Abstract
Method for production of alpha emitter 149Tb by irradiation of 151Eu with 70 MeV 3He nuclei is proposed. For the first time, the cross sections for the formation of isotopes 149,150,151,152Tb were measured experimentally using a stack foil technique in the 3He particles energy range 70 → 12 MeV. The thick target yield of 149Tb is 39 MBq/μAh, or 230 MBq/μA 149Tb at saturation. The optimal energy range from the point of view of radioisotopic purity is 70 → 40 MeV. At these conditions about 150 MBq/μA 149Tb can be produced in 8 hours irradiation, which is sufficient for therapeutic applications. The main impurities are 150Tb (~100% in activity) and 151Tb (~30% in activity). The proposed method surpasses its counterparts by the high content of the target isotope in the natural mixture and the simplicity of the radiochemical separation of 149Tb from the bulk target material.
Subject terms: Nuclear chemistry, Experimental nuclear physics
Introduction
Targeted alpha-radiotherapy is considered one of the most promising ways for cancer treatment1–3. Alpha particles have 2-3 orders of magnitude greater linear energy transfer (LET) than beta particles1, therefore the application of alpha emitters allows to target small objects such as micrometastases and single cancer cells more effectively as compared to beta-emitting radionuclides used today. For this purpose, a number of radiopharmaceuticals are considered4, in which molecules with a small weight (225Ac-PSMA-617)5, peptides (213Bi-DOTATOC)6, or antibodies (213Bi-HuM195mAb)7 are used as a vector. However, the use of targeted alpha radiotherapy is limited primarily by the limited availability of suitable alpha emitters. Radionuclide 149Tb is considered one of the few candidates for targeted alpha-radiotherapy8. It has a half-life of 4.12 hours and emits alpha particles with an energy of 3.97 MeV (17%). It also decays by electron capture (76%) and positron emission (7%) Unlike 225Ac, 149Tb does not have daughter alpha emitters in the decay chain, which means that the recoil effect during radioactive decay should not lead to excessive dose burden. The methods for radiolabeling of biomolecules by using bifunctional chelators based on DOTA, DTPA and others have been well developed for rare earth elements (REE). This is a clear advantage of 149Tb over 211At and 223Ra.
149Tb is one of four medically relevant terbium isotopes8. The beta emitter 161Tb is considered therapeutic radionuclide that exceeds 177Lu9 in its nuclear properties and 152Tb can be used for PET. 155Tb is suitable for molecular imaging and Auger therapy10. The availability of several isotopes with various nuclear properties allows using isotope pairs for theranostics thus enhancing the perspectives of the medical application of the isotopes of terbium. 149Tb can also be considered a theranostic radionuclide. Its positron radiation allows visualizing the distribution of the radiopharmaceutical using PET11. In vivo experiments12 showed that 149Tb-rituximab can effectively kill single lymphoma cells. The efficacy of 149Tb-DOTA-folate conjugate against carcinoma has also been shown in animal studies13.
Various reactions have been proposed for the production of 149Tb, in particular, under the action of protons14 and heavy ions15–17 (Table 1); a review can be found in18–20. However, the production of this radionuclide is associated with serious difficulties. In the preclinical studies mentioned above, 149Tb was obtained in the spallation reaction by irradiating the tantalum target with a proton beam of 1.0–1.4 GeV energy and online mass-separation of isotopes in the ISOLDE (CERN) facility. As a result, 25 MBq of radionuclide had been obtained at the time of radiolabeling. It was proposed21 to obtain 149Tb by irradiation of 151Eu targets with 3He nuclei and the thick target yields in the energy range 70 → 40 MeV were experimentally determined. Preliminary results showed that 149Tb yields can be high enough to produce therapeutic amounts of a radionuclide. This work is a further study of 3He induced reactions on 151Eu and the first experimental measurement of their cross sections.
Table 1.
Main routes of 149Tb production.
