide 1.2 mg price in the liraglutide arm of PIONEER 4; increasing and decreasing the costs of complications by 10% in separate analyses; applying an alternative cost of stroke in the year of the event and in subsequent years, based on a publication by Patel et al. [43]; applying the UKPDS 82 risk equations to predict model outcomes; application of alternative disutilities for increases in BMI (based on a publication by Lee et al. [26]) and hypoglycaemic events (based on publications by Currie et al. [44] and Lauridsen et al. [45]); application of the 26-week clinical data; and application of data evaluated by the treatment policy estimand from the PIONEER 2, 3 and 4 clinical trials [13–15].
Probabilistic sensitivity analyses (PSA) were also performed using a second-order Monte Carlo approach. Cohort characteristics, treatment effects and complication costs and utilities were sampled from distributions, with cohorts of 1000 patients run through the model 1000 times.
Results
Base Case Analysis
Long-term projections of clinical outcomes indicated that oral semaglutide 14 mg was associated with improvements in discounted life expectancy of 0.06, 0.17 and 0.07 years and improvements in discounted QALE of 0.09, 0.20 and 0.07 QALYs versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg, respectively (Table 2). Clinical benefits arose from a reduced incidence of diabetes-related complications with oral semaglutide in all comparisons. The mean times to any diabetes-related complication in the analyses were lengthened with oral semaglutide 14 mg treatment by 0.2 years versus empagliflozin 25 mg and sitagliptin 100 mg and by 0.1 years versus liraglutide 1.8 mg.
Table 2.
Long-term cost-effectiveness outcomes
| Health outcomes | PIONEER 2 | ||
|---|---|---|---|
| Oral semaglutide 14 mg | Empagliflozin 25 mg | Difference | |
| Discounted life expectancy (years) | 13.19 | 13.13 | + 0.06 |
| Discounted QALE (QALYs) | 8.58 | 8.49 | +0.09 |
| Discounted direct costs (GBP) | 25,856 | 24,885 | + 971 |
| ICER | GBP 11,006 per QALY gained | ||
| Health outcomes | PIONEER 3 | ||
|---|---|---|---|
| Oral semaglutide 14 mg | Sitagliptin 100 mg | Difference | |
| Discounted life expectancy (years) | 12.74 | 12.57 | + 0.17 |
| Discounted QALE (QALYs) | 8.20 | 8.00 | + 0.20 |
| Discounted direct costs (GBP) | 27,226 | 26,263 | + 963 |
| ICER | GBP 4930 per QALY gained | ||
| Health outcomes | PIONEER 4 | ||
|---|---|---|---|
| Oral semaglutide 14 mg | Liraglutide 1.8 mg | Difference | |
| Discounted life expectancy (years) | 13.28 | 13.21 | + 0.07 |
| Discounted QALE (QALYs) | 8.53 | 8.46 | + 0.07 |
| Discounted direct costs (GBP) | 27,868 | 29,418 | − 1551 |
| ICER | Oral semaglutide dominant | ||
Values are given as mean values
GBP Pounds sterling, ICER incremental cost-effectiveness ratio, QALE quality-adjusted life expectancy, QALY quality-adjusted life year
Oral semaglutide was associated with direct cost increases of GBP 971 and GBP 963 versus empagliflozin 25 mg and sitagliptin 100 mg, respectively, over patient lifetimes, driven by the assumed higher acquisition cost of oral semaglutide and the differential times to treatment intensification, with patients receiving oral semaglutide for 1 additional year in the comparison with empagliflozin and for 2 additional years in the comparison with sitagliptin (Fig. 1). However, higher treatment costs were partially offset by cost savings due to avoidance of diabetes-related complications, most notably avoided cardiovascular complications (mean cost savings of GBP 99 per patient compared with empagliflozin 25 mg and of GBP 146 per patient compared with sitagliptin 100 mg). In the analyses based on PIONEER 4, oral semaglutide 14 mg was associated with estimated cost savings of GBP 1551 versus liraglutide 1.8 mg over patient lifetimes, driven by the lower acquisition cost of oral semaglutide. Further cost savings were achieved through reduced needle use (with none required for oral semaglutide versus one per day for liraglutide) and avoidance of diabetes-related complications with oral semaglutide, most notably avoided cardiovascular complications (mean cost savings of GBP 101 per patient).
