THE NEED FOR AN UPDATE
The BJP has recently implemented several policies to improve the rigour and transparency of experimental design, analysis, and data reporting (Alexander et al., 2018; Curtis et al., 2018; Docherty et al., 2019). This journal has also developed checklists to help authors comply with the BJP's submission requirements on experimental design and reporting (Declaration, 2018a), animal experimentation (Declaration, 2018b), and immunoblotting and immunohistochemistry (Declaration, 2018c). The editors recognize also that a keystone in the process of improving further the transparency and openness in reporting scientific progress is to make raw data compliant with FAIR principles (i.e., be findable, available, interoperable and reproducible; FAIR, 2019). The Senior Editorial Team at BJP is committed to supporting fully initiatives that open up data sharing as widely as possible.
In 2017, we considered issues on data sharing relevant to pharmacology research. We concluded that, at that time, no digital solutions existed to enable the integration of published findings with the underpinning raw data for the types of approaches typically published in the BJP (George et al., 2017). With due consideration of the practical limitations and fallibilities of available repositories and archiving systems, and issues on data storage and transfer, we adopted a policy of encouraging authors to share their data. This position did not unnecessarily burden authors with having to comply with systems for data sharing that we decided were not fit for purpose. However, we pledged to provide an update in due course, and this editorial reviews the rationale for expecting data sharing for articles published in BJP and provides guidelines for authors to comply with this expectation.
BJP DATA SHARING SURVEY: VIEWS FROM THE EDITORIAL BOARD
Although there have been helpful guidelines introduced to facilitate the maintenance of digital data (UK Research and Innovation, 2015; Hart et al., 2016), the development of web‐based systems for the indexing, structuring, sharing, and curation of research data has not matured to the extent that might have been anticipated. Reconciling the acknowledged benefits of sharing research data with the current mechanisms for doing so remains a challenge. So that we could better gauge how the BJP could maintain its compliance with policies that promote data availability and sharing (Committee on Publication Ethics, 2019; Centre for Open Science, 2019), whilst recognizing real practical limitations and also serving the needs of authors publishing in this journal, the views on data sharing of the full editorial board of the BJP were sought. Figure 1 shows the responses to a survey that posed questions that concerned data acquisition, sharing, structuring, and storage. We contend that due to the international nature of the BJP editorial board (representation from 23 different countries) that these responses also give insights into the collective perspectives from across the global pharmacology research community.
Figure 1.
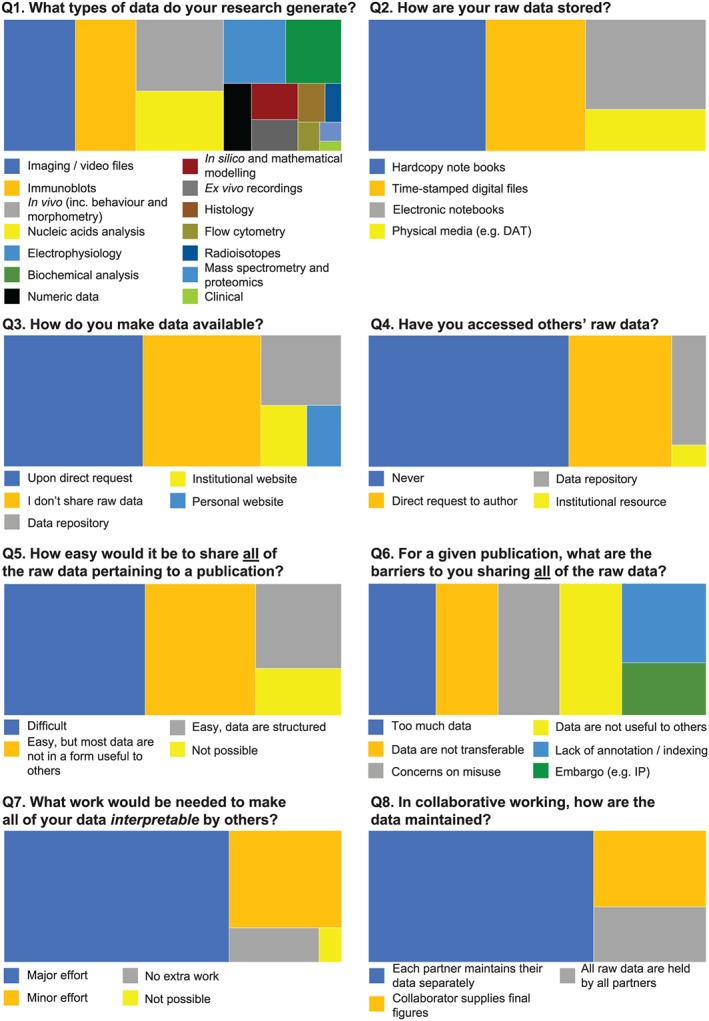
Survey of BJP editorial board on data sharing and availability. Survey was conducted online in February 2019. N = 57 respondents
Summarizing key points:
The breadth of activities in the pharmacology community probably precludes a “one‐size fits all” solution to data sharing (Q1). Finding common ground in relation to standardisation of data format and annotation is likely to be exceptionally difficult.
Raw data are typically stored using a variety of media, some of which pose challenges for transferability and digitization (Q2).
Almost two‐thirds of respondents (65%) presently make their data available, but most (59%) have never requested raw data from others (Q3 and Q4).
Sharing all data pertaining to a published paper is not commonplace, and substantial efforts would be required to overcome the barriers to enabling this (Q5, Q6, and Q7).