Results
The radioactive isotopes of terbium and gadolinium are formed in the irradiation of a stack of thin (100 μg/cm2) 151Eu targets by 3He nuclei with incoming energy of 70 ± 1 MeV. 147, 148, 149, 150, 151Tb and 147, 149Gd were identified (Table 2) in gamma-ray spectra (Fig. 1a) of irradiated targets. The alpha activity of irradiated targets was due to 149Tb (Fig. 1b) and to a small extent to 151Tb. It is not possible to see 151Tb peak due to low alpha decay branching (9.5∙10−3%), for more spectral data see Supplementary Information.
Table 2.
Activation products identified in irradiated targets.
| Nuclide | Half life | Principal contributing reaction | Q-value MeV | Decay mode | Eγ, keV | Iγ, % |
|---|---|---|---|---|---|---|
| 147Tb | 1.7 h | 151Eu(3He,7n)147Tb | −45.48 | EC (100%) | 694.4 keV | 43.0 |
| 148Tb | 60 m | 151Eu(3He,6n)148Tb | −37.62 | EC (100%) | 784.4 keV | 84.4 |
| 149Tb | 4.118 h | 151Eu(3He,5n)149Tb | −28.59 |
EC (83.3%) α (16.7%) |
352.2 keV | 29.43 |
| 150Tb | 3.48 h | 151Eu(3He,4n)150Tb | −20.90 |
EC (100%) α (<0.05%) |
638.1 keV | 72.0 |
| 151Tb | 17.609 h | 151Eu(3He,3n)151Tb | −12.31 |
EC (100%) α (0.0095%) |
108.1 keV 251.9 keV 287.4 keV |
24.3 26.3 28.3 |
| 152Tb | 17.5 h | 151Eu(3He,2n)152Tb | −5.15 |
EC (100%) α (<7E-7%) |
344.3 keV | 65.0 |
| 147Gd | 38.06 h |
151Eu(3He,p6n)147Gd 147Tb → 147Gd |
−40.08 | EC (100%) | 229.3 keV | 63.0 |
| 149Gd | 9.28 d |
151Eu(3He, p4n)149Gd 149Tb → 149Gd |
−24.17 |
EC (100%) α (4.3E-4%) |
149.7 keV | 48.2 |
Figure 1.
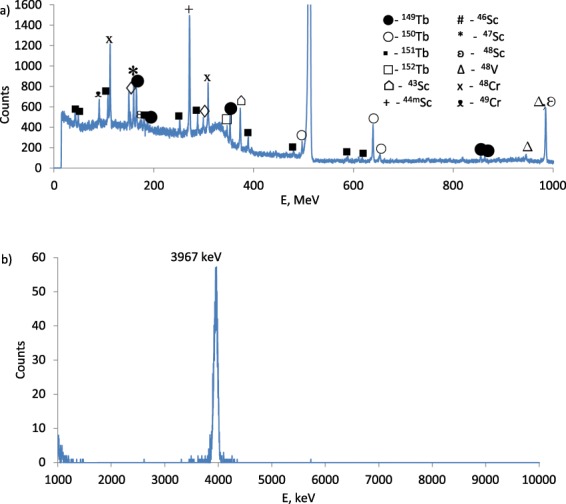
Typical gamma-ray spectrum (a) and alpha particle spectrum (b) of 151Eu target on Ti support irradiated by 3He nuclei with incident energy ~50 MeV, measured at ~11 cm distance during 10 minutes 5 h after the end of bombardment (EOB) for (a) and measured at ~2 cm distance during 2 minutes 5 h after the EOB for (b).
The cross sections of nuclear reactions leading to the corresponding terbium isotopes were calculated based on the radioactivity measurements of the irradiated targets. The experimentally obtained excitation functions for the main nuclear reactions are presented in Fig. 2a. By integrating the excitation functions, the physical yields were calculated in the energy range E0 → 0, where the incident beam energy E0 varied from 70 to a minimum value of ~12 MeV (Fig. 2b).
