Abstract
Background and Purpose
Aldosterone stimulates epithelial Na+ channel (ENaC)‐dependent Na+ retention in the cortical collecting duct (CCD) of the kidney by activating mineralocorticoid receptors that promote expression of serum and glucocorticoid‐inducible kinase 1 (SGK1). This response is critical to BP homeostasis. It has previously been suggested that inhibiting lysine deacetylases (KDACs) can post‐transcriptionally disrupt this response by promoting acetylation of the mineralocorticoid receptor. The present study critically evaluates this hypothesis.
Experimental Approach
Electrometric and molecular methods were used to define the effects of a pan‐KDAC inhibitor, trichostatin A, on the responses to a physiologically relevant concentration of aldosterone (3 nM) in murine mCCDcl1 cells.
Key Results
Aldosterone augmented ENaC‐induced Na+ absorption and increased SGK1 activity and abundance, as expected. In the presence of trichostatin A, these responses were suppressed. Trichostatin A‐induced inhibition of KDAC was confirmed by increased acetylation of histone H3, H4, and α‐tubulin. Trichostatin A did not block the electrometric response to insulin, a hormone that activates SGK1 independently of increased transcription, indicating that trichostatin A has no direct effect upon the SGK1/ENaC pathway.
Conclusions and Implications
Inhibition of lysine de‐acetylation suppresses aldosterone‐dependent control over the SGK1–ENaC pathway but does not perturb post‐transcriptional signalling, providing a physiological basis for the anti‐hypertensive action of KDAC inhibition seen in vivo.
What is already known
Aldosterone stimulates Na+ retention by activating mineralocorticoid receptors in cortical collecting ducts of the kidney.
This anti‐natriuretic response depends upon mineralocorticoid receptor‐mediated changes in gene expression.
What this study adds
As expected, trichostatin A consistently promoted acetylation of cytoplasmic and nuclear proteins.
Trichostatin A suppressed the mineralocorticoid receptor‐mediated transcription of Sgk1 and blocked aldosterone‐induced Na+ absorption.
What is the clinical significance
Hypertension can be treated with drugs that disrupt the response to aldosterone.
Agents that promote protein acetylation may provide a novel means of suppressing aldosterone‐induced Na+ retention.
Abbreviations
- CCD
cortical collecting duct
- ENaC
epithelial Na+ channel
- Iamil
amiloride‐sensitive current
- Ieq
equivalent short circuit current
- KDAC
lysine deacetylase, HDAC
- NDRG1
N‐myc downstream‐regulated gene 1
- Rt
transepithelial resistance
- SGK1
serum‐ and glucocorticoid‐inducible kinase 1
- TSA
trichostatin A
- Vt
transepithelial voltage
1. INTRODUCTION
Protein acetylation is a post‐translational modification, catalysed by lysine acetyl transferases (also known as histone acetyl transferases), that convert positively charged amine groups in lysine residues into neutral amides (see Falkenberg & Johnstone, 2014). The subsequent removal of acetyl residues from proteins modified in this way is catalysed by lysine deacetylases (KDACs, also known as https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=848), a family of zinc‐dependent enzymes (Falkenberg & Johnstone, 2014). The acetylation status of many cellular proteins is therefore determined by the relative activities of lysine acetyl transferases and KDACs, and the acetylation of many cytoplasmic proteins has physiological relevance. Acetylation of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=625, for example, modifies the hormone‐induced transcription of several genes (Kadiyala et al., 2013; Winkler et al., 2012), whereas acetylation of the cytoplasmic receptors for oestrogen, progesterone, and testosterone reduces the ability of sex steroids to regulate gene transcription, an effect that is exploited to suppress the growth of hormone‐sensitive tumour cells (Falkenberg & Johnstone, 2014). Changes to the acetylation status of cytoplasmic receptors therefore provide a means of modifying responses to steroid hormones (Barnes, 2013; Falkenberg & Johnstone, 2014).
https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=626 in the cortical collecting ducts (CCD) of the kidney allow https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2872 to increase renal Na+ reabsorption by evoking expression of genes, such as that encoding https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1534; Lang et al., 2006), that control the abundance of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=122 subunits at the apical membrane (Alvarez De La Rosa, Li, & Canessa, 2002; Blazer‐Yost, Liu, & Helman, 1998; Lang, Artunc, & Vallon, 2009; Loffing & Korbmacher, 2009; Soundararajan, Melters, Shih, Wang, & Pearce, 2009; Soundararajan, Wang, Melters, & Pearce, 2007; Soundararajan, Zhang, Wang, Vandewalle, & Pearce, 2005). The mineralocorticoid receptor–SGK1–ENaC pathway is critical to the long‐term regulation of BP, and many drugs used to treat hypertension (ACE inhibitors and mineralocorticoid receptor antagonists) promote diuresis/natriuresis by disrupting this mechanism. It is therefore interesting that KDAC inhibitors increase acetylation of heterologously expressed mineralocorticoid receptors and reduce the transcriptional activity of this protein (Lee et al., 2013). Moreover, KDAC inhibitors also suppress aldosterone‐induced gene transcripts in HEK cells, apparently by promoting acetylation of the endogenous mineralocorticoid receptors (Lee et al., 2013). Furthermore, such inhibitors also block the development of hypertension in unilaterally nephrectomised mice exposed to a high salt diet and injected with deoxycorticosterone acetate, a mineralocorticoid receptor agonist (Lee et al., 2013).
While this work suggests that KDAC inhibitors might lower BP by suppressing aldosterone‐induced Na+ retention (Lee et al., 2013), it is important to note that HEK cells, although derived from renal tissue, do not display a phenotype representative of any nephron segment. Indeed, these cells express voltage‐gated Na+ channels suggesting that they display a neural, rather than an epithelial, phenotype (Mansell, Barratt, & Wilson, 2011; Moran, Nizzari, & Conti, 2000). Although aldosterone did induce gene expression in these cells (Lee et al., 2013), the effect upon SGK1 developed over ~24 hr (Lee et al., 2013), and this extremely slow response is in stark contrast with data from CCD cells where hormone‐induced Na+ transport is apparent after only ~1 hr. This relatively rapid response is accompanied by large (~40‐fold) increases in the abundance of SGK1 mRNA and protein and a clear increase in cellular SGK1 activity (Lang et al., 2006; Lang et al., 2009; Mansley et al., 2016). It is therefore difficult to relate the effects of KDAC inhibitors on HEK cells to events within the CCD. In order to test the hypothesis that KDAC inhibitors are able to suppress the mineralocorticoid receptor‐mediated stimulation of Na+ absorption in the CCD, we have now explored the effects of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7005, a pan‐KDAC inhibitor, on the responses to physiologically relevant concentrations of aldosterone in mouse CCD cells (mCCDcl1).
