Abstract
Despite the fact that complete visual deprivation leads to volumetric reductions in brain structures associated with spatial learning, blind individuals are still able to navigate. The neural structures involved in this function are not fully understood. Our study aims to correlate the performance of congenitally blind individuals (CB) and blindfolded sighted controls (SC) in a life-size obstacle-course using a visual-to-tactile sensory substitution device, with the size of brain structures (voxel based morphometry-VBM-) measured through structural magnetic resonance Imaging (MRI). VBM was used to extract grey matter volumes within several a-priori defined brain regions in all participants. Principal component analysis was utilized to group brain regions in factors and orthogonalize brain volumes. Regression analyses were then performed to link learning abilities to these factors. We found that (1) both CB and SC were able to learn to detect and avoid obstacles; (2) their learning rates for obstacle detection and avoidance correlated significantly with the volume of brain structures known to be involved in spatial skills. There is a similar relation between regions of the dorsal stream network and avoidance for both SC and CB whereas for detection, SC rely more on medial temporal lobe structures and CB on sensorimotor areas.
Subject terms: Human behaviour, Cognitive neuroscience, Human behaviour, Human behaviour, Cognitive neuroscience
Introduction
Vision is undoubtedly a great facilitator of navigational tasks1 (for review see2). While approaching an obstacle, visual cues regulate foot placements by providing constant spatial updates of the distance to the obstacle3 in order to adapt locomotor behavior4. In sighted people this behavior is mediated by a complex network of interacting brain regions that integrates visual information and translates visual cues into appropriate behavior5. The hippocampal formation and the posterior parietal cortex are traditionally thought to play a pivotal role in navigation6. These two brain regions are involved in the processing of higher order spatial-cognitive information7, and in the registering of spatial information which is crucial for navigation8. They are largely affected by the absence of vision from birth and might therefore interfere with the blind navigational skills9–11. Indeed, spatial abilities in auditory and tactile spatial tasks are slightly compromised by early blindness12,13. A possible explanation for these compromised spatial abilities is that in the absence of vision, the neuronal networks responsible for spatial tasks do not develop in the same way as in the sighted14. All the components of the visual system in congenitally blind (CB) are volumetrically reduced15–17. In addition, a cascade of other non-visual brain structures undergo anatomical18, morphological19, and morphometric20–23 alterations, as well as modifications in functional connectivity24. There is no doubt that the brain of born blind individuals undergoes substantial reorganization compared to the sighted and is still able to carry out a number of behavioral tasks including navigation see25,26 for recent reviews with the use of sensory substitution devices (SSDs).
SSDs can potentially help make navigation easier for people who are visually impaired or CB by providing visual information via the auditory or tactile channels27–30. Indeed, CB have preserved navigational skills in an obstacle course27, that are associated with the activation of the hippocampal/parahippocampal area using fMRI31, and are also capable of completing and integrating paths (for review see32). Furthermore, it has been demonstrated that CB are able to generate cognitive representations of space stemming from the remaining intact senses28–31 and they preserve the ability to recognize a travelled route and represent spatial information mentally31. They even transfer spatial knowledge from virtual reality to the real world and vice versa28,29. CBs’ preserved navigational skills correlate with a larger anterior hippocampus10 that is accompanied by a volume reduction of its posterior portion9. These findings were explained by the possibility that the blind may rely more heavily on structures other than the hippocampus (such as the posterior parietal cortex) for navigation (31,25). The taxing demands of learning to detect and avoid obstacles without vision may drive hippocampal plasticity and volumetric changes in CB9,11,15,32. The precise hippocampal cellular layers concerned by this plasticity remain however unknown.
In this study, we provide comparative behavioral results on the learning rates of CB vs sighted control (SC) equipped with a Visual-to-tactile sensory substitution system (the tongue Display Unit or TDU), in an obstacle course. Using principal component analysis (PCA), we explore the relationship between learning performances of CB and SC individuals and some specific brain areas known to be involved in navigation.
Results
All participants could ‘see’ objects in front of them with the device. Participants performed a ‘visual’-tactile acuity test and were ascribed a score33. We found that there is a significant correlation between the performance of our CB participants and their ‘visual’-tactile acuity score in the training part of the experiment for obstacle detection (r(32) = 0.286; p < 0.05), and for avoidance (r(32) = 0.314; p < 0.05). There was no correlation however between the ‘visual’-tactile acuity score in the test phase of the experiment for detection (r(32) = 0.031; p > 0.05), or avoidance (r(32) = 0.216; p > 0.05).
Learning rate
All participants were able to describe the distance (near, far) and type of obstacles in the training phase of the experiment. They could also accurately point to and avoid the obstacles. Both SC and CB groups significantly improved in terms of detection and avoidance between the training and test phases of the experiment (Fig. 1). There were significant differences in the scores for detection in the training phase (SC: M = 53.48, SE = 4.15; CB: M = 62.18, SE = 5.72) and in the test phase (SC: M = 68.57, SE = 5.23; CB: M = 71.64, SE = ; 4.56) of the experiment (SC: t(20) = 7.067, p = 0.000; CB: t(11) = 3.506, p = 0.002). There were also significant differences in the scores for avoidance in the training phase (SC: M = 45.38, SE = 3.74; CB: M = 33, SE = 3.83) and in the test phase (SC: M = 59.86, SE = 3.99; CB: M = 54.09, SE = 6.67) of the experiment (SC: t(20) = 7.406, p = 0.000; CB: t(11) = 3.94, p = 0.001) (see Fig. 1).
Figure 1.
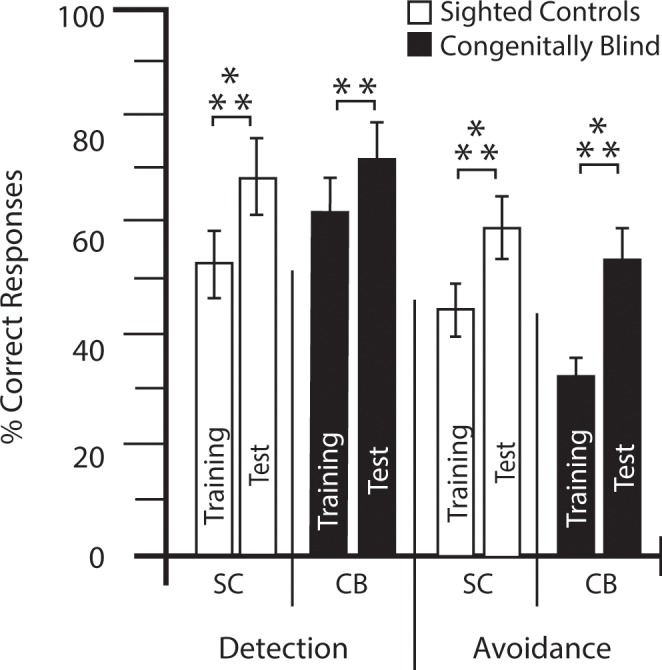
Performance. Bar graph comparing performances (percent correct response) for detection and avoidance in CB and SC participants for the training and test parts of the experiment. **P ≤ 0.01; ***P ≤ 0.001.