Figure 2.
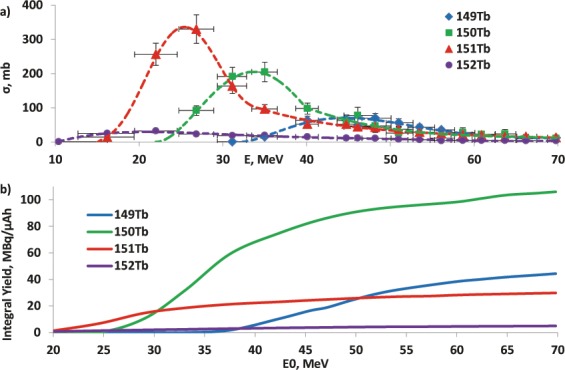
Measured excitation functions for 151Eu(3He,xn)154-xTb reactions (a) and physical thick target yields for reactions 151Eu(3He,xn)154-xTb (b), calculated using measured excitation functions.
Discussion
The physical yield of the 151Eu(3He,5n) 149Tb reaction in the range 70 → 30 MeV was 38.7 ± 7.7 MBq/μAh, that allows one to produce up to 230 MBq/μA on a 151Eu metal target up on saturation. In previous work21, thick targets from pressed 151Eu oxide were irradiated. Our yields based on the experimentally cross sections (Fig. 2a) are in good agreement with obtained in21. The saturation yield was 125.0 ± 25.0 MBq/μA for the range 70 → 40 MeV for the target from Eu2O321 and 161.7 ± 32.3 MBq/μA in this work, recalculated for the same target.
Besides terbium isotopes, peaks of 147,149Gd were also detected in the gamma-ray spectra of irradiated targets. They can be formed both by direct reactions and as a result of the decay of 147Tb and 149Tb, respectively. The relative contribution of these processes to the work was not determined. The 148Gd lines were not observed in the spectrum, apparently, due to the low specific activity of this radionuclide.
As follows from the excitation functions shown in Fig. 2a the proposed method does not allow to obtain a product free of radioisotope impurities 150Tb and 151Tb. They reduce the specific activity of the product and thereby can affect the efficiency of labeling, as well as lead to an undesirable dose burden on the patient. However, both of these impurities have a relatively short half-life. It should also be noted that LET for alpha particles is two orders of magnitude higher than for electrons. Therefore, therapeutic doses of alpha emitters are usually significantly lower than those of the electron emitters. This means that impurity of 150 and 151 isotopes of the same radioactivity will cause a significantly lower damaging biological effect in targeted delivery.
In part, the problem of radioisotope impurities can be solved by selecting a suitable range of incident particle energies. The maximum production cross sections for 149Tb are ~47 MeV, and for 150Tb ~ 35 MeV, 151Tb ~ 23 MeV. By changing the lower limit of the energy of the incident particles, one can reduce the amount of impurities, but the yield of the target radionuclide also decreases (Fig. 3). It is proposed to use the range of 70 → 40 MeV as a reasonable compromise between the amount and the purity of the product. Moreover, the activity of the target product 149Tb after eight hours will be 150 MBq/µA, which is only 10% less than the maximum possible. At the same time, the content of impurities reduces more than twice in comparison with the range 70 → 30 MeV.
Figure 3.
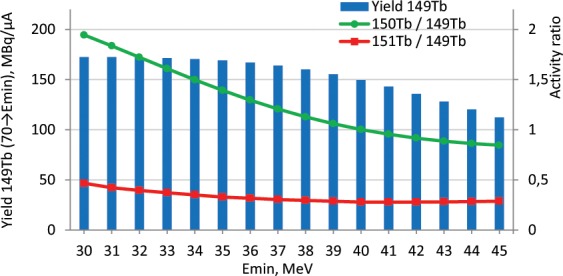
The yield of 149Tb and radioisotopic impurities content calculated for 8 h irradiation of metal 151Eu target.