2. METHODS
2.1. Electrophysiological measurements
Standard methods were used to maintain murine mCCDcl1 cells, (University of Lausanne, Lausanne, Switzerland), in serial culture (Mansley, Neuhuber, Korbmacher, & Bertog, 2015), and experiments undertaken using confluent cells (7–8 days culture) that had been deprived of hormones/growth factors for ~48 hr (Gaeggeler et al., 2005; Mansley et al., 2015). ENaC‐mediated Na+ transport was usually quantified in cells grown on Transwell membranes by measuring transepithelial voltage (V t) and resistance (R t) using an epithelial volt‐ohm‐meter (WPI EVOM2, Hitchin, Herts., UK). Some experiments were undertaken using cells grown on Costar Snapwell membranes that were mounted in Ussing chambers where V t and R t were monitored continuously (Mansley & Wilson, 2010b). Equivalent short circuit current (I Eq) was then calculated from Ohm's Law (i.e., V t/R t). All electrometric experiments were terminated by adding https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2421 (10 μM), an ENaC blocker, to the solution bathing the apical side of the membrane. The small current (~5% of control) that persisted under these conditions was then subtracted from the previously measured values of I Eq to quantify the transepithelial Na+ current flowing via ENaC (I Amil). As V t was expressed relative to an earth electrode in the basolateral bath, the absorption of Na+ moving from the apical to the basal compartment will generate negative current.
2.2. Western analysis of extracted proteins
Confluent cells were washed three times with ice‐cold PBS before being scraped into ice‐cold lysis buffer containing protease and phosphatase inhibitors (Mansley & Wilson, 2010b). The cellular lysates were then ultrasonicated to ensure disruption, centrifuged to remove insoluble debris, and their protein contents determined (Bradford reagent, Bio‐Rad, Hertfordshire, UK). Aliquots of extracted protein (40 μg) were then fractionated on SDS‐PAGE and transferred to Hybond PVDF membranes that were probed using antibodies detailed below. Immunoreactive proteins were detected using peroxide‐conjugated secondary antibodies/enhanced chemiluminescence. Once this analysis was completed, gels were stripped and then re‐probed using antibodies against β‐actin or total N‐myc downstream‐regulated gene 1 (NDRG1) to provide markers of protein loading. Fuller details are provided elsewhere (Mansley et al., 2016; Mansley & Wilson, 2010b). The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
Protein samples derived from each experiment were processed in parallel and analysed on the same gels. This was important since western analysis cannot produce entirely uniform data and ensuring that samples from the entire experiments were analysed together therefore guarantees that variations between batches of antibody, enhanced chemiluminescence reagents, efficiencies of protein transfer to blotting membranes, and so forth do not contribute to the observed variation within each experimental group. It is evident from the presented images that some of the antibodies used identified additional bands in certain experiments. These additional bands were unaltered by any experimental manoeuvres and were therefore assumed to be irrelevant to the present study and ignored in our analyses. To derive quantitative data, high resolution, uncompressed digital images (TIF format) of each developed blot were obtained using a Chemi DOC MP Imaging System (BioRad, Hemel Hemptead, Herts. UK). The ODs of all relevant bands were measured digitally (ImageJ, RRID:SCR_003070), and the data from each series of experiments collated. The mean OD associated with protein extracted from cells deprived of hormones throughout the entire experimental period (i.e., control) was then determined. The individual data points from the entire series of experiments, whether from control or experimental cells, were then normalised to this overall mean value. The results of all such experiments are therefore expressed relative to a mean control signal. The effects of TSA upon the abundance of each acetylated protein were quantified densitometrically using ImageJ (Rueden et al., 2017) using the expression ODTSA − ODcontrol/ODmax − ODcontrol. In this expression, ODTSA refers to the OD quantified in cells exposed to a particular concentration of TSA, whist ODcontrol and ODmax, respectively, refer to the corresponding values from control cells (i.e., cells simply exposed to solvent vehicle) and cells exposed to a maximally effective concentration of TSA. The results of this analysis are plotted (fractional response, mean ± 95% confidence interval [95% CI]) against the concentration of TSA. The presented images show individual gels judged to represent the results obtained in the entire series of experiments. None of these images were manipulated in such a way to conceal, move, or introduce any specific feature. Cited molecular weights were estimated by reference to standards that were run on each gel.
2.3. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The effects of TSA upon responses to hormones were explored using strictly paired protocols in which control cells were exposed to a relevant concentration of the appropriate solvent vehicle. For all experiments, cells were grown on filters matched by passage number, date of thaw, seeding density, and culture conditions. For experiments, each matched filter of cells was randomly allocated to an experimental group, and values of n refer to the number of times the entire protocol was repeated. Pooled data are shown as mean ± 95% CI, and the results of individual experiments are shown in all figures. Statistical analysis was undertaken using GraphPad Prism 7.03 (GraphPad Software Ltd., San Diego, CA, USA, RRID:SCR_002798). Where appropriate, the pooled data derived from each set of experiments were firstly analysed by two‐way ANOVA. If this analysis identified statistically significant variations between the experimental groups, we undertook further analysis using Tukey's post hoc test with correction for multiple comparisons in order to identify differences between particular experimental groups. Values of P < 0.05 were considered to indicate statistical significance.
2.4. Materials
Amiloride, aldosterone, insulin, and general laboratory reagents were from Sigma (Poole, Dorset, UK). Antibodies against the Thr346/356/366‐phosphorylated and total forms of the protein encoded by NDRG1 and against SGK1 were purchased from the MRC Protein Phosphorylation Unit, University of Dundee (Dundee, UK). The antibodies against β‐actin (RRID:AB_476697) and acetylated α‐tubulin (RRID:AB_609894) were from Sigma; the antibodies against acetylated histone H3 (RRID:AB_823528) and H4 (RRID:AB_2448400) were from Cell Signaling Technologies (Leiden, The Netherlands).