Learning rate was calculated individually for each subject as the difference between the training and testing phases of the experiment on the scores for detection and avoidance. Only one SC participant did not improve between the training phase and testing phase of the experiment (detection: SC M = 15.10 SE = 2.12), and all SC participants improved in terms of avoidance (M = 14.48 SE = 1.92). Nine out of the twelve CB participants improved between the training phase and testing phase of the experiment in terms of their Learning Rate of Detection (LRD: M = 9.45, SE = 2.63), and for Learning Rate of Avoidance (LRA) only two out of the twelve participants did not improve (M = 21.09, SE = 5.13). There was no significant difference between the scores for LRD (t(32) = 1.601, p = 0.060), and for LRA (t(32) = 1.44, p = 0.079) compared to SC. Of note, LRD and LRA were not correlated, meaning that the learning rate of obstacle detection is not linked to obstacle avoidance.
Principal component analysis of the brain volumes related to learning rate of detection (LRD)
Bivariate correlations identified 38 volumes of brain areas related to LRD. Principal components analysis revealed three different factors that explained 80% of the behavioral variance. The first factor explained 68% of the variance, the second one 6% and the third one 5%. These three components gathered the following brain regions: (1) Medial Temporal Lobe Factor (MTL): The first factor was composed of hippocampal and medial temporal lobe regions: Cornu Ammonis bilaterally, Entorhinal cortex on the right side, and the Subiculum bilaterally. (2) Sensorimotor Factor (SM): The second factor was composed of the following brain volumes: InferiorParietal (PFt) (supramarginal gyrus) on the right side, Brodmann area 3a (BA3a) of the human somatosensory cortex on the left side, and Brodmann area 4p (BA4p) on the left side in the primary motor cortex. (3) Attention Factor: The third factor included: The superior parietal lobule area 5 L (SPL 5 L) on the left side, Superior parietal lobule area (5Ci) bilaterally, and Brodmann area 4a (BA4a) on the left side.
The Cronbach’s alphas that are the three groups of variables composing the three factors appeared high (respectively: 0.949; 0.965; 0.905). This allowed for determining the composite variables from the independent variables in the three principal components.
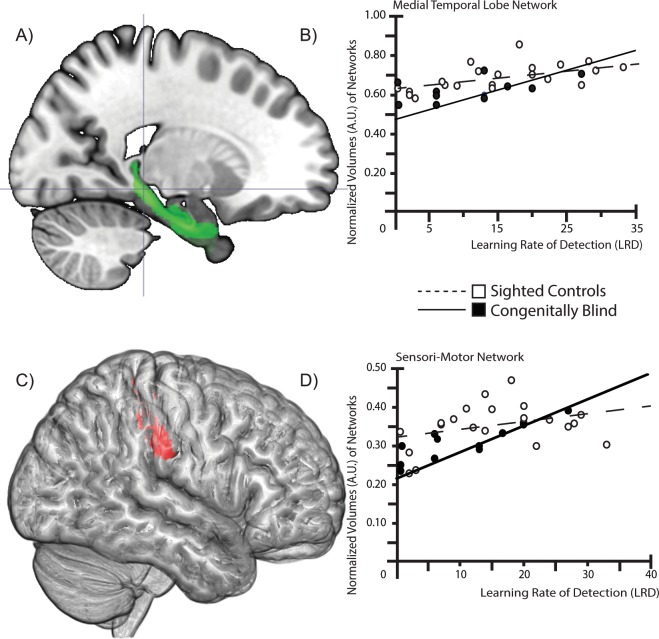
In the case of SC individuals, the Medial Temporal Lobe factor explained 38% of the variance and was significantly correlated to LRD (beta = 0.62, t = 3.42, p = 0.003). The addition of the SM and the attention factors did not significantly increase the regression model (SM: ΔR2 = 0.02, ΔF = 0.47, p = 0.50; SM + Attention: ΔR2 = 0.00, ΔF = 0.00, p = 0.97). For CB subjects, MTL and SM factors explained 75% of the variance (compared to MTL only: ΔR2 = 0.38, ΔF = 12.33, p = 0.008). SM factor correlated significantly with LRD (beta = 0.66, t = 3.51, p = 0.008) but this was not the case for MTL (beta = 0.36, t = 1.93, p = 0.09) (Fig. 2). The inclusion of the attention factor in the model did not improve the explained variance (ΔR2 = 0.00, ΔF = 0.11, p = 0.75). Hierarchical regression modeling showed that the slopes were significantly different between CB and SC groups for the SM network (t = 2.38, p = 0.025) whereas not for MTL regions (t = 1.11, p = 0.28).
Figure 2.
Obstacle Detection is Mediated by Different Networks for the Blind and Sighted. (A) Sagittal view showing hippocampal and entorhinal areas composing the Medial temporal Lobe factor. (B) Scatter plot showing the correlation between LRD and normalized volumes (in arbitrary units, A.U.). For SC, there was a significant correlation between the volume of this network and the LRD. (C) Sagittal view (left brain) showing areas composing the SensoriMotor factor. (D) Scatter plot showing the correlation between LRD and normalized brain volumes. For CB, there was a significant correlation between the volume of this network and the LRD.
Principal component analysis of the brain volumes related to learning rate of avoidance (LRA)
Five brain volumes were correlated to LRA. The principal component analysis on these five variables showed one single factor - the Dorsal Stream - that explains 74% of the variance. The Dorsal Stream Factor was composed of mainly dorsal stream occipital and parietal areas: V3 (hOC3v) on the right side, V4 (hOC V4) on the right side, the Inferior Parietal Cortex (IPC PFm) on the left side, the superior parietal lobule area 7 M (SPL 7PC) on the right side, and 5 M (SPL 5 M) on the left side. All five variables were included in the factor (loadings are superior to 0.66). The Cronbach alpha for these variables was 0.90, which allowed averaging them and creating a composite variable.