A possible route to obtain 149Tb is to use the nuclear reaction 152Gd(p,4n)149Tb. Its cross section reaches a maximum value of ~250 millibarn at a proton energy of 42 MeV, which inherently provides an opportunity for obtaining of this radionuclide in sufficient quantities for medical use14. The yield of this reaction is two orders of magnitude higher than proposed in our work. However, the practical implementation of this path is associated with serious difficulties. The main disadvantage of the proton pathway is the low content of the 152Gd target isotope in the natural mixture (0.20%) compared to 151Eu (47.8%). Unfortunately, the cross sections for the formation of terbium isotopes with mass numbers of 150 and 151 are not given14. Therefore, the question of the content of radioisotope impurities and the optimal range of beam energy remains unsolved. However, it is apparent that in this case, the formation of radioisotope impurities of 150,151Tb is inevitable. Another competitive advantage of our method is the further radiochemical processing of the europium target. Europium is one of the few rare earth metals that can be reduced in aqueous solutions to an oxidation state of +2. This makes it possible to quickly and efficiently separate the target radionuclide from the bulk of the target material and traces of gadolinium, and such a technique has already been developed22.
Also, the possibility of using 12C15,18 beams and other heavy ions23 to produce 149Tb, was repeatedly considered. In this case, both a direct way to obtain by the reaction 141Pr(12C,4n)149Tb is possible, as well as an indirect one: 142Nd(12C,5n)149Dy → 149Tb. The latter looks more promising. According to Zaitseva et al.15, this way can yield tens of GBq for irradiation at a beam current of 50-100 μA. Upon 1.25 hours irradiation of 12 mg/cm2 thick natural neodymium target with a beam of 12C ions with an energy of 108 MeV and a particle current of 1 μA 2.6 MBq of 149Tb were produced. However, no information concerning radioisotopic impurities is given. Irradiation of targets from praseodymium gives a significantly lower activity of 149Tb16. The weak point of these methods is the lack of accelerators giving intense 12C ion beams.
Methods
Target preparation and irradiation
151Eu of 97.5 ± 0.1% of enrichment in oxide form was used. The material was provided by the National stable isotopes reserve. It contains 0.02% Nd, 0.02% Gd, <0.01% Sm impurities. 151Eu targets were prepared by electrodeposition from isopropanol solution on 2 μm thick titanium foil (Fig. 4). Enriched europium oxide powder was dissolved in 4 M nitric acid and evaporated near dryness, then triply evaporated with water, the residue was dissolved in a minimal volume of ethyl alcohol and diluted with isopropanol and placed in an electrolytic cell. Electrodeposition was carried out at a constant current density of 0.058 A/dm2 for 2.5 hours at a voltage 100–250 V. The precipitated layer was heated on a hot plate to 400 °C. The thickness of 151Eu layer was ~100 μg/cm2 that is thin enough to allow the measurement of 149Tb alpha particles emitted from the target. The content of Eu on the target was controlled by X-ray fluorescence analysis. To excite X-ray fluorescence, a standard 120 MBq 109Cd O-ring source was used. Characteristic x-ray radiation was recorded with Canberra semiconductor Si (Li) detector with an energy resolution of 145 eV for 6.4 keV Fe line. The spectra were analyzed using the WinAxil Canberra software. As a reference 149 μg/cm2 europium sample with a diameter of 21 mm was used. It was prepared by evaporation of a standard solution of europium nitrate in isopropanol on a filter paper substrate. Thickness inhomogeneity was controlled using x-ray excitation in the target material through collimator moving along the target.
Figure 4.
151Eu target with a thickness of 100 μg/cm2, electrodeposited on a 2 μm Ti support.