Nearly one‐fifth of respondents expressed serious concerns on the potential for their shared data to be misused (Q6). Making data available should not mean that the data can be reused for any purpose by whomever has accessed them. How consent/permissions from the originators of shared data, for the subsequent use of their data, might be regulated remains a complex and unresolved problem.
Assigning who should hold overall responsibility for all data generated as part of collaborative working remains a challenge (Q8).
The survey served to reinforce the view that the pharmacology community is fully engaged with the ethos of data sharing and availability. However, major efforts—that include developing policy on who should organise, standardise, support, and pay for data sharing—are required if it is to become a seamless part of the publishing experience.
ENABLING A FLEXIBLE WAY OF MAKING DATA AVAILABLE
Following a constructive collaboration among the editors of the BJP, Wiley, our publisher, and the British Pharmacological Society (BPS), we have now produced a data sharing policy that appropriately considers the landscape of research data and attitudes to sharing amongst pharmacology researchers. We believe that this new policy—one that enables multiple ways to share data—has the requisite flexibility as a simple, workable solution for authors to make available data compliant with “level 1” of the Transparency and Openness Promotion (TOP; Centre for Open Science, 2019). As shown in Table 1, an appropriate statement can be selected from templates among several options regarding the sharing of data.
Table 1.
Template statements on data availability
| Availability of data | Template for data availability statement |
|---|---|
| Data available on request from the authors | The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions. |
| Data openly available in a public repository that issues datasets with DOIs | The data that support the findings of this study are openly available in [repository name] at http://doi.org/ [doi], reference number [reference number]. |
| Data subject to third party restrictions | The data that support the findings of this study are available from [third party]. Restrictions apply to the availability of these data, which were used under license for this study. Data are available [from the authors/at URL] with the permission of [third party]. |
| Data sharing not applicable—no new data generated a | Data sharing is not applicable to this article because no new data were created or analysed in this study. |
| Data not shared | No data have been shared. |
Relevant only to articles that contain no original research, such as review articles.
Authors might want simply to make data available on request. This option is likely to be most attractive to many authors seeking to publish in the BJP, since it obviates problems inherent in making some of their “non‐digital”/“non‐standard” research data available using an externally hosted resource (e.g., an online repository).
Authors though may choose to archive the supporting data, from which the published results are derived, in a public repository that offers guaranteed preservation. For help in choosing a suitable repository, please see Registry for Research Data Repository (2019). We strongly discourage the use of data repositories that do not assign a Digital Object Identifier (DOI) and regard the impermanence of such archiving resources as a cause for real concern.
It is important to stress that this policy update does not mandate that data are shared. If there are legitimate reasons that prevent the sharing of some data described in the manuscript, for example, for legal or ethical reasons, or simply that authors do not wish to share data, then the inclusion of a statement to this effect is appropriate. However, if authors do not choose to share data—and their paper includes a statement to this effect—there would still be an expectation that all raw data supporting papers published in the BJP would be retained for a minimum of 10 years (or 20 years for clinical data) as per the Concordat on Open Research Data (Concordat, 2016; Committee on Publication Ethics, 2019).
WHAT DOES THIS CHANGE IN POLICY MEAN FOR AUTHORS?
The BJP now expect that authors will make data available under one or more of the mechanisms set out in Table 1. All accepted manuscripts will be required to include for publication a data availability statement selected from one of the templates listed in Table 1 and the Author Guidelines. Authors will be required to confirm adherence to this data policy on submission of their manuscript. All statements will be placed in the heading of the manuscript, in front of all firewalls. When data are available and electronically linked to the source, authors will need to provide a citation of the data in their reference list. For further information, please see our Instructions to Authors (British Journal of Pharmacology, 2019). We thank the BJP editors for their assistance in completing the survey and authors for their cooperation with this new guideline.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Journal of Pharmacology . (2019). Author Guidelines. https://bpspubs.onlinelibrary.wiley.com/hub/journal/14765381/author-guidelines.html
- Centre for Open Science . (2019). Transparency Openness Promotion. https://cos.io/top/
- Committee on Publication Ethics (COPE) . (2019). https://publicationethics.org/
- Concordat on Open Research Data . (2016). https://www.ukri.org/files/legacy/documents/concordatonopenresearchdata-pdf/
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declaration (2018a). Declaration of transparency and scientific rigour: Checklist for design and analysis. British Journal of Pharmacology, 175, 2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declaration (2018b). Declaration of transparency and scientific rigour: Checklist for animal experimentation. British Journal of Pharmacology, 175, 2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declaration (2018c). Declaration of transparency and scientific rigour: Checklist for immunoblotting and immunohistochemistry. British Journal of Pharmacology, 175, 2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty, J. R. , Stanford, S. C. , Panattieri, R. A. , Alexander, S. P. H. , Cirino, G. , George, C. H. , … Ahluwalia, A. (2019). Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. British Journal of Pharmacology, 176, 4081–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIR . (2019). FAIR: Findable, accessible, interoperable, re‐useable. http://www.force11.org/group/fairgroup/fairprinciples
- George, C. H. , Stanford, S. C. , Alexander, S. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , … Ahluwalia, A. (2017). Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. British Journal of Pharmacology, 174, 2801–2804. 10.1111/bph.13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, E. M. , Barmby, P. , Lebauer, D. , Michonneau, F. , Mount, S. , Mulrooney, P. , … Hollister, J. W. (2016). Ten simple rules for digital data storage. PLoS Computational Biology, 12, e1005097 10.1371/journal.pcbi.1005097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Registry of Research Data Repository . (2019). https://www.re3data.org/
- UK Research and Innovation . (2015). Guidance on best practice in the management of research data. https://www.ukri.org/files/legacy/documents/rcukcommonprinciplesondatapolicy-pdf/