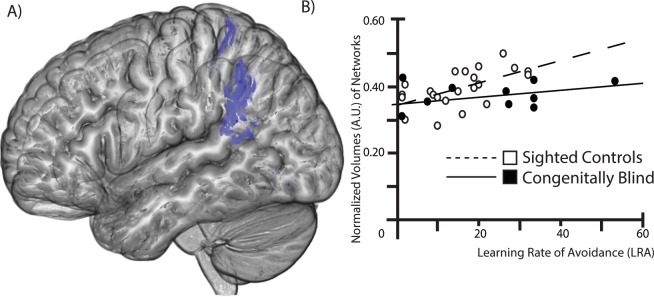
For the SC, 30% of the variance was explained by the dorsal stream factor and was significantly correlated to LRA (beta = 0.55, t = 2.85, p = 0.01). In the case of CB individuals, we found that 49% of the variance was explained by the dorsal stream network (beta = 0.70, t = 2.93, p = 0.02) (Fig. 3). Hierarchical regression modeling showed that the slopes were significantly different between CB and SC groups for the dorsal stream network (t = 2.51, p = 0.02).
Figure 3.
Obstacle Avoidance is Mediated by the Dorsal Stream Network: (A) Sagittal view (left brain) showing areas composing this factor. (B) Scatter plot showing the correlation between LRD and normalized volumes (in arbitrary units, A.U.). For both SC and CB, there was a significant correlation between the volume of this network and the LRA.
Table 1 summarizes volumetric differences of the brain networks between SC and CB, and the correlations between the brain networks and performances. Of note, we did not find any significant correlation between the volumes of these brain networks and the baseline performances (obstacle avoidance or detection) neither for SC nor CB.
Table 1.
Brain network characteristics and differences between sighted and congenitally blind groups.
| Network | Task | Brain Regions | Volume Difference | Correlation with Learning Rates |
|---|---|---|---|---|
| Medial Temporal Lobe | Detection | Cornu Ammonis (LR) | P < 0.01 |
LRD for SC Beta = 0.62 t = 3.42 P = 0.003 |
| Enthorhinal Cortex (R) | ||||
| Subiculum (LR) | ||||
| Fascia Dentata (LR) | ||||
| Sensori-Motor | Detection | Inferior Parietal Cortex (Pft, R) | P = 0.02 |
LRD for CB* Beta = 0.66 t = 3.51 P = 0.008 |
| Area 3a (L) | ||||
| Area 4p (L) | ||||
| Dorsal Stream | Avoidance | Superior Parietal Lobule (5 M, L) | P = 0.20 |
LRA for CB* LRA for SC* Beta = 0.55 Beta = 0.70 t = 2.85 t = 2.93 P = 0.01 P = 0.02 |
| Superior Parietal Lobule (7 pc, R) | ||||
| Inferior Parietal Cortex (PFm, L) | ||||
| hOC (V3, R) | ||||
| hOC (V4, R) |
L: left, R: right, CB: congenital blind, SC: sighted control, *slopes are significantly different (P < 0.05).
Discussion
In this study, we report that CB have a similar learning capabilities to SC for detection (LRD), and avoidance (LRA) of obstacles. In addition to the hippocampus and entorhinal cortex, LRD seems to involve a sensorimotor network in CB. As expected, for LRA and since there are no volumetric differences in the dorsal stream structures between CB and SC, this brain network is likely to be recruited in both groups. A possible limiting factor of our study is the relatively small sample size of CB participants. However, since CB individuals represent an exceptionally rare population, even more so when strict selection requirements are enforced, a sample of 12 participants can be considered as large and within the range of other classical brain morphometry studies in this population (for review see:34).
Brain volumes and learning rate of detection correlations
Obstacle detection and avoidance in the context of normal vision is an automatic process that is still preserved in the absence of the visual cortex or visual awareness. This unconscious process is thought to require a subcortical visual input35–37. If then, the visual cortex is not necessary for obstacle avoidance, what cortical areas are then crucial for detection and avoidance of obstacles in CB? Obstacle avoidance relies on a complex and sophisticated avoidance system that is ‘sensitive and conservative’ in adjusting movement according to potential obstacles in the way38. This complex network of regions includes the hippocampus39,]40 and the visual dorsal stream37. In our experiment, obstacle detection learning was correlated with regions of the Medial Temporal Lobe for SC (hippocampus and entorhinal cortex). In CB participants, there is a large-scale anatomical reorganization of almost all brain regions11,15,41 and more specifically, the hippocampus that shows volumetric alterations9–11. We report here that, in addition to the hippocampus, CB recruit a sensorimotor network when learning the detection task. Our results fit remarkably well with recent literature on visually guided behavioral learning42–45, and spatial learning46–48. It is well known that brain volume can be associated with learning behavior49,50. A recent study also shows that hippocampal and entorhinal volumetric differences mediate spatial learning46, lending more support to the idea that the hippocampus, entorhinal cortex, and thalamus are important structures for navigation. In a recent study, memory guided attention task, hippocampal volume was related to the ability to implicitly learn contextual information47, and cognitive mapping style is mediated by the ratio of anterior-posterior hippocampal volume48. Moreover, our results are in line with the idea that the sensorimotor cortex is important for the plasticity of associative sensory learning42. A recent fMRI experiment also supports this view by showing that this same network is recruited during visually guided spatial behavior43. Furthermore, several recent studies have linked hippocampal volumetric differences in healthy47, and clinical populations with the ability to learn49. A recent study also demonstrated traces of learning in thalamo-cortical circuits42, and the impact of sensorimotor networks in guiding learning behavior44.
In the present study, we found that better learning detection performance was correlated with a larger hippocampus. Previous reports51 found respectively volumetric enhancement of the hippocampus head in CB and late blind participants10, while we had reported a volumetric reduction in the hippocampus tail of CB9. These differences have both been confirmed to co-exist in the hippocampus of CB by other reports11,]15. Moreover, there is impaired learning of object location (such as obstacles in a hallway) in human patients with hippocampal damage52. Spatial memory depends on synaptic plasticity of the hippocampus53,]54 and longer-lasting spatial memory is associated with a larger hippocampus55. For example, the posterior segment of the hippocampus is volumetrically enhanced in humans with extensive navigational training39. When CB participants were tested for route recognition in an fMRI scanner using a sensory substitution device, they recruited regions adjacent to the hippocampus and the parahippocampal place area31.