Up to 10 151Eu/Ti target foils were irradiated in one run using the conventional stack foils technique. Apart from the target foils, the stack also contained 9 μm Al and 13 μm Cu monitors for controlling the beam parameters, and aluminum degraders (53–159 μm), so that the energy 3He on each subsequent target was 2–3 MeV less than on the previous one.
The assembled target was irradiated at the U-150 isochronous cyclotron of the National Research Center “Kurchatov Institute”. The initial energy of 3He particle beam was 70 ± 1 MeV in the first run and 45 ± 1 MeV in the second. The beam energy was determined by the cyclotron settings and additionally was controlled by the monitoring reactions on Al, Ti, and Cu (see Supplementary Information). The beam current averaged 1 μA irradiation time 1 h. The passed charge was determined by the activation of aluminum monitors, based on the IAEA recommended cross section for 27Al(3He,x)24Na reaction24. If necessary, a correction was done in order to take into account the change in the current value during irradiation. In total, two irradiations were performed; the thicknesses of the degraders were selected so that the energy range of 3He particles overlaps in both experiments. In Fig. 2a shows the combined result of two irradiations.
Radioactivity measurements
The radioactivity of the activation products was measured by two methods: α-spectrometry and γ-spectrometry. Gamma-ray spectrometry was carried out on a spectrometer with a high purity germanium detector ORTEC GEM 35P4 Series, the energy resolution of 850 eV for 122 keV line and of 1.5 keV for 1.33 MeV. The measurement was carried out in two geometries at a distance of 11 cm and 6 cm so that the dead time did not exceed 6–7%. Efficiency calibration for the selected measurement conditions was carried out using certified reference point sources 137Cs, 241Am, 60Co, 152Eu with an activity of 10 to 100 kBq. Spectra were processed using Spectra Line 1.4.2792 software (LSRM Company, Russia).
Registration of alpha particles was chosen as the main method for the radioactivity measurements for 149Tb since the efficiency of registration under the experimental conditions was approximately two orders of magnitude higher than that of gamma rays. Also, the main gamma line of 149Tb can interfere with the natural background radiation line 351.9 keV (214Pb). Alpha spectrometry was performed without radiochemical separation, registering α particles emitted from the irradiated target surface on an ORTEC Alpha Suite Alpha Duo instrument with ULTRA Ion Implanted Silicon Charged Particle Radiation Detector with an energy resolution of up to 20 keV. The detection efficiency of alpha particles was determined using certified reference sources of 239Pu and 226Ra.
Cross-sections calculations and uncertainty budget
The cross sections of nuclear reactions were calculated by activation equation25. Nuclear data were used from IAEA database26. Energy losses were calculated by SRIM 2008.04 code27.
The overall experimental uncertainty was calculated as the square root of the sum of the squares of the individual relative uncertainties. The following uncertainty values of the individual components were taken: detector efficiency - 5–7%, photopeak areas uncertainty varied 1–10% depending on counting statistics, determination of Eu targets thickness and composition - 10%, and nuclear decay data 3%. The overall experimental cross sections uncertainties amounted to about 20%.
Calculating energy uncertainties, it was assumed that the initial beam energy varies within ± 1 MeV (an estimate based on the cyclotron settings). The uncertainty of the thickness of the degraders was estimated at 1 μm by multiple weighing, of copper and titanium foils at 0.1 μm. Based on these data, the possible limits of the energy loss of the beam along the path to each foil were calculated by SRIM 2008.04 code.
Conclusions
Thus, the use of the 151Eu(3He,5n)149Tb reaction is an effective solution to the problems associated with the production of this very promising radionuclide. In our experiments, low-intensity beams were used, however, up to 20 µA values can be achieved at the Kurchatov Institute isochronous cyclotron U-150. This means that up to 3.0 GBq can be obtained in eight-hour irradiation, which is sufficient for clinical use. Although the therapeutic quantity has yet to be established for 149Tb-based preparations, one can estimate from other alpha emitters. For example, for 213Bi this value is 10–50 MBq/kg; for 225Ac - 20–150 kBq/kg. For 223Ra, the recommended dose is 55 kBq/kg. Dosages for 212Pb (as [212Pb]Pb-TCMC-trastuzumab) are 7.4–21.1 MBq/m2, or about 200-500 kBq/kg28. It can be assumed that the latter value is closest to therapeutic doses of 149Tb. That is, for a single patient, an estimated 10–50 MBq.