Fig. 1.

Direct cost outcomes over patient lifetimes. GBP Pounds sterling
In the analyses based on PIONEER 2 and 3, oral semaglutide 14 mg was associated with improved clinical outcomes and increased costs from a healthcare payer perspective, yielding ICERs of GBP 11,006 and GBP 4930 per QALY gained versus empagliflozin 25 mg and sitagliptin 100 mg, respectively. Based on the commonly-quoted willingness-to-pay threshold of GBP 20,000 per QALY gained in the UK, oral semaglutide 14 mg represents a cost-effective treatment option versus both empagliflozin 25 mg and sitagliptin 100 mg. In the analyses based on PIONEER 4, oral semaglutide 14 mg was associated with improved clinical outcomes and reduced costs versus liraglutide 1.8 mg, and it was therefore considered to be dominant.
Deterministic Sensitivity Analyses
Sensitivity analyses showed that the results of the cost-effectiveness analyses comparing oral semaglutide 14 mg with empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg were robust to changes in the input parameters and assumptions used (Table 3).
Table 3.
Sensitivity analyses results
| Analysis | Oral semaglutide 14 mg vs. empagliflozin 25 mg (PIONEER 2) | Oral semaglutide 14 mg vs. sitagliptin 100 mg (PIONEER 3) | Oral semaglutide 14 mg vs. liraglutide 1.8 mg (PIONEER 4) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Δ Discounted QALE (QALYs) | Δ Discounted direct costs (GBP) | ICER (GBP per QALY gained) | Δ Discounted QALE (QALYs) | Δ Discounted direct costs (GBP) | ICER (GBP per QALY gained) | Δ Discounted QALE (QALYs) | Δ Discounted direct costs (GBP) | ICER (GBP per QALY gained)a | |
| Base case | + 0.09 | + 971 | 11,006 | + 0.20 | + 963 | 4930 | + 0.07 | − 1551 | Oral semaglutide dominant |
| Statistically significant differences only | + 0.08 | + 973 | 11,605 | + 0.19 | + 960 | 5048 | + 0.06 | − 1572 | Oral semaglutide dominant |
| 35-year time horizon | + 0.09 | + 1074 | 11,572 | + 0.19 | + 855 | 4532 | + 0.07 | − 1473 | Oral semaglutide dominant |
| 20-year time horizon | + 0.08 | + 999 | 12,924 | + 0.15 | + 811 | 5438 | + 0.03 | − 1492 | Oral semaglutide dominant |
| 10-year time horizon | + 0.05 | + 1176 | 21,821 | + 0.09 | + 1060 | 11,232 | + 0.03 | − 1492 | Oral semaglutide dominant |
| 0% Discount rates | + 0.14 | + 906 | 6693 | + 0.32 | + 1049 | 3333 | + 0.12 | − 1646 | Oral semaglutide dominant |
| 6% Discount rates | + 0.07 | + 988 | 14,182 | + 0.15 | + 954 | 6315 | + 0.05 | − 1467 | Oral semaglutide dominant |
| Upper 95% CI of HbA1c ETD | + 0.11 | + 828 | 7615 | + 0.14 | + 667 | 4840 | + 0.06 | − 1452 | Oral semaglutide dominant |
| Lower 95% CI of HbA1c ETD | + 0.08 | + 1098 | 13,211 | + 0.20 | + 880 | 4307 | + 0.13 | − 1227 | Oral semaglutide dominant |
| Upper 95% CI of BMI ETD | + 0.10 | + 976 | 9371 | + 0.18 | + 961 | 5278 | + 0.06 | − 1564 | Oral semaglutide dominant |
| Lower 95% CI of BMI ETD | + 0.07 | + 942 | 13,752 | + 0.20 | + 985 | 4846 | + 0.08 | − 1529 | Oral semaglutide dominant |
| BMI difference maintained for patient lifetimes | + 0.12 | + 978 | 8257 | + 0.25 | + 971 | 3817 | + 0.07 | − 1562 | Oral semaglutide dominant |
| Treatment switching at 7.0% HbA1c | + 0.11 | + 557 | 5232 | + 0.11 | + 168 | 1514 | + 0.09 | − 305 | Oral semaglutide dominant |
| Treatment switching at 8.0% HbA1c | + 0.10 | + 1726 | 17,545 | + 0.19 | + 1333 | 6977 | + 0.07 | − 2387 | Oral semaglutide dominant |