Learning rate of avoidance and the dorsal stream network
The visual dorsal stream is important for controlling visually guided actions56 and selecting a path through a cluttered environment guided by vision57. For example, optic flow which is processed in area hMT+ and is an integral part of the visual dorsal stream provides information about the direction of self-motion and is very important for obstacle detection and avoidance58. Lesions to the dorsal stream38,]59, severely disrupts the ability to avoid obstacles, but lesions to the ventral stream do not60.
The posterior parietal cortex, which is also part of the dorsal visual stream, is involved in the processing of higher order spatial-cognitive information7. The parietal lobe is believed to register spatial information in interaction with the hippocampus6,8 and play a critical role in route decision making61. The posterior parietal cortex is also involved in visuo-spatial decision-making mediated by sensory motor integrations such as when pointing to a target5, and the visual guidance of movements in obstacle avoidance is a function of the occipito-parietal pathway37. We found correlations with parietal area 7 M. This area has been found to be sensitive to context dependent places in the macaque62, and activated in the human during execution and observation of an action63. The task of pointing to an obstacle and then avoiding it takes much planning indeed. After having pointed to the obstacle, one must keep in mind where the obstacle is located in space, and plan an appropriate response when arriving close to it64. In our study, there were no differences between SC and CB, in terms of their LRA performance, and although CB started out with a lower rate of avoidance in the training phase of the experiment, they performed as well as their SC counterparts in the test phase of the experiment, resulting in a higher LRA for CB compared to SC. Furthermore, although there was a volumetric reduction of almost all cortical and subcortical structures in CB, we find that the volume of the dorsal stream structures was preserved. We suggest that through mechanisms of brain plasticity, visual brain areas can be recruited to accomplish visuo-spatial tasks (just as they would do in the SC) using the visuo-tactile information provided by the sensory substitution device in lieu of vision.
Conclusions
Our results indicate that there is a double dissociation between the brain networks involved in LRD and LRA for blind and sighted. Different networks are involved in the LRD behavior, namely for sighted individuals it is the medial temporal lobe network that is associated with this task, while for the blind it is certainly also sensorimotor areas that are associated with this behavior to achieve the same level of performance. Meanwhile, for both blind and sighted participants it is the dorsal stream network that is associated with their LRA behavior. It is interesting to note that the volume of this network is not affected by early blindness, as are almost all the other studied structures in CB.
Methods
Participants
Twelve right-handed CB participants (7 Males; average: 35; age range: 20–54 years old) with no history of light perception were compared to twenty-one normally SC participants (13 Males; average: 33; age range: 22–68 years old). All blind participants were recruited through the “Montreal Association for the Blind” and “Institut Nazareth et Louis-Braille”. Causes of blindness were retinopathy of prematurity in 4 cases; Glaucoma in two cases, congenital cataract, Leber’s amaurosis, detached retina, retinoblastoma, retinitis pigmentosa, and electrocution, each in one case, and a (medical) accident in two cases. The study was approved by the Ethics committee of the Université de Montréal in accordance with the Declaration of Helsinki. All subjects gave informed written consent prior testing.
MR Image acquisition and volumetric analysis
Subjects were scanned in a 1.5 T Siemens MR Scanner (Siemens, Erlangen, Germany) in order to obtain fast spoiled grass gradient echo images using a standard head coil. Voxel-based morphometry was applied to 3D T1 MR images acquired in the axial plane, to assess purported correlations between the volume of brain regions known to be involved in navigation and performance. Sequence parameters were TI/TR/TE 450/106/42 ms, a flip angle of 20 degrees and a spatial resolution of 0.94 × 0.94 × 1 mm3, as previously reported in Ptito et al.,15. For Voxel Based Morphometry, spatial pre-processing of the brain scans was performed according to the optimized VBM protocol65 using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK) and in-house software written in Matlab (MathWorks, Natick, MA, USA). For each subject, the averaged grey matter volumes were extracted within selected brain structures using some of the pre-defined probabilistic regions of interest included in the Anatomy Toolbox66: the medial temporal lobe67,68, hippocampus and surrounding areas69: Cornu Ammonis (CA), Fascia Dentata (FD), Hippocampal Amygdaloïd Transition Area (HATA) and Subiculum (SUB), BA 17 and 18, V3v, V4v, V5/MT+ 70, the superior parietal lobule (5ci, 5M, 7A, 7M, 7P and 7PC;71, hMT+ 69. The brain regions were selected because they are known to be involved in navigation from our own previous findings and other work (for review:72). Moreover, studies using the Shapiro-Wilk’s test of normality we find that the brain volumes were normally distributed for both CB (skewness = 0.501; p = 0.859) and SC (skewness = 0.637; p = 0.848).
Visual-to-tactile sensory substitution system
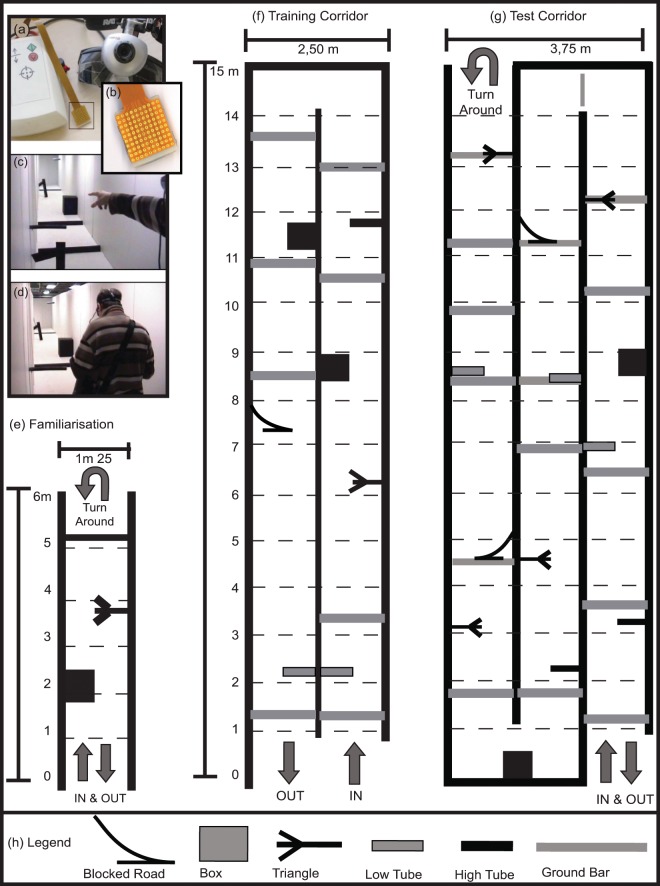
We used a lingual visual-to-tactile sensory substitution system, the Tongue display Unit (TDU), that has been described in detail in earlier publications15,27,33. The TDU (Brainport, WICAB inc, Wisconsin, U.S.A.) consists of a webcam connected to a laptop computer and a tongue stimulator array (Fig. 4a,b). The tongue array consists of 100 small circular electrodes arranged in a 10 by 10 matrix with a diameter of 1 mm, and spaced 1 mm apart. The entire tongue array measures 2 × 2 cm (Fig. 4b). Participants wore the webcam mounted on a pair of light-tight safety goggles (Fig. 4a), in order to point and avoid the obstacles in the obstacle course (Fig. 4c,d). The camera sends images to the laptop which in turn converts them the visual information into electro-tactile pulses delivered to the tongue via an electrode array. Every time an object enters within the visual field, the visual image is translated into electro-tactile pulses that are transmitted to the tongue through the electrode array. The obstacles are thus ‘drawn’ with electrical current on the tongue in real time from the images provided by the webcam enabling then participants to point to and avoid obstacles.