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski, et al., 2017; Alexander, Fabbro, et al., 2017; Alexander, Peters, et al., 2017).
3. RESULTS
3.1. The response of CCD cells to aldosterone
Table 1 shows the results of electrometric experiments that explored the effects of aldosterone (3 nM, 3 hr) on cells grown on Transwell membranes. Aldosterone hyperpolarised V t and reduced R t and analysis using Ohm's law revealed a clear increase in the magnitude of I Eq (Table 1). Subsequently exposing unstimulated and aldosterone‐stimulated cells to apical amiloride (10 μM), an ENaC blocker, depolarised V t, increased R t and caused >90% block of I Eq (Table 1). While these findings confirm that I Eq is due, almost exclusively, to electrogenic Na+ absorption via ENaC, it is interesting that aldosterone did augment the small current that persisted in the presence of amiloride (Table 1). The physiological basis of this small, amiloride resistant current was not investigated, but it is possible that it reflects the secretion of Cl− and/or HCO3 − across the apical membrane. To complete the analysis of these data, we quantified I Amil (see Section 2), and our data show clearly that aldosterone caused a 2.7 ± 0.2‐fold increase (mean ± 95% CI) in the magnitude of this current (unstimulated: −7.5 ± 1.3 μA·cm−2; aldosterone‐stimulated: −20.2 ± 3.0 μA·cm−2, n = 12, mean ± 95% CI, P < 0.05 Student's unpaired t test).
Table 1.
The electrometric response to aldosterone
| V t (mV) | R t (Ω·cm2) | I eq (μA·cm−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3‐hr treatment | Amil. | Baseline | 3‐hr treatment | Amil. | Baseline | 3‐hr treatment | Amil. | |
| Vehicle | −17.2 ± 5.9 | −16.3 ± 5.1 | 0.5 ± 0.7# | 2105 ± 342 | 2125 ± 363 | 3340 ± 650# | −7.8 ± 1.6 | −7.4 ± 1.4 | 0.1 ± 0.2# |
| Aldo. | −18.1 ± 5.2 | −36.7 ± 7.4* | −5.9 ± 3.2* , # | 2277 ± 257 | 1666 ± 113* | 3261 ± 461# | −7.7 ± 1.5 | −5.9 ± 3.2* | −1.6 ± 0.8* , # |
Note. Electrometric measurements (V t and R t) were recorded, allowing calculation of equivalent short circuit current (I eq), from cells grown on filters under control (vehicle) or aldosterone‐treated conditions. Measurements were made before treatment (baseline); 3 hr after treatment (3‐hr treatment) with solvent vehicle or aldosterone (Aldo, 3 nM); and 5 min after exposure to amiloride (Amil., 10 μM). Data are mean ± 95% confidence interval (n = 12). Statistical significance was determined using a repeated‐measures two‐way ANOVA. Where appropriate, multiple comparisons were determined by Tukey's post hoc test.
P<0.05, significantly different from vehicle‐treated cells.
P<0.05, significant difference between 3‐hr treatment and amiloride.
3.2. TSA‐induced acetylation of cellular proteins
TSA was one of the first KDAC inhibitors to be described (Yoshida, Kijima, Akita, & Beppu, 1990) and is widely used as a reference in research within this field. It has been used to determine anti‐tumour activity in preclinical models of cancer, but it has not been assessed in a clinical setting (Sanderson et al., 2004). Studies of protein extracted from hormone‐deprived cells exposed to TSA (0.01–100 μM) and/or solvent vehicle for 6 hr showed that this pan‐KDAC inhibitor increased the abundance of acetylated histone H3 (Figure 1a), histone H4 (Figure 1b), and α‐tubulin (Figure 1c). Analysis of sigmoid curves fitted to these data by least squares regression (Figure 1a–c) showed that half maximal responses occurred at ~0.15 μM (histone H3: EC50 = 0.14 ± 0.07 μM; histone H4: EC50 = 0.15 ± 0.19 μM; α‐tubulin: EC50 = 0.20 ± 0.13 μM) and established that concentrations of TSA > 1 μM were maximally effective (Figure 1a–c). Parallel analyses using an antibody against β‐actin confirmed that identical amounts of protein had been loaded onto each gel (Figure 1d), and we therefore conclude that exposure to TSA promotes acetylation of all three proteins. Figure 2 shows that the effects on histone H3 and H4 became apparent after ~1 hr and reached plateau values after 6–12 hr (Figure 2a,b). The acetylation of α‐tubulin, on the other hand, peaked at ~1 hr (Figure 2c). Although the effects on all three proteins declined throughout the remainder of the experimental period, increased acetylation persisted until at least 24 hr (Figure 2a–c). Parallel analyses using the β‐actin antibody confirmed that essentially identical amounts of cellular protein had been loaded onto each gel (Figure 2d).
Figure 1.
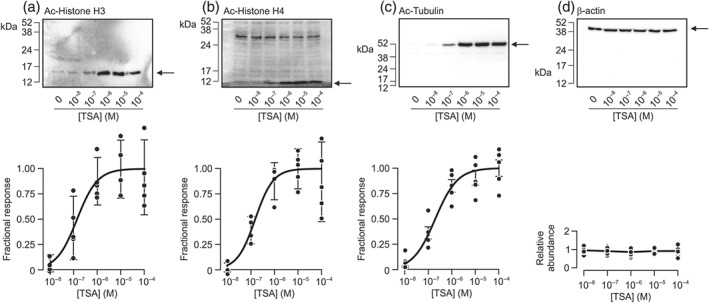
TSA‐induced acetylation of cellular proteins. Cells were exposed to TSA (0.01–100 μM) and/or solvent vehicle for 6 hr. Upper panels show representative blots probed with acetylated histone H3 (a, Ac‐Histone H3), histone H4 (b, Ac‐Histone H4), and α‐tubulin (c, Ac‐α‐tubulin). All blots were reanalysed using an antibody against β‐actin (d). MW markers are marked, and arrows denote relevant bands. Lower panels show the densitometric analyses of the data derived from the entire series of experiments (n = 5). The solid curves are sigmoid curves fitted to these data by least squares regression whilst the vertical bars are centred on the mean values and show the 95% confidence interval. The mean densitometric values of β‐actin are expressed relative to the mean value from cells treated with solvent vehicle of TSA
Figure 2.