| Second treatment intensification at 7.5% HbA1c to basal–bolus | + 0.15 | + 654 | 4316 | + 0.27 | + 210 | 779 | + 0.07 | − 1420 | Oral semaglutide dominant |
| NPH basal insulin cost applied | + 0.09 | + 1111 | 12,600 | + 0.20 | + 1237 | 6334 | + 0.07 | − 1562 | Oral semaglutide dominant |
| Lantus cost applied | + 0.09 | + 1082 | 12,267 | + 0.20 | + 1054 | 5397 | + 0.07 | − 1418 | Oral semaglutide dominant |
| Semglee cost applied | + 0.09 | + 1013 | 11,480 | + 0.20 | + 1044 | 5348 | + 0.07 | − 1554 | Oral semaglutide dominant |
| Oral semaglutide price + 5% | + 0.09 | + 1102 | 12,490 | + 0.20 | + 1092 | 5594 | + 0.07 | − 1420 | Oral semaglutide dominant |
| Oral semaglutide price − 5% | + 0.09 | + 840 | 9522 | + 0.20 | + 833 | 4266 | + 0.07 | − 1681 | Oral semaglutide dominant |
| Liraglutide 1.2 mg price applied | – | – | – | – | – | – | + 0.07 | − 246 | Oral semaglutide dominant |
| Cost of complications + 10% | + 0.09 | + 933 | 10,583 | + 0.20 | + 915 | 4687 | + 0.07 | − 1570 | Oral semaglutide dominant |
| Cost of complications − 10% | + 0.09 | + 1011 | 11,467 | + 0.20 | + 1017 | 5206 | + 0.07 | − 1531 | Oral semaglutide dominant |
| Alternative costs of stroke applied | + 0.09 | + 968 | 10,973 | + 0.20 | + 946 | 4846 | + 0.07 | − 1551 | Oral semaglutide dominant |
| UKPDS 82 risk equations applied | + 0.07 | + 1026 | 14,041 | + 0.14 | + 806 | 5671 | + 0.03 | − 1520 | Oral semaglutide dominant |
| Lee et al. [26] BMI disutility applied | + 0.10 | + 971 | 10,219 | + 0.20 | + 963 | 4729 | + 0.07 | − 1551 | Oral semaglutide dominant |
| Diminishing hypoglycaemia disutility model | + 0.09 | + 971 | 10,920 | + 0.20 | + 963 | 4922 | + 0.07 | − 1551 | Oral semaglutide dominant |
| Currie et al. [44] hypoglycaemia disutilities | + 0.08 | + 971 | 12,195 | + 0.18 | + 963 | 5409 | + 0.07 | − 1551 | Oral semaglutide dominant |
| 26-week treatment effects applied | + 0.07 | + 1079 | 15,413 | + 0.15 | + 872 | 5874 | + 0.08 | − 1108 | Oral semaglutide dominant |
| Treatment policy estimand | + 0.06 | + 722 | 12,274 | + 0.12 | + 584 | 4704 | + 0.05 | − 1356 | Oral semaglutide dominant |
CI Confidence interval, Δ difference in, ETD estimated treatment difference, NPH neutral protamine Hagedorn, UKPDS United Kingdom Prospective Diabetes Study
aDominant indicates that the intervention is associated with improved clinical outcomes and cost savings
Oral Semaglutide Versus Empagliflozin (PIONEER 2)
For the analyses based on PIONEER 2, the biggest change in the ICER from the base case analysis was seen when applying a 10-year time horizon. At this time horizon, clinical benefits with oral semaglutide 14 mg were reduced and incremental costs were increased, yielding an ICER of GBP 21,821 per QALY gained versus empagliflozin 25 mg. However, this was due to the shorter time horizon of the analysis not capturing all of the long-term diabetes-related complications patients may experience, and their subsequent impact on clinical and cost outcomes. The largest reduction in the ICER was observed when applying a second treatment intensification step to basal–bolus insulin at an HbA1c switching threshold of 7.5% (58 mmol/mol), which resulted in increased clinical benefits and reduced incremental costs with oral semaglutide and an ICER of GBP 4316 per QALY gained.