Figure 4.
Apparatus and Schematic drawing of the experimental setup. (a) The tongue display unit (TDU) and Webcam mounted on a pair of safety goggles and fastened with an elastic band around the head. The image is translated into electro-tactile stimulation applied to the tongue using a 10 × 10 electrode array [boxed area in (b)]. The electrodes become active when a dark object enters the visual field of the camera. (c,d) An actual picture of a participant in the obstacle course pointing (c) and avoiding (d) obstacles. (e) Familiarization area with the juxtaposition of three different obstacles. (f) The training corridor includes two hallways of obstacles. (g) The testing corridor with three hallways. Hatched lines indicate 1 m distances. The exact location of the obstacles is represented with a different symbol for each obstacle type. (h) The different obstacle types.
Experimental setup
A life-sized obstacle course (Fig. 4c–g), was used to assess the ability of congenitally blind and sighted control participants to detect and avoid obstacles with the TDU (Fig. 4a,b). Six different types of obstacles made of cardboard and styrofoam were used (Fig. 4h) (see Chebat et al.,27 for complete procedural details): (i) boxes (60 × 60 × 60 cm), (ii) triangles (60 × 45 × 2 cm), (iii) tubes (10 × 45 × 10 cm), (iv) branches (10 × 45 × 10 cm), (v) road blocks (150 × 10 × 10 cm) and (vi) ground bars (15 × 145 × 20 cm). They were made to resemble obstacles that can be realistically encountered on a sidewalk of any large city. The obstacles were positioned in each hallway according to Fig. 4. For the detection task, accurately pointing to an obstacle was scored as a correct response. For the avoidance task, negotiating a path without hitting or touching each obstacle was scored as a correct response after each obstacle was successfully passed. The obstacle course was composed of three main areas: the familiarization area (Fig. 4e), the training corridor (Fig. 4f) and the test corridor (Fig. 4g). In the familiarization phase, participants walked to the end of the corridor, turned around and came back three times, and each time obstacles were replaced in a different position. The number and positioning of the obstacles are outlined in Fig. 4. Participants were then placed at the entrance of the training corridor and walked down both corridors, and were placed at the entrance of the training corridor a total of four times. In the test phase of the experiment the participants walked through all three corridors, turned around and walked back to the entrance.
Learning rate
We re-analyzed data accumulated in two previous experiments15,27, as well as novel and unpublished data in order to assess the learning rate of our participants in the detection and avoidance paradigm. Only those participants who had completed both behavioral testing and MRI scanning were included in the analysis. We compared their performances during the training phase with the test phase of the experiment. Learning rate was determined by subtracting the performance (percent correct responses) in the training phase of the experiment from the performance in the last phase of the experiment. The resulting scores were used to assess learning rate for detection (LRD) and the learning rate for avoidance (LRA).
Statistical analyses
We used the SPSS statistical package (IBM SPSS Statistics 20 Software) for the analyses; i.e., t-tests, principal component analysis, and regression analysis. Differences between the learning rates (LRD and LRA) of SC and CB participants were evaluated using t-tests. We assessed the relation between the volumes of some specific brain areas and two dependent variables, LRD and LRA related to the ability of the participants to learn to detect and avoid obstacles in the obstacle course. This can be achieved with a regression analysis for each of the dependent variables. However, because of multicollinearity between brain region volumes (many were highly correlated), we performed two principal component analyses; one for the variables related to LRD and one for LRA. These analyses identify groups of independent and homogenous variables (forming brain networks in this case). Then, regression analyses can be performed since principal components (or factors) are independent from each other. These analyses evaluate the relationship between the volumes of brain areas and learning scores (LRD and LRA) for both SC and CB participants. We tested the strength of these relationships between the two groups in a hierarchical regression model (this approach is sometimes called Potthoff analysis73,74) including the principal components (or factors) obtained by PCA, an indicator for group (EB, SC) plus the products of the group with the factors. The test of homogeneity of slopes between groups is then obtained by a t-test for each factor75. Moreover, the most efficient model was evaluated for each subject group. To this aim, we tested different linear models including an increasing number of factors. Significant variations of R2 were evaluated using an F-test75 to select for the most efficient model.
Acknowledgements
This work was supported by the Harland Sanders Research Chair in Visual Science (University of Montreal; M.P.) and the Ariel University Research Authority Absorption Grant # RA1700000192 (D.R.C.). The authors are indebted to the late Professor Jean-Charles Chebat for fruitful discussions and to whom this paper is dedicated.