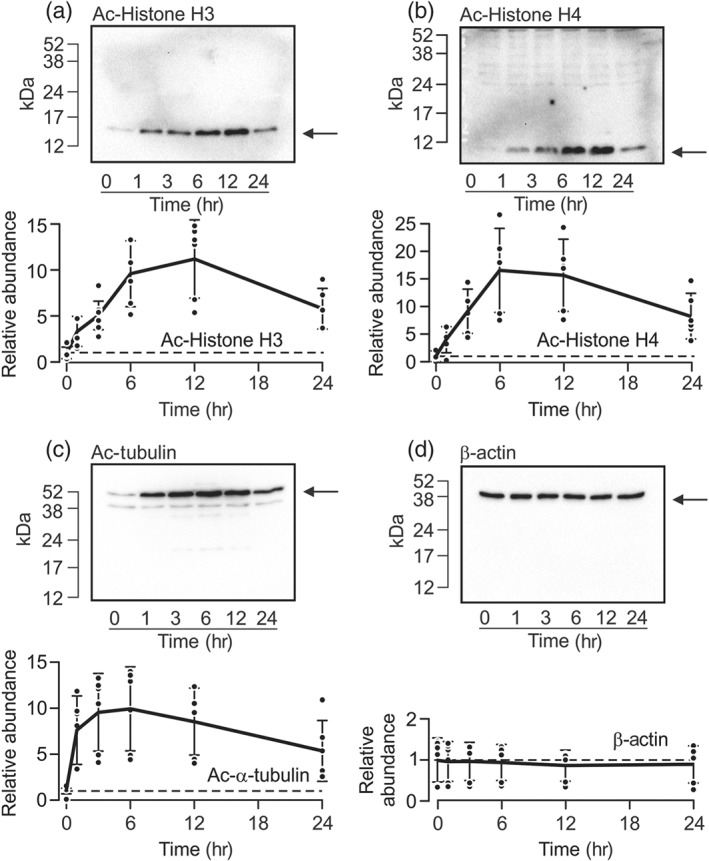
Time course of TSA‐induced acetylation. Protein extracted from cells exposed to 1‐μM TSA for 0–24 hr was subject to western analysis using antibodies against the acetylated forms of histone H3 (a, Ac‐Histone H3, n = 6), histone H4 (b, Ac‐Histone H4, n = 6), and α‐tubulin (c, Ac‐α‐tubulin, n = 6). All blots were then re‐probed using the antibody against β‐actin (d). In each figure, the upper panel shows the results of a representative experiment, whilst the densitometric analyses presented below show the changes to the abundance of each protein plotted against time. Data are mean ± 95% CI
3.3. TSA disrupts the electrometric response to aldosterone
We explored the effects of TSA (1 μM) upon the electrometric response to aldosterone using the experimental protocol shown in Figure 3a. Measurements made at the onset of these experiments showed that I Eq was normally ~8 μA·cm−2 (Figure 3b), and this parameter was unaffected by exposure (2 hr) to 1‐μM TSA (Figure 3c). Control and TSA‐treated cells were then exposed to 3‐nM aldosterone and/or solvent vehicle and the electrometric measurements repeated after a further 3 hr. While the control data confirmed that aldosterone normally augments I Eq (Figure 3d), this hormone had only very small effects on TSA‐treated cells (Figure 3d). Further analysis of these data confirmed (Mansley et al., 2015) that aldosterone normally augments I Amil (Figure 3f). Although a small response was seen in the TSA‐treated cells, its magnitude was only 15.7 ± 1.0% of control (Figure 3), and therefore, we conclude that TSA causes substantial (~85%) loss of sensitivity to aldosterone.
Figure 3.
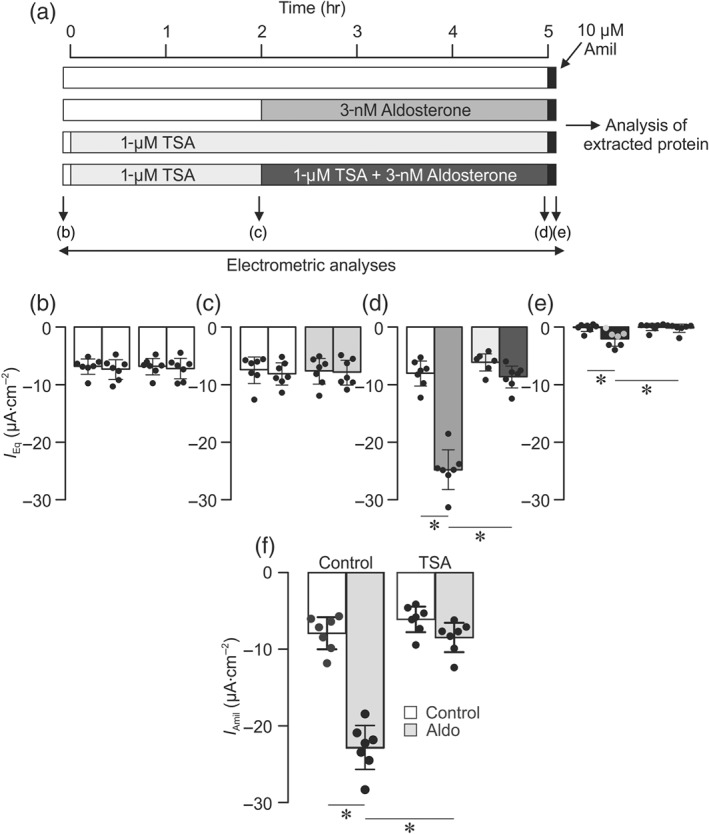
Effects of TSA upon the electrometric response to aldosterone. (a) Each horizontal bar represents a separate cultured epithelial layer, and cells were exposed to TSA, aldosterone, and amiloride as indicated by the different shading. Electrometric data were recorded (arrows) at the onset of each experiment (b); after 2‐hr exposure to TSA (1 μM) and/or solvent vehicle (c); after the cells had been exposed to aldosterone (3 nM) and/or solvent vehicle for a further 3 hr (d); and after a final application of 10‐μM apical amiloride (e). Shading of the vertical bars (b–d) corresponds with those used in (a) to differentiate the various stages of the experiment. Vertical columns show the mean values ± 95% CI (n = 7) for each experimental group. *P<0.05, significantly different as indicated; repeated measures two‐way ANOVA and Tukey's post hoc test. (f) The amiloride‐sensitive component of the transepithelial equivalent current (I Amil, mean ± 95% CI, n = 7) was quantified. *P<0.05, significantly different as indicated; two‐way ANOVA and Tukey's post hoc test
3.4. The control of cellular SGK1 abundance and activity