Lowering the HbA1c treatment switching threshold to 7.0% (53 mmol/mol) also greatly influenced cost-effectiveness outcomes, reducing the incremental costs associated with oral semaglutide while increasing the incremental clinical benefits. This resulted in an ICER of GBP 5232 per QALY gained versus empagliflozin. Conversely, application of an 8.0% HbA1c (64 mmol/mol) treatment switching threshold yielded increased incremental costs and an ICER of GBP 17,545 per QALY gained for oral semaglutide versus empagliflozin.
All other sensitivity analyses yielded similar ICERs to the base case analysis.
Oral Semaglutide Versus Sitagliptin (PIONEER 3)
For the analyses based on PIONEER 3, the largest change in the ICER was also observed when applying a 10-year time horizon. Clinical benefits with oral semaglutide 14 mg were greatly reduced at this time horizon, while incremental costs were increased, resulting in an ICER of GBP 11,232 per QALY gained versus sitagliptin 100 mg. Performing a second treatment intensification to basal–bolus insulin at a 7.5% (58 mmol/mol) HbA1c switching threshold led to greatly increased clinical benefits and reduced incremental costs, and an ICER of GBP 779 per QALY gained.
Using a lower 7.0% (53 mmol/mol) HbA1c threshold for treatment switching lowered the clinical benefits associated with oral semaglutide but also reduced incremental costs, yielding an ICER of GBP 1514 per QALY gained. Applying a higher 8.0% (64 mmol/mol) HbA1c switching threshold also led to lowered clinical benefits but with increased incremental costs, and oral semaglutide was associated with an ICER of GBP 6977 per QALY gained versus sitagliptin. Maintaining the treatment effects of BMI for patient lifetimes resulted in increased clinical benefits with oral semaglutide, and incremental costs similar to the base case analysis, yielding an ICER of GBP 3817 per QALY gained versus sitagliptin.
The ICERs in all other sensitivity analyses remained similar to the base case analysis.
Oral Semaglutide Versus Liraglutide (PIONEER 4)
For the analyses based on PIONEER 4, the base case conclusion that oral semaglutide was dominant versus liraglutide did not change. Application of shorter time horizons lowered the clinical benefits observed with oral semaglutide, while application of a 7.0% (53 mmol/mol) HbA1c threshold for treatment switching and the liraglutide 1.2 mg price in the liraglutide 1.8 mg arm yielded lower cost savings with oral semaglutide. However, clinical and cost benefits remained consistently in favour of oral semaglutide 14 mg throughout all analyses, and it remained dominant versus liraglutide 1.8 mg.