Author contributions
D.R.C., F.C.S. and M.P. conceived and designed the experiment. D.R.C. ran the experiment. F.C.S. analyzed and segmented the MRI data, D.R.C. analyzed the behavioral data and ran statistical analyses. D.R.C., M.P. and F.C.S. wrote the manuscript. The data that support the findings of this study are available on request from the corresponding author (D.R.C.). The data are not publicly available due to restrictions that could compromise the privacy of research participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McVea David A., Pearson Keir G. Advances in Experimental Medicine and Biology. Boston, MA: Springer US; 2009. Object Avoidance During Locomotion; pp. 293–315. [DOI] [PubMed] [Google Scholar]
- 2.Jeamwatthanachai Watthanasak, Wald Mike, Wills Gary. Indoor navigation by blind people: Behaviors and challenges in unfamiliar spaces and buildings. British Journal of Visual Impairment. 2019;37(2):140–153. doi: 10.1177/0264619619833723. [DOI] [Google Scholar]
- 3.Patla Aftab E., Greig Michael. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neuroscience Letters. 2006;397(1-2):110–114. doi: 10.1016/j.neulet.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Graci Valentina, Elliott David B., Buckley John G. Utility of Peripheral Visual Cues in Planning and Controlling Adaptive Gait. Optometry and Vision Science. 2010;87(1):21–27. doi: 10.1097/OPX.0b013e3181c1d547. [DOI] [PubMed] [Google Scholar]
- 5.Tosoni Annalisa, Galati Gaspare, Romani Gian Luca, Corbetta Maurizio. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nature Neuroscience. 2008;11(12):1446–1453. doi: 10.1038/nn.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poucet Bruno, Lenck-Santini Pierre-Pascal, Paz-Villagrán Vietminh, Save Etienne. Place cells, neocortex and spatial navigation: a short review. Journal of Physiology-Paris. 2003;97(4-6):537–546. doi: 10.1016/j.jphysparis.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez Paul F. Human navigation that requires calculating heading vectors recruits parietal cortex in a virtual and visually sparse water maze task in fMRI. Behavioral Neuroscience. 2010;124(4):532–540. doi: 10.1037/a0020231. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock J. R., Sutherland R. J., Witter M. P., Moser M.-B., Moser E. I. Navigating from hippocampus to parietal cortex. Proceedings of the National Academy of Sciences. 2008;105(39):14755–14762. doi: 10.1073/pnas.0804216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chebat D-R, et al. Alterations in right posterior hippocampus in early blind individuals. Neuroreport. 2007;18:329–333. doi: 10.1097/WNR.0b013e32802b70f8. [DOI] [PubMed] [Google Scholar]
- 10.Fortin M, et al. Wayfinding in the blind: larger hippocampal volume and supranormal spatial navigation. Brain. 2008;131:2995–3005. doi: 10.1093/brain/awn250. [DOI] [PubMed] [Google Scholar]
- 11.Leporé N, et al. Pattern of hippocampal shape and volume differences in blind subjects. Neuroimage. 2009;46:949–957. doi: 10.1016/j.neuroimage.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gori M, Sandini G, Martinoli C, Burr DC. Impairment of auditory spatial localization in congenitally blind human subjects. Brain. 2014;137:288–293. doi: 10.1093/brain/awt311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gori, M., Cappagli, G., Baud-Bovy, G. & Finocchietti, S. Shape perception and navigation in blind adults. Front. Psychol., 10.3389/fpsyg.2017.00010 (2017). [DOI] [PMC free article] [PubMed]
- 14.Amadeo Maria Bianca, Campus Claudio, Gori Monica. Impact of years of blindness on neural circuits underlying auditory spatial representation. NeuroImage. 2019;191:140–149. doi: 10.1016/j.neuroimage.2019.01.073. [DOI] [PubMed] [Google Scholar]
- 15.Ptito Maurice, Schneider Fabien C. G., Paulson Olaf B., Kupers Ron. Alterations of the visual pathways in congenital blindness. Experimental Brain Research. 2008;187(1):41–49. doi: 10.1007/s00221-008-1273-4. [DOI] [PubMed] [Google Scholar]
- 16.Noppeney U. The effects of visual deprivation on functional and structural organization of the human brain. Neurosci. Biobehav. Rev. 2007;31:1169–1180. doi: 10.1016/j.neubiorev.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Cecchetti Luca, Ricciardi Emiliano, Handjaras Giacomo, Kupers Ron, Ptito Maurice, Pietrini Pietro. Congenital blindness affects diencephalic but not mesencephalic structures in the human brain. Brain Structure and Function. 2015;221(3):1465–1480. doi: 10.1007/s00429-014-0984-5. [DOI] [PubMed] [Google Scholar]
- 18.Yang Chunlan, Wu Shuicai, Lu Wangsheng, Bai Yanping, Gao Hongjian. Anatomic Differences in Early Blindness: A Deformation-Based Morphometry MRI Study. Journal of Neuroimaging. 2012;24(1):68–73. doi: 10.1111/j.1552-6569.2011.00686.x. [DOI] [PubMed] [Google Scholar]
- 19.Park Hae-Jeong, Lee Jong Doo, Kim Eung Yeop, Park Bumhee, Oh Maeng-Keun, Lee SungChul, Kim Jae-Jin. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. NeuroImage. 2009;47(1):98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Rombaux P, et al. Increased olfactory bulb volume and olfactory function in early blind subjects. Neuroreport. 2010;21:1069–1073. doi: 10.1097/WNR.0b013e32833fcb8a. [DOI] [PubMed] [Google Scholar]
- 21.Tomaiuolo Francesco, Campana Serena, Collins D. Louis, Fonov Vladimir S., Ricciardi Emiliano, Sartori Giuseppe, Pietrini Pietro, Kupers Ron, Ptito Maurice. Morphometric Changes of the Corpus Callosum in Congenital Blindness. PLoS ONE. 2014;9(9):e107871. doi: 10.1371/journal.pone.0107871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maller Jerome J., Thomson Richard H., Ng Amanda, Mann Collette, Eager Michael, Ackland Helen, Fitzgerald Paul B., Egan Gary, Rosenfeld Jeffrey V. Brain morphometry in blind and sighted subjects. Journal of Clinical Neuroscience. 2016;33:89–95. doi: 10.1016/j.jocn.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Aguirre Geoffrey K., Datta Ritobrato, Benson Noah C., Prasad Sashank, Jacobson Samuel G., Cideciyan Artur V., Bridge Holly, Watkins Kate E., Butt Omar H., Dain Aleksandra S., Brandes Lauren, Gennatas Efstathios D. Patterns of Individual Variation in Visual Pathway Structure and Function in the Sighted and Blind. PLOS ONE. 2016;11(11):e0164677. doi: 10.1371/journal.pone.0164677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine, L. et al. Prevalence of increases in functional connectivity in visual, somatosensory and language areas in congenital blindness. Front. Neuroanat., 10.3389/fnana.2015.00086 (2015). [DOI] [PMC free article] [PubMed]
- 25.Chebat Daniel-Robert, Heimler Benedetta, Hofsetter Shir, Amedi Amir. The Neuroimaging of Brain Diseases. Cham: Springer International Publishing; 2018. The Implications of Brain Plasticity and Task Selectivity for Visual Rehabilitation of Blind and Visually Impaired Individuals; pp. 295–321. [Google Scholar]
- 26.Chebat Daniel-Robert, Harrar Vanessa, Kupers Ron, Maidenbaum Shachar, Amedi Amir, Ptito Maurice. Mobility of Visually Impaired People. Cham: Springer International Publishing; 2017. Sensory Substitution and the Neural Correlates of Navigation in Blindness; pp. 167–200. [Google Scholar]
- 27.Chebat D-R, Schneider F, Kupers R, Ptito M. Navigation with a sensory substitution device in congenitally blind individuals. Neuroreport. 2011;22:342–347. doi: 10.1097/WNR.0b013e3283462def. [DOI] [PubMed] [Google Scholar]
- 28.Chebat Daniel-Robert, Maidenbaum Shachar, Amedi Amir. Navigation Using Sensory Substitution in Real and Virtual Mazes. PLOS ONE. 2015;10(6):e0126307. doi: 10.1371/journal.pone.0126307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chebat, D. R., Maidenbaum, S. & Amedi, A. The transfer of non-visual spatial knowledge between real and virtual mazes via sensory substitution. In International Conference on Virtual Rehabilitation, ICVR, 10.1109/ICVR.2017.8007542 (2017).