The advantage of the method is the availability of target material and the relatively simple radiochemical processing of the irradiated europium. The main drawback is the very limited availability of high-intensity 3He beams worldwide.
This method, like other methods not related to electromagnetic mass separation, does not allow one to obtain 149Tb free of impurities from short-lived neighboring radioisotopes. The possibility of clinical application of such a product has yet to be proven.
Supplementary information
Acknowledgements
The work was supported by the NRC “Kurchatov Institute” (No. 1363).
Author contributions
Conception, design of the work: A.A. Ogloblin, R.A. Aliev, V.A. Zagryadskiy, A.N. Moiseeva; Preparation of materials: N.V. Aksenov, N.S. Gustova, M.G. Voronuk, G.Ya. Starodub, V.N. Unezhev and S.T. Latushkin; Collected and analyzed data: A.N. Moiseeva, R.A. Aliev and V.A. Zagryadskiy; Wrote majority of the manuscript: A.N. Moiseeva and R.A. Aliev. Reviewed and edited the manuscript: all authors.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57436-6.
References
- 1.Marcu L, Bezak E, Allen BJ. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018;123:7–20. doi: 10.1016/j.critrevonc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Elgqvist J, Frost S, Pouget J-P, Albertsson P. The Potential and Hurdles of Targeted Alpha Therapy – Clinical Trials and Beyond. Front. Oncol. 2014;3:1–9. doi: 10.3389/fonc.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacoeuille F, Arlicot N, Faivre-Chauvet A. Targeted alpha and beta radiotherapy: An overview of radiopharmaceutical and clinical aspects. Médecine Nucléaire. 2018;42:32–44. doi: 10.1016/j.mednuc.2017.12.002. [DOI] [Google Scholar]
- 4.Parker C, et al. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018;4:1765–1772. doi: 10.1001/jamaoncol.2018.4044. [DOI] [PubMed] [Google Scholar]
- 5.Kratochwil C, et al. 225Ac-PSMA-617 for PSMA-Targeted -Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 6.Kratochwil C, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:2106–2119. doi: 10.1007/s00259-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurcic JG, et al. Targeted α particle immunotherapy for myeloid leukaemia. Blood. 2002;100:1233–1239. doi: 10.1182/blood.V100.4.1233.h81602001233_1233_1239. [DOI] [PubMed] [Google Scholar]
- 8.Muller C, et al. A Unique Matched Quadruplet of Terbium Radioisotopes for PET and SPECT and for - and–Radionuclide Therapy: An In Vivo Proof-of-Concept Study with a New Receptor-Targeted Folate Derivative. J. Nucl. Med. 2012;53:1951–1959. doi: 10.2967/jnumed.112.107540. [DOI] [PubMed] [Google Scholar]
- 9.Lehenberger S, et al. The low-energy β - and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl. Med. Biol. 2011;38:917–924. doi: 10.1016/j.nucmedbio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Webster B, et al. Chemical Purification of Terbium-155 from Pseudo-Isobaric Impurities in a Mass Separated Source Produced at CERN. Sci. Rep. 2019;9:10884. doi: 10.1038/s41598-019-47463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller C, et al. Alpha-PET with terbium-149: evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm. Chem. 2017;1:5. doi: 10.1186/s41181-016-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyer G-J, et al. Targeted alpha therapy in vivo: direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:547–54. doi: 10.1007/s00259-003-1413-9. [DOI] [PubMed] [Google Scholar]