Probabilistic Sensitivity Analysis
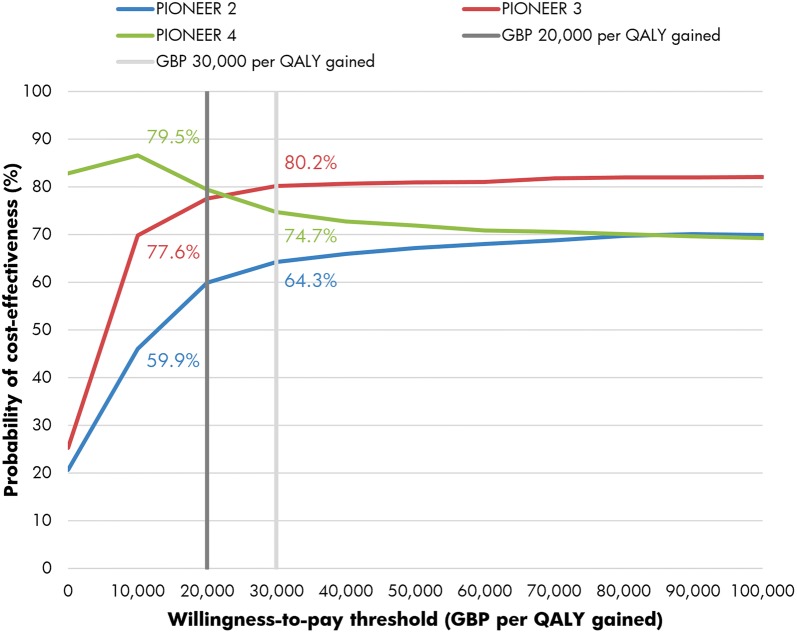
The PSA with sampling across cohort characteristics, treatment effects, complication costs and utilities showed similar mean results to the base case analyses but increased measures of variance around the mean outcomes (Fig. 2). The mean incremental improvements in QALE with oral semaglutide 14 mg were 0.09, 0.18 and 0.07 QALYs versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg, respectively. Mean costs were estimated to be GBP 1021 and GBP 817 higher with oral semaglutide than with empagliflozin and sitagliptin, respectively, but GBP 1177 lower versus liraglutide. This resulted in ICERs of GBP 11,580 and GBP 4620 per QALY gained for oral semaglutide 14 mg versus empagliflozin 25 mg and sitagliptin 100 mg, respectively, while oral semaglutide 14 mg was considered dominant versus liraglutide 1.8 mg. At a willingness-to-pay threshold of GBP 20,000 per QALY gained, the probabilities of oral semaglutide 14 mg being cost-effective versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg were 59.9, 77.6 and 79.5%, respectively, while at a willingness-to-pay threshold of GBP 30,000 per QALY gained, these probabilities were estimated to be 64.3, 80.2 and 74.7%, respectively (Fig. 3).
Fig. 2.
Cost-effectiveness scatterplot based on the probabilistic sensitivity analyses. QALY quality-adjusted life year
Fig. 3.
Cost-effectiveness acceptability curves based on the probabilistic sensitivity analyses
Discussion
Based on long-term projections of clinical and cost outcomes, oral semaglutide 14 mg offers a cost-effective treatment option versus other modern treatments for type 2 diabetes, including empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg. The observed clinical benefits were the result of a reduced incidence and delayed time to onset of long-term diabetes-related complications with oral semaglutide. Diabetes-related complications were fewer with oral semaglutide 14 mg, which yielded cost savings that partially offset its higher treatment costs versus empagliflozin and sitagliptin. Oral semaglutide was associated with lower treatment costs versus liraglutide, with further cost savings achieved through a reduced incidence of diabetes-related complications. Oral semaglutide 14 mg was therefore associated with ICERs of GBP 11,006 and GBP 4930 per QALY gained versus empagliflozin 25 mg and sitagliptin 100 mg, respectively, and was considered to be cost effective based on a willingness-to-pay threshold of GBP 20,000 per QALY gained. With improved clinical outcomes and reduced costs, oral semaglutide 14 mg was considered to be dominant versus liraglutide 1.8 mg.