- 30.Kolarik Andrew J., Scarfe Amy C., Moore Brian C. J., Pardhan Shahina. Blindness enhances auditory obstacle circumvention: Assessing echolocation, sensory substitution, and visual-based navigation. PLOS ONE. 2017;12(4):e0175750. doi: 10.1371/journal.pone.0175750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupers R, Chebat D-R, Madsen KH, Paulson OB, Ptito M. Neural correlates of virtual route recognition in congenital blindness. Proc. Natl. Acad. Sci. 2010;107:12716–12721. doi: 10.1073/pnas.1006199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schinazi Victor R., Thrash Tyler, Chebat Daniel-Robert. Spatial navigation by congenitally blind individuals. Wiley Interdisciplinary Reviews: Cognitive Science. 2015;7(1):37–58. doi: 10.1002/wcs.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chebat D-R, Rainville C, Kupers R, Ptito M. Tactile-’visual’ acuity of the tongue in early blind individuals. Neuroreport. 2007;18:1901–1904. doi: 10.1097/WNR.0b013e3282f2a63. [DOI] [PubMed] [Google Scholar]
- 34.Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neuroscience & Biobehavioral Reviews. 2014;41:36–52. doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Striemer Christopher L., Chapman Craig S., Goodale Melvyn A. “Real-time” obstacle avoidance in the absence of primary visual cortex. Proceedings of the National Academy of Sciences. 2009;106(37):15996–16001. doi: 10.1073/pnas.0905549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McIntosh R.D, McClements K.I, Dijkerman H.C, Birchall D, Milner A.D. Preserved obstacle avoidance during reaching in patients with left visual neglect. Neuropsychologia. 2004;42(8):1107–1117. doi: 10.1016/j.neuropsychologia.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Schindler Igor, Rice Nichola J, McIntosh Robert D, Rossetti Yves, Vighetto Alain, Milner A David. Automatic avoidance of obstacles is a dorsal stream function: evidence from optic ataxia. Nature Neuroscience. 2004;7(7):779–784. doi: 10.1038/nn1273. [DOI] [PubMed] [Google Scholar]
- 38.Chapman Craig S., Goodale Melvyn A. Missing in action: the effect of obstacle position and size on avoidance while reaching. Experimental Brain Research. 2008;191(1):83–97. doi: 10.1007/s00221-008-1499-1. [DOI] [PubMed] [Google Scholar]
- 39.Maguire E. A., Gadian D. G., Johnsrude I. S., Good C. D., Ashburner J., Frackowiak R. S. J., Frith C. D. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maguire Eleanor A., Spiers Hugo J., Good Catriona D., Hartley Tom, Frackowiak Richard S.J., Burgess Neil. Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus. 2003;13(2):250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- 41.Hasson Uri, Andric Michael, Atilgan Hicret, Collignon Olivier. Congenital blindness is associated with large-scale reorganization of anatomical networks. NeuroImage. 2016;128:362–372. doi: 10.1016/j.neuroimage.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yonk Alex J., Margolis David J. Traces of Learning in Thalamocortical Circuits. Neuron. 2019;103(2):175–176. doi: 10.1016/j.neuron.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Gorbet Diana J., Sergio Lauren E. Looking up while reaching out: the neural correlates of making eye and arm movements in different spatial planes. Experimental Brain Research. 2018;237(1):57–70. doi: 10.1007/s00221-018-5395-z. [DOI] [PubMed] [Google Scholar]
- 44.Huda Rafiq, Goard Michael J., Pho Gerald N., Sur Mriganka. Neural mechanisms of sensorimotor transformation and action selection. European Journal of Neuroscience. 2018;49(8):1055–1060. doi: 10.1111/ejn.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auger Meagan L., Meccia Juliet, Galea Liisa A. M., Floresco Stan B. Disinhibition of the prefrontal cortex leads to brain-wide increases in neuronal activation that are modified by spatial learning. Brain Structure and Function. 2018;224(1):171–190. doi: 10.1007/s00429-018-1769-z. [DOI] [PubMed] [Google Scholar]
- 46.Sherrill Katherine R., Chrastil Elizabeth R., Aselcioglu Irem, Hasselmo Michael E., Stern Chantal E. Structural Differences in Hippocampal and Entorhinal Gray Matter Volume Support Individual Differences in First Person Navigational Ability. Neuroscience. 2018;380:123–131. doi: 10.1016/j.neuroscience.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Rosero Mario A., Winkelmann Tobias, Pohlack Sebastian, Cavalli Juliana, Nees Frauke, Flor Herta. Memory-guided attention: bilateral hippocampal volume positively predicts implicit contextual learning. Brain Structure and Function. 2019;224(6):1999–2008. doi: 10.1007/s00429-019-01887-9. [DOI] [PubMed] [Google Scholar]
- 48.Brunec Iva K., Robin Jessica, Patai Eva Zita, Ozubko Jason D., Javadi Amir-Homayoun, Barense Morgan D., Spiers Hugo J., Moscovitch Morris. Cognitive mapping style relates to posterior-anterior hippocampal volume ratio. Hippocampus. 2019;29(8):748–754. doi: 10.1002/hipo.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sormani Maria Pia, De Stefano Nicola, Giovannoni Gavin, Langdon Dawn, Piani-Meier Daniela, Haering Dieter A, Kappos Ludwig, Tomic Davorka. Learning ability correlates with brain atrophy and disability progression in RRMS. Journal of Neurology, Neurosurgery & Psychiatry. 2018;90(1):38–43. doi: 10.1136/jnnp-2018-319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohbot V. D., Lerch J., Thorndycraft B., Iaria G., Zijdenbos A. P. Gray Matter Differences Correlate with Spontaneous Strategies in a Human Virtual Navigation Task. Journal of Neuroscience. 