Cellular SGK1 activity was assessed using an established method that is based upon the identification of residues within an endogenous protein (NDRG1‐Thr346/356/366) that are phosphorylated by SGK1 but not by other, closely related kinases (Inglis et al., 2009; Murray et al., 2004). Once the electrometric measurements described above were completed, protein was extracted from cells for western analysis so that we could correlate the electrometric data with changes to the phosphorylation status of these residues. These experiments showed clearly that the aldosterone‐induced augmentation of I Amil was associated with an increase in the abundance of Thr346/356/366‐phosphorylated NDRG1 (Figure 4a) that occurred without change to the overall NDRG1 expression level (Figure 4a). Aldosterone thus increased phosphorylation of NDRG1‐Thr346/356/366, and this finding confirms (Inglis et al., 2009; Murray et al., 2004) that this hormone normally increases cellular SGK1 activity. No such response was seen in TSA‐treated cells (Figure 4). Parallel analyses using an antibody against SGK1 itself showed that aldosterone also increased the abundance of SGK1 protein, and analyses undertaken using the β‐actin antibody confirmed that this effect could not be attributed to variations in the mass of protein loaded onto the gels (Figure 4b). Aldosterone therefore increases the abundance of this protein, and this response, in common with the associated increase in cellular SGK1 activity, was abolished by TSA (Figure 4b).
Figure 4.
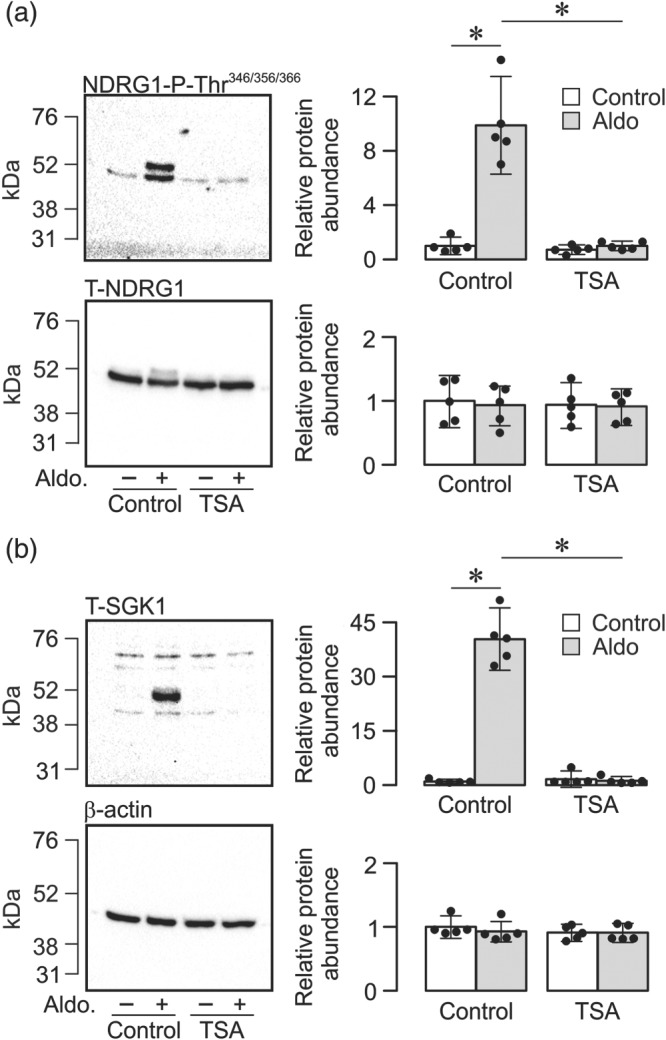
Effects of TSA upon aldosterone‐induced SGK1 activity and abundance. (a) Typical blots obtained using antibodies against the Thr346/356/366‐phosphorylated form of the protein encoded by N‐myc downstream regulated gene 1 (upper panel, NDRG1‐P‐Thr346/356/366) and the equivalent full length protein (lower panel, T‐NDRG1). (b) Typical blots obtained using antibodies against the serum and glucocorticoid‐inducible kinase 1 (upper panel, T‐SGK1) and β‐actin (lower panel). Pooled data (right‐hand panels) from the entire series of experiments showing (mean ± 95% CI, n = 5) the effects of aldosterone (3 nM, 3 hr) upon the abundance of (a) NDRG1‐P‐Thr346/356/366 (upper) and total NDRG1 (lower) or (b) SGK1 (upper) and β‐actin (lower) in control and TSA‐treated (1 μM, 2 hr) cells. *P<0.05, significantly different as indicated; two‐way ANOVA and Tukey's post hoc test
3.5. Effects of TSA on the response of CCD cells to insulin
Figure 5a shows continuous records of I Amil derived from cells mounted in Ussing chambers and acutely exposed to insulin (20 nM). As anticipated (Faletti, Perrotti, Taylor, & Blazer‐Yost, 2002; Gonzalez‐Rodriguez, Gaeggeler, & Rossier, 2007; Mansley et al., 2016; Mansley & Wilson, 2010b; Record, Froelich, Vlahos, & Blazer‐Yost, 1998), insulin caused a clear and rapid augmentation of I Amil although the response (ΔI Amil ~11 μA·cm−2, Figure 5a) was smaller than the response to aldosterone. Figure 5a also includes data from experiments undertaken using age‐matched cells pretreated (2 hr) with 1‐μM TSA. The responses seen in these cells were essentially identical to control indicating that TSA does not modify the electrometric response to this hormone. The insulin‐induced augmentation of I Amil was associated with an increase in the abundance of Thr346/356/366‐phosphorylated NDRG1 (Figure 5b) that occurred with no change to the overall NDRG1 expression level (Figure 5b). These data accord with previous findings (Mansley & Wilson, 2010a; Mansley & Wilson, 2010b) and therefore confirm that insulin normally activates SGK1 in CCD cells. This response persisted in the presence of TSA (1 μM), a novel finding which shows that insulin can still activate SGK1 following inhibition of KDAC.