Oral semaglutide is the first GLP-1 receptor agonist available for oral administration. Offering patients the benefits of a GLP-1 receptor agonist in a once-daily tablet could overcome some of the obstacles that lead to therapeutic inertia, as evidence suggests that patient concerns over potential side effects of therapies, including hypoglycaemia and weight gain, as well as fear of injections, often lead to delayed intensification of treatment, despite poor glycaemic control [12, 46–48]. The PIONEER clinical trial programme enrolled patients receiving differing background therapies, with PIONEER 2 enrolling patients with inadequate glycaemic control on metformin, PIONEER 3 enrolling patients with inadequate glycaemic control on metformin with or without a sulfonylurea, and PIONEER 4 enrolling patients with inadequate glycaemic control on metformin with or without an SGLT2 inhibitor [13–15]. As shown in the present study, oral semaglutide represents a cost-effective treatment option in all of these patient populations. Moreover, while the lower 1.2 mg dose of liraglutide is commonly recommended in the UK for the majority of patients, the present study has demonstrated that oral semaglutide remains more effective and less costly versus the more efficacious 1.8 mg dose of liraglutide when the lower 1.2 mg price is applied.
The present analysis used a clinically-relevant approach for HbA1c progression and treatment intensification, which is in line with recent publications and cost-effectiveness assessments of GLP-1 receptor agonists and SGLT2 inhibitors [10, 31]. This strategy is representative of clinical practice in a real-world population with type 2 diabetes, with patients contining treatments while they remain within their glycaemic target, intensification becoming necessary as the disease progresses and glycaemic control becoming increasingly challenging over the long term. Indeed, the latest EASD/ADA guidelines recommend that patients are evaluated every 3–6 months to ensure treatments are performing effectively, and clinical guidelines published by NICE in the UK recommend treatment intensification at a 7.5% (58 mmol/mol) HbA1c threshold [6, 7]. The use of the multivariate equations published by Willis et al. [30] to estimate changes in HbA1c on the initiation of basal insulin therapy also represents a key strength of the present analysis. These equations are informed by a variety of sources captured in a literature review, allowing the analyses to avoid the use of specific treatment effects designed to artificially improve model outcomes. Therefore, the present study offers a highly relevant approach to real-world practice, where glycaemic control cannot be maintained indefinitely with one medication. However, a potential limitation of this approach is the use of the UKPDS equations for HbA1c progression, as these are based on data from 20 years ago and as such may no longer be as applicable in modern clinical practice. Nonetheless, there are no readily available long-term type 2 diabetes studies equivalent in length to the UKPDS to test this.
When evaluating the clinical and cost outcomes associated with the interventions included in the present analysis, it is important to consider the impact of differential treatment switching occurring due to the 7.5% (58 mmol/mol) HbA1c threshold. In the long-term projections based on PIONEER 2, patients received empagliflozin 25 mg for 2 years and oral semaglutide 14 mg for 3 years, while in the projections based on PIONEER 3, patients received sitagliptin 100 mg for 1 year and oral semaglutide 14 mg for 3 years. This improved glycaemic control resulted in initial treatment costs being maintained for 1 additional year in the analyses based on PIONEER 2 and for 2 additional years in the analyses based on PIONEER 3. However, alternative HbA1c thresholds were tested, including adding a further treatment intensification step to basal–bolus insulin, and these analyses did not change the conclusion that oral semaglutide is cost-effective.
A limitation inherent in all long-term health economic analyses is the reliance on short-term clinical trial data to project outcomes over patient lifetimes. However, this is an essential tenet of all long-term diabetes modelling and arguably represents the best source of evidence for decision-making in the absence of long-term clinical trial data. The use of 52-week data from the PIONEER trials, matching the annual cycle length of the model, also represents a strength of the analysis. Moreover, the variety of sensitivity analyses performed with different treatment switching assumptions and time horizons did not change the conclusion that oral semaglutide is cost-effective.