- 13.Müller C, et al. Folate receptor targeted alpha-therapy using terbium-149. Pharmaceuticals. 2014;7:353–365. doi: 10.3390/ph7030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyn GF, et al. Cross-sections of proton-induced reactions on 152Gd, 155Gd and 159Tb with emphasis on the production of selected Tb radionuclides. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms. 2014;319:128–140. doi: 10.1016/j.nimb.2013.11.013. [DOI] [Google Scholar]
- 15.Zaitseva NG, et al. Terbium-149 for nuclear medicine. The production of 149Tb via heavy ions induced nuclear reactions. Czechoslov. J. Phys. 2003;53:A455–A458. doi: 10.1007/s10582-003-0058-z. [DOI] [Google Scholar]
- 16.Maiti M, Lahiri S, Tomar BS. Investigation on the production and isolation of 149,150,151 Tb from 12 C irradiated natural praseodymium target. Radiochim. Acta. 2011;99:527–534. doi: 10.1524/ract.2011.1839. [DOI] [Google Scholar]
- 17.Dmitriev SN, et al. Lanthanides in Nuclear Medicine: Preparation of 149Tb by Irradiation with Heavy Ions. Radiochemistry. 2002;44:171–173. doi: 10.1023/A:1019627514277. [DOI] [Google Scholar]
- 18.Maiti M. New measurement of cross sections of evaporation residues from the natPr +12C reaction: A comparative study on the production of 149Tb. Phys. Rev. C - Nucl. Phys. 2011;84:1–7. [Google Scholar]
- 19.Qaim SM, Scholten B, Neumaier B. New developments in the production of theranostic pairs of radionuclides. J. Radioanal. Nucl. Chem. 2018;318:1493–1509. doi: 10.1007/s10967-018-6238-x. [DOI] [Google Scholar]
- 20.Beyer GJ, et al. Production routes of the alpha-emitting 149Tb for medical application. Radiochim. Acta. 2002;90:247–252. doi: 10.1524/ract.2002.90.5_2002.247. [DOI] [Google Scholar]
- 21.Zagryadskii VA, et al. Measurement of Terbium Isotopes Yield in Irradiation of 151Eu Targets by 3He Nuclei. At. Energy. 2017;123:55–58. doi: 10.1007/s10512-017-0299-8. [DOI] [Google Scholar]
- 22.Kazakov AG, Aliev RA, Bodrov AY, Priselkova AB, Kalmykov SN. Separation of radioisotopes of terbium from a europium target irradiated by 27 MeV α-particles. Radiochim. Acta. 2018;106:135–140. doi: 10.1515/ract-2017-2777. [DOI] [Google Scholar]
- 23.Alexander JM, Simonoff GN. Excitation Functions for Tb 149g from Reactions between Complex Nuclei*. Phys. Rev. 1963;130:2383–2387. doi: 10.1103/PhysRev.130.2383. [DOI] [Google Scholar]
- 24.Hermanne A, et al. Reference Cross Sections for Charged-particle Monitor Reactions. Nucl. Data Sheets. 2018;148:338–382. doi: 10.1016/j.nds.2018.02.009. [DOI] [Google Scholar]
- 25.Choppin, G. R., Liljenzin, J.-O. & Rydberg, J. Radiochemistry and Nuclear Chemistry. (Butterworth-Heinmann, 2002).
- 26.IAEA. Live Chart of Nuclides. Available at, https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (2019).
- 27.Pavlovič M, Strašík I. Supporting routines for the SRIM code. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms. 2007;257:601–604. doi: 10.1016/j.nimb.2007.01.047. [DOI] [Google Scholar]
- 28.Poty S, Francesconi LC, McDevitt MR, Morris MJ, Lewis JS. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 2. J. Nucl. Med. 2018;59:1020–1027. doi: 10.2967/jnumed.117.204651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.