Figure 5.
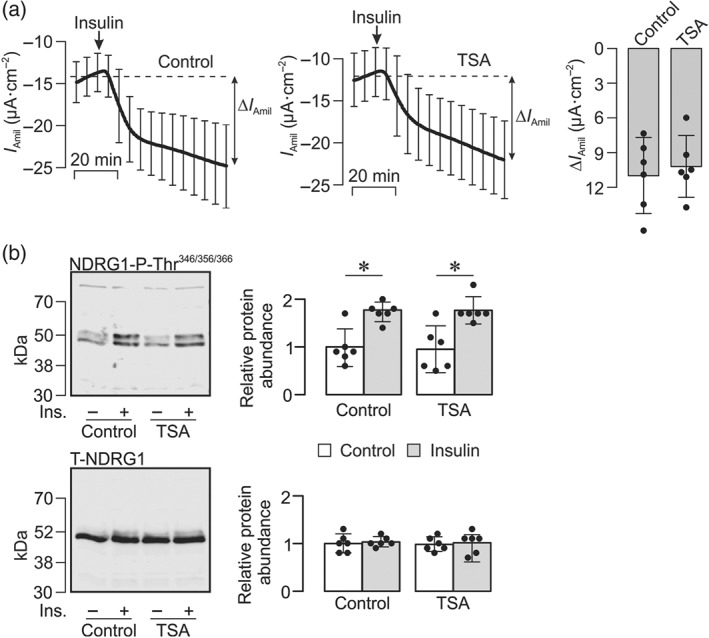
Insulin‐induced Na+ transport and SGK1 activity. (a) Confluent cells on Costar Snapwell membranes were mounted in Ussing chambers so that the effects of insulin (20 nM) upon I Amil could be continuously recorded (mean ± 95% CI, n = 6) under control conditions (left panel) and in cells that had been pretreated (2 hr) with 1‐μM TSA (middle panel). The amiloride‐sensitive responses to insulin (ΔI Amil) were quantified and presented as mean ± 95% CI (right panel). (b) Typical blots obtained using antibodies against the Thr346/356/366‐phosphorylated form of the protein encoded by N‐myc downstream regulated gene 1 (upper panel, NDRG1‐P‐Thr346/356/366) and the equivalent full length protein (lower panel, T‐NDRG1). Pooled data (right‐hand panels) from the entire series of experiments showing (mean ± 95% CI, n = 6) the effects of insulin (20 nM, 1 hr) upon the abundance of NDRG1‐P‐Thr346/356/366 (upper) and total NDRG1 (lower) in control and TSA‐treated (1 μM, 2 hr) cells. *P<0.05, significantly different as indicated; two‐way ANOVA and Tukey's post hoc test
4. DISCUSSION
4.1. Design of the present study
The controlled reabsorption of Na+ within the CCD is critical to BP homeostasis and depends upon ENaC, a Na+‐selective channel present in the apical membrane of principal cells in the CCD. In unstimulated cells, the amount of ENaC in this membrane is restricted by the continual internalisation of channel subunits, and this internalisation process limits the Na+ permeability of the apical membrane and restricts the amount of Na+ that can be recovered from the tubular fluid. Aldosterone increases Na+ retention by binding to the mineralocorticoid receptor and inducing expression of several genes, including that encoding SGK1 (Lang et al., 2006). Although other mechanisms may well be involved (Frindt, Gravotta, & Palmer, 2016; Soundararajan, Pearce, & Ziera, 2012), a substantial body of work shows that increased cellular SGK1 activity can inhibit this ENaC internalisation mechanism and thus allow the Na+ permeability of the apical membrane to rise. This, in turn, increases the amount of Na+ recovered from the tubular fluid (Debonneville et al., 2001; Snyder, 2005; Snyder, Olson, Kabra, Zhou, & Steines, 2004). Earlier studies of cultured CCD cells showed that the natriuretic responses to aldosterone and insulin were abolished by pharmacological inhibition of SGK1 (Mansley, Korbmacher, & Bertog, 2018; Mansley & Wilson, 2010b), and this cannot be attributed to a non‐specific effect as the responses to peptide hormones that signal via cAMP/PKA are preserved when signalling via SGK1 is blocked. Hormone‐induced changes to cellular SGK1 activity are therefore central to the regulation of Na+ retention.
Administration of KDAC inhibitors abolished the high BP seen in a mouse model of hypertension, an observation attributed to loss of mineralocorticoid receptor function (Lee et al., 2013). This therefore implied that KDAC inhibition would abolish mineralocorticoid receptor‐mediated activation of the SGK1–ENaC pathway in the CCD. The aim of the present study was to test this hypothesis using a clinically applicable in vitro model of CCD cells, the mCCDcl1 cell line. Our initial experiments were thereby focused on characterisation of the control response of these cells to aldosterone. Relatively brief (3 hr) exposure of these cells to this hormone clearly augmented ENaC‐mediated Na+ transport, and so, in contrast to HEK cells (Lee et al., 2013), mCCDcl1 cells display a relatively rapid response to aldosterone. As the concentration of aldosterone used (3 nM) approximates to the circulating concentration in salt‐deprived mice (Bertog et al., 2008; Gaeggeler et al., 2005), these data also confirm that mCCDcl1 cells are sensitive to physiologically relevant concentrations of this hormone (Mansley et al., 2018). This point is very relevant to the present study because high concentrations of aldosterone (>~10 nM) activate glucocorticoid receptors as well as mineralocorticoid receptors (Gaeggeler et al., 2005). We estimate (Gaeggeler et al., 2005) that 3‐nM aldosterone will provide essentially complete (>~90%) occupancy of the mineralocorticoid receptors but negligible binding to the glucocorticoid receptors and therefore attribute the present responses to mineralocorticoid receptor‐mediated stimulation of Na+ transport via ENaC.