Conclusions
Oral semaglutide 14 mg was projected to be a cost-effective treatment option versus empagliflozin 25 mg, sitagliptin 100 mg and liraglutide 1.8 mg for the treatment of patients with type 2 diabetes in the UK.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the Rapid Service Fee for publication were supported by funding from Novo Nordisk A/S, Søborg, Denmark.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Stephen Bain has received honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Cellnovo, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Sanofi; funding for the development of educational programmes from Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia-Med, Medscape; provided expert advice for All-Wales Medicines Strategy Group and the National Institute for Health and Care Excellence UK; and is a shareholder of Glycosmedia. Brian Bekker Hansen is an employee of Novo Nordisk A/S and owns stock in Novo Nordisk A/S. Solomon Nuhoho is an employee of Novo Nordisk A/S. Barrie Chubb is an employee of Novo Nordisk Ltd. and owns stock in Novo Nordisk A/S. Samuel Malkin is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. William Valentine is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. Barnaby Hunt is an employee of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. Matthew Capehorn has received honoraria from advisory board meetings for BI/Lilly Alliance, Janssen, MSK, Novo Nordisk; payments for speaker meetings from Abbot, BI/Lilly Alliance, Novo Nordisk, Sanofi-Aventis; travel and/or accommodation expenses to attend educational meetings from BI/Lilly, Lighterlife, Novo Nordisk; and is a partner and Clinical Manager of the Rotherham Institute for Obesity (RIO), Director of RIO Weight Management Ltd., Medical Director at Lighterlife (paid), board member of the Association of the Study for Obesity (ASO) (unpaid) and an Expert Advisor to the National Institute for Health and Care Excellence (unpaid); RIO has received research funding in the past or currently from Abbot, Bayer, BI/Lilly Alliance, Cambridge Weight Plan, GSK, Janssen, Leo Pharma, Lighterlife, Merck, Novartis, Novo Nordisk.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10305206.
References
- 1.Diabetes UK. Facts and figures. 2019. https://www.diabetes.org.uk/resources-s3/2019-02/1362B_Facts%20and%20stats%20Update%20Jan%202019_LOW%20RES_EXTERNAL.pdf. Accessed 21 Oct 2019.
- 2.UK Prospective Diabetes Study (UKPDS) Group Intensive blood–glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood–glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 4.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 5.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management (NICE guideline [NG28]). 2019. https://www.nice.org.uk/guidance/ng28. Accessed 21 Oct 2019.
- 7.Davies MJ, D’Alessio DA, Fradkin J. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viljoen A, Hoxer CS, Johansen P, Malkin S, Hunt B, Bain SC. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for the treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab. 2019;21(3):611–621. doi: 10.1111/dom.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malkin S, Russel-Szymczyk M, Liidemann G, Volke V, Hunt B. Once-weekly semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes: a long-term cost-effectiveness analysis in Estonia. Diabetes Ther. 2019;10(1):159–176. doi: 10.1007/s13300-018-0542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gæde P, Johansen P, Tikkanen CK, Pollock RF, Hunt B, Malkin SJP. Management of patients with type 2 diabetes with once-weekly semaglutide versus dulaglutide, exenatide ER, liraglutide and lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther. 2019;10(4):1297–1317. doi: 10.1007/s13300-019-0630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkin SJP, Russel-Szymczyk M, Psota M, Hlavinkova L, Hunt B. The management of type 2 diabetes with once-weekly semaglutide versus dulaglutide: a long-term cost-effectiveness analysis in Slovakia. Adv Ther. 2019;36(8):2034–2051. doi: 10.1007/s12325-019-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodbard HW, Rosenstock J, Canani LH. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019 doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstock J, Allison D, Birkenfeld AL. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratley R, Amod A, Hoff ST. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 16.Palmer AJ, Roze S, Valentine WJ. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 17.Palmer AJ, Roze S, Valentine WJ. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–S40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 18.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265. doi: 10.2337/diacare.27.9.2262. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation. Global Health Observatory data repository: Life tables by country (United Kingdom). 2018. http://apps.who.int/gho/data/view.main.61780?lang=en. Accessed 09 Aug 2019.