2007;27(38):10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pruessner J.C. Volumetry of Hippocampus and Amygdala with High-resolution MRI and Three-dimensional Analysis Software: Minimizing the Discrepancies between Laboratories. Cerebral Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 52.Milner Brenda, Johnsrude Ingrid, Crane Joelle. Right medial temporal–lobe contribution to object–location memory. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1997;352(1360):1469–1474. doi: 10.1098/rstb.1997.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farioli-Vecchioli Stefano, Saraulli Daniele, Costanzi Marco, Pacioni Simone, Cinà Irene, Aceti Massimiliano, Micheli Laura, Bacci Alberto, Cestari Vincenzo, Tirone Felice. The Timing of Differentiation of Adult Hippocampal Neurons Is Crucial for Spatial Memory. PLoS Biology. 2008;6(10):e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsanov Marian, Manahan-Vaughan Denise. Synaptic Plasticity from Visual Cortex to Hippocampus: Systems Integration in Spatial Information Processing. The Neuroscientist. 2008;14(6):584–597. doi: 10.1177/1073858408315655. [DOI] [PubMed] [Google Scholar]
- 55.Biegler R., McGregor A., Krebs J. R., Healy S. D. A larger hippocampus is associated with longer-lasting spatial memory. Proceedings of the National Academy of Sciences. 2001;98(12):6941–6944. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goodale Melvyn A., Milner A.David. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 57.Fajen Brett R., Warren William H. Behavioral dynamics of steering, obstable avoidance, and route selection. Journal of Experimental Psychology: Human Perception and Performance. 2003;29(2):343–362. doi: 10.1037/0096-1523.29.2.343. [DOI] [PubMed] [Google Scholar]
- 58.Longuet-Higgins, H. C. & Prazdny, K. The interpretation of a moving retinal image. Proc. R. Soc. London - Biol. Sci., 10.1098/rspb.1980.0057 (1980). [DOI] [PubMed]
- 59.Rice Nichola J., Edwards Martin G., Schindler Igor, Punt T. David, McIntosh Robert D., Humphreys Glyn W., Lestou Vaia, Milner A. David. Delay abolishes the obstacle avoidance deficit in unilateral optic ataxia. Neuropsychologia. 2008;46(5):1549–1557. doi: 10.1016/j.neuropsychologia.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Rice Nichola J., McIntosh Robert D., Schindler Igor, Mon-Williams Mark, Démonet Jean-François, Milner A. David. Intact automatic avoidance of obstacles in patients with visual form agnosia. Experimental Brain Research. 2006;174(1):176–188. doi: 10.1007/s00221-006-0435-5. [DOI] [PubMed] [Google Scholar]
- 61.Nitz Douglas. Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiology of Learning and Memory. 2009;91(2):179–185. doi: 10.1016/j.nlm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Sato N., Sakata H., Tanaka Y. L., Taira M. Context-Dependent Place-Selective Responses of the Neurons in the Medial Parietal Region of Macaque Monkeys. Cerebral Cortex. 2009;20(4):846–858. doi: 10.1093/cercor/bhp147. [DOI] [PubMed] [Google Scholar]
- 63.Evangeliou M. N., Raos V., Galletti C., Savaki H. E. Functional Imaging of the Parietal Cortex during Action Execution and Observation. Cerebral Cortex. 2008;19(3):624–639. doi: 10.1093/cercor/bhn116. [DOI] [PubMed] [Google Scholar]
- 64.McFadyen Bradford J., Bouyer Laurent, Bent Leah R., Inglis J. Timothy. Visual-vestibular influences on locomotor adjustments for stepping over an obstacle. Experimental Brain Research. 2006;179(2):235–243. doi: 10.1007/s00221-006-0784-0. [DOI] [PubMed] [Google Scholar]
- 65.Good Catriona D., Johnsrude Ingrid, Ashburner John, Henson Richard N.A., Friston Karl J., Frackowiak Richard S.J. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. NeuroImage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 66.Eickhoff SB, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 67.Squire Larry R. The Legacy of Patient H.M. for Neuroscience. Neuron. 2009;61(1):6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Epstein R. A., Parker W. E., Feiler A. M. Where Am I Now? Distinct Roles for Parahippocampal and Retrosplenial Cortices in Place Recognition. Journal of Neuroscience. 2007;27(23):6141–6149. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., Habel U., Schneider F., Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210(5-6):343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 70.Malikovic A, et al. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: A probabilistic, stereotaxic map of area hOc5. Cereb. Cortex. 2007 doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- 71.Scheperjans F., Eickhoff S. B., Homke L., Mohlberg H., Hermann K., Amunts K., Zilles K. Probabilistic Maps, Morphometry, and Variability of Cytoarchitectonic Areas in the Human Superior Parietal Cortex. Cerebral Cortex. 2008;18(9):2141–2157. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epstein Russell A. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12(10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potthoff, R. F. Statistical aspects of problem of biases in psychological tests. (North Carolina State University. Dept. of Statistics, 1966).
- 74.Weaver B, Wuensch KL. SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behav. Res. Methods. 2013;45:880–895. doi: 10.3758/s13428-012-0289-7. [DOI] [PubMed] [Google Scholar]
- 75.Ahrens H, Dempster AP. Elements of Continuous Multivariate Analysis. Addison‐Wesley Publ. Co., Reading, Mass. 1969. XII, 388 S. Biom. Z. 1975;17:468. doi: 10.1002/bimj.19750170714. [DOI] [Google Scholar]