Analysis of protein extracted from TSA‐treated cells revealed clear increases in the abundance of acetylated histones H3 and H4. These are nuclear proteins that are archetypical class I KDAC substrates, found in several cell types (Bantscheff et al., 2011; Vigushin et al., 2001; Yoshida, Horinouchi, & Beppu, 1995). In addition to nuclear proteins, TSA treatment also increased abundance of acetylated α‐tubulin in mCCDcl1 cells, a protein target of class II KDACs. Moreover, although the acetylation of nuclear histone H3/H4 proteins developed over ~6 hr, the acetylation of α‐tubulin developed over a much shorter time period (~1 hr). This observation supports the reported mechanistic basis of KDAC activity and the requirement for class I KDACs to translocate to the nuclear compartment to exert their effect upon histones, as opposed to cytoplasmic proteins which can be directly modified.
The ability of the pan‐KDAC inhibitor TSA to modify both cytoplasmic and nuclear proteins, in combination with our earlier studies, confirming a physiologically relevant response of the mCCDcl1 cells to mineralocorticoid receptor activation, allowed us to design a protocol to test the theory that KDAC inhibitors suppress mineralocorticoid receptor‐mediated activation of the SGK1–ENaC pathway in CCD cells. Aldosterone normally increased the magnitude of I Amil by ~2.5‐fold, and the 95% CI associated with this response was ~0.2‐fold. This implies that the smallest inhibitory action detectable by our study is ~20%. However, the previously reported animal studies had indicated that administration of KDAC inhibitors essentially abolished experimental hypertension (Lee et al., 2013) which, if correctly attributed to loss of mineralocorticoid receptor function, would predict that KDAC inhibition was associated with substantial inhibition of the response to aldosterone. Our experimental protocol therefore has sufficient statistical power to allow us to properly evaluate this hypothesis.
4.2. TSA suppressed the response to aldosterone without directly affecting SGK1–ENaC
TSA caused substantial (~85%) inhibition of the electrometric response to aldosterone and abolished the associated increases in the abundance and activity of SGK1. These findings are consistent with the hypothesis that inhibition of KDACs cause loss of mineralocorticoid receptor function (Lee et al., 2013). Although aldosterone is the principal regulator of ENaC in the CCD, insulin also promotes Na+ absorption via a mechanism dependent on the SGK1–ENaC signalling pathway (Blazer‐Yost et al., 1998; Blazer‐Yost, Esterman, & Vlahos, 2003; Blazer‐Yost, Shah, Jarett, Cox, & Smith, 1992; Faletti et al., 2002; Mansley et al., 2016; Mansley & Wilson, 2010a; Pearce & Kleyman, 2007; Record et al., 1998; Wang et al., 2001). However, although both hormones increased cellular SGK1 activity, TSA did not perturb the response to insulin. In contrast to aldosterone, insulin activates SGK1 without increasing the abundance of SGK1 protein or mRNA. Instead, this hormone acts via a non‐genomic mechanism dependent upon https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=673 (Cohen, 2006), an enzyme that increases the catalytic activity of SGK1 protein by controlling its phosphorylation (Kobayashi & Cohen, 1999; Park et al., 1999). TSA therefore suppresses mineralocorticoid receptor‐ mediated activation of the SGK1–ENaC pathway by disrupting control over SGK1 protein abundance without affecting the PI3K‐dependent, post‐translational control of this pathway.
The mechanism that allows aldosterone to regulate ENaC‐mediated Na+ retention is complex and incompletely understood. However, it is now clear that the acetylation status of cellular proteins has a direct bearing upon several components of this pathway. For example, KDAC inhibition has been shown to promote acetylation of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=784), a protein that controls the translocation of activated steroid receptors to the nucleus (Barnes, 2013; Jimenez‐Canino et al., 2016). Moreover, in contrast to the studies of wild type/mutant forms of the mineralocorticoid receptor (Lee et al., 2013), these works indicate that KDAC inhibitors do not alter the transcriptional activity of the receptor (Jimenez‐Canino et al., 2016). The inhibition of mineralocorticoid receptor signalling which we now report may therefore be due, at least in part, to altered translocation of the mineralocorticoid receptor between the cytoplasm and the nucleus (Jimenez‐Canino et al., 2016). However, KDAC inhibition has also been shown to promote acetylation of ENaC itself (Butler, Staruschenko, & Snyder, 2015). This modification seems to block the ENaC internalisation process that appears to limit Na+ transport in unstimulated cells (Blazer‐Yost & Nofziger, 2005; Gonzalez‐Rodriguez et al., 2007; Wang et al., 2001). Rather than suppressing Na+ absorption, these data therefore suggest that ENaC acetylation will augment Na+ retention by increasing the surface abundance of the channel (Butler et al., 2015). While we cannot exclude the possibility that this process may augment Na+ retention under certain conditions (Butler et al., 2015), our data show that TSA had no effect on basal Na+ transport and did not modify the response to insulin. It thus appears that inhibition of KDAC did not directly modify ENaC function under the conditions of our model.
The KDAC enzyme family contain at least eight members (KDAC1–8), and it is now clear that different KDACs can fulfil different physiological roles. The acetylation status of the mineralocorticoid receptor, for example, seems to be maintained by https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2617 (Lee et al., 2013), while https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2618 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2660 are respectively thought to determine the acetylation status of hsp90 (Jimenez‐Canino et al., 2016) and ENaC (Butler et al., 2015). TSA is a broad spectrum KDAC inhibitor and thus has the potential to interfere with all of these events. Moreover, although selective inhibitors are becoming available, their use is not straightforward since the KDAC isoforms are known to interact with each other. For example, although acetylated α‐tubulin is not a substrate for KDAC3, MI192, a highly selective inhibitor of KDAC3 (Boissinot et al., 2012), promotes acetylation of this protein via an indirect mechanism that is ultimately dependent upon KDAC6 (Bacon et al., 2015).