- 21.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Making. 2002;22(4):340–349. doi: 10.1177/027298902400448902. [DOI] [PubMed] [Google Scholar]
- 22.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–230. doi: 10.1002/hec.910. [DOI] [PubMed] [Google Scholar]
- 23.Wasserfallen JB, Halabi G, Saudan P. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19(6):1594–1599. doi: 10.1093/ndt/gfh175. [DOI] [PubMed] [Google Scholar]
- 24.Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311(7020):1595–1599. doi: 10.1136/bmj.311.7020.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenwick EK, Xie J, Ratcliffe J. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Investig Ophthalmol Vis Sci. 2012;53(2):677–684. doi: 10.1167/iovs.11-8992. [DOI] [PubMed] [Google Scholar]
- 26.Lee AJ, Morgan CL, Morrissey M, Wittrup-Jensen KU, Kennedy-Martin T, Currie CJ. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with type 1 diabetes, type 2 diabetes and with no diagnosed diabetes. Diabet Med. 2005;22(11):1482–1486. doi: 10.1111/j.1464-5491.2005.01657.x. [DOI] [PubMed] [Google Scholar]
- 27.Evans M, Khunti K, Mamdani M. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11(1):90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203–2210. doi: 10.1111/dom.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH harmonised tripartite guideline. Statistical principles for clinical trials E9. 5 Feb 1998. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed 12 June 2019.
- 30.Willis M, Asseburg C, Nilsson A, Johnsson K, Kartman B. Multivariate prediction equations for HbA1c lowering, weight change, and hypoglycemic events associated with insulin rescue medication in type 2 diabetes mellitus: informing economic modeling. Value Health. 2017;20:357–371. doi: 10.1016/j.jval.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence (NICE). Canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes. Technology appraisal guidance [TA390]. 2016. https://www.nice.org.uk/guidance/ta390. Accessed 30 Sept 2019.
- 32.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 33.Monthly Index of Medical Specialities (MIMS). 2019. https://www.mims.co.uk/. Accessed 03 Sept 2019.
- 34.NHS England and Wales. Electronic Drug Tariff. 2019. http://www.drugtariff.nhsbsa.nhs.uk/#/00616707-DB/DB00616702/Home. Accessed 11 July 2019.
- 35.Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84) Diabetes Med. 2015;32(4):459–466. doi: 10.1111/dme.12647. [DOI] [PubMed] [Google Scholar]
- 36.Danese M, Gleeson M, Kutikova L, et al. Costs of cardiovascular (CV) events in the United Kingdom (UK) using real-world data. Value Health. 2015;18:A335–A766. doi: 10.1016/j.jval.2015.06.003. [DOI] [Google Scholar]
- 37.NHS England. Reference costs. 2018. https://improvement.nhs.uk/resources/reference-costs/#rc1718. Accessed 11 Oct 2019.
- 38.Kent S, Schlackow I, Lozano-Kuhne J. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65. doi: 10.1186/s12882-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chubb B, Tikkanen C. The cost of non-severe hypoglycaemia in Europe. Value Health. 2015;18(7):A611. doi: 10.1016/j.jval.2015.09.2118. [DOI] [Google Scholar]
- 40.Hammer M, Lammert M, Mejias SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12(4):281–290. doi: 10.3111/13696990903336597. [DOI] [PubMed] [Google Scholar]
- 41.Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care. 2002;11(2):70–74. doi: 10.12968/jowc.2002.11.2.26675. [DOI] [PubMed] [Google Scholar]
- 42.Curtis L, Burns A. Unit costs of health and social care 2018. Canterbury: Personal Social Services Research Unit; 2018. [Google Scholar]
- 43.Patel A, Berdunov V, King D, Quayyum Z, Wittenberg R, Knapp M. Current, future and avoidable costs of stroke in the UK. Stroke Association. 2015. https://www.stroke.org.uk/sites/default/files/jn_1819.144b_current_future_avoidable_costs_of_stroke_0.pdf. Accessed 21 Oct 2019.
- 44.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–1534. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]
- 45.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–2650. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 46.Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12. doi: 10.1016/j.pcd.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Khunti K, Gomes MB, Pocock S. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–437. doi: 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409. doi: 10.1111/dom.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article/as supplementary information files.