In summary, TSA promoted the acetylation of nuclear (histone H3 and H4) and cytoplasmic (α‐tubulin) proteins indicating clear inhibition of KDAC. Moreover, TSA also blocked the aldosterone‐dependent control over ENaC‐mediated Na+ transport and cellular SGK1 activity but did not affect the corresponding responses to insulin. The present data therefore show that TSA selectively disrupts the regulation of Na+ transport via genomic mechanisms. These data accord with studies of the heterologously expressed mineralocorticoid receptors (Lee et al., 2013) and establish a physiological basis for the antihypertensive actions seen in vivo (Lee et al., 2013). KDAC inhibition may therefore provide a novel means of lowering BP in hypertensive patients, and it is therefore important to fully define the mechanisms that allow these compounds to act in this way. The effects described in this earlier study (Lee et al., 2013) were attributed to increased acetylation of the mineralocorticoid receptor itself, and although we did not directly monitor the acetylation status of this receptor, mineralocorticoid receptor acetylation could well explain the inhibitory action of TSA described here. However, it is important to stress that genomic responses to steroids such as aldosterone occur via complex mechanisms that are still incompletely understood. In this context, it is interesting that inhibition of KDAC5/6 appears to suppress the translocation of the activated mineralocorticoid receptor from the cytoplasm into the nucleus without altering the transcriptional activity of the receptor itself (Jimenez‐Canino et al., 2016). We cannot exclude the possibility that such a mechanism may contribute to the effect of TSA which we now report, and it is also possible that the response may reflect changes to the acetylation of histone itself. Further studies, in which the acetylation status of the mineralocorticoid receptor along with other physiologically important proteins are critically assessed, will therefore be required to establish the mechanism that allows TSA to suppress aldosterone‐induced Na+ transport.
AUTHOR CONTRIBUTIONS
S.M.W., M.K.M., and M.A.B. did the conception and design of the research. M.K.M., A.J.R., and S.L.F. performed the experiments. S.M.W., M.A.B., J.H.G., M.K.M., A.J.R., and S.L.F. interpreted the results of the experiments. S.M.W., M.K.M., A.J.R., and S.L.F. prepared the figures. S.M.W. drafted the manuscript. S.M.W., M.K.M., M.A.B., and J.H.G. edited and revised the manuscript. S.M.W., M.A.B., J.H.G., M.K.M., A.J.R., and S.L.F. approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was supported by a Kidney Research UK Project Grant RP39/2011 (S. M. W. and M. A. B.) and a Kidney Research UK Intermediate Fellowship (M. K. M.). We are grateful to Dr. Ilona Obara for generously providing the acetylated histone H3 antibody.
Mansley MK, Roe AJ, Francis SL, Gill JH, Bailey MA, Wilson SM. Trichostatin A blocks aldosterone‐induced Na+ transport and control of serum‐ and glucocorticoid‐inducible kinase 1 in cortical collecting duct cells. Br J Pharmacol. 2019;176:4708–4719. 10.1111/bph.14837
REFERENCES
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez De La Rosa, D. A. , Li, H. , & Canessa, C. M. (2002). Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells. The Journal of General Physiology, 119, 427–442. 10.1085/jgp.20028559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon, T. , Seiler, C. , Wolny, M. , Hughes, R. , Watson, P. , Schwabe, J. , … Peckham, M. (2015). Histone deacetylase 3 indirectly modulates tubulin acetylation. The Biochemical Journal, 472, 367–377. 10.1042/BJ20150660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantscheff, M. , Hopf, C. , Savitski, M. M. , Dittmann, A. , Grandi, P. , Michon, A. M. , … Drewes, G. (2011). Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nature Biotechnology, 29, 255–U124. [DOI] [PubMed] [Google Scholar]
- Barnes, P. J. (2013). Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. The Journal of Allergy and Clinical Immunology, 131, 636–645. 10.1016/j.jaci.2012.12.1564 [DOI] [PubMed] [Google Scholar]
- Bertog, M. , Cuffe, J. E. , Pradervand, S. , Hummler, E. , Hartner, A. , Porst, M. , … Korbmacher, C. (2008). Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle's syndrome. The Journal of Physiology, 586, 459–475. 10.1113/jphysiol.2007.140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer‐Yost, B. L. , Esterman, M. A. , & Vlahos, C. J. (2003). Insulin‐stimulated trafficking of ENaC in renal cells requires PI 3‐kinase activity. American Journal of Physiology. Cell Physiology, 284, C1645–C1653. 10.1152/ajpcell.00372.2002 [DOI] [PubMed] [Google Scholar]
- Blazer‐Yost, B. L. , Liu, X. , & Helman, S. I. (1998). Hormonal regulation of ENaCs: Insulin and aldosterone. The American Journal of Physiology, 274, C1373–C1379. 10.1152/ajpcell.1998.274.5.C1373 [DOI] [PubMed] [Google Scholar]
- Blazer‐Yost, B. L. , & Nofziger, C. (2005). The role of the phosphoinositide pathway in hormonal regulation of the epithelial sodium channel. Advances in Experimental Medicine and Biology, 559, 359–368. 10.1007/0-387-23752-6_33 [DOI] [PubMed] [Google Scholar]
- Blazer‐Yost, B. L. , Shah, N. , Jarett, L. , Cox, M. , & Smith, R. M. (1992). Insulin and IGF1 receptors in a model renal epithelium: Receptor localization and characterization. Biochemistry International, 28, 143–153. [PubMed] [Google Scholar]
- Boissinot, M. , Inman, M. , Hempshall, A. , James, S. R. , Gill, J. H. , Selby, P. , … Cockerill, P. N. (2012). Induction of differentiation and apoptosis in leukaemic cell lines by the novel benzamide family histone deacetylase 2 and 3 inhibitor MI‐192. Leukemia Research, 36, 1304–1310. 10.1016/j.leukres.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Butler, P. L. , Staruschenko, A. , & Snyder, P. M. (2015). Acetylation stimulates the epithelial sodium channel by reducing its ubiquitination and degradation. The Journal of Biological Chemistry, 290, 12497–12503. 10.1074/jbc.M114.635540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, P. (2006). Timeline—The twentieth century struggle to decipher insulin signalling. Nature Reviews. Molecular Cell Biology, 7, 867–873. 10.1038/nrm2043 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonneville, C. , Flores, S. Y. , Kamynina, E. , Plant, P. J. , Tauxe, C. , Thomas, M. A. , … Staub, O. (2001). Phosphorylation of Nedd4‐2 by Sgk1 regulates epithelial Na+ channel cell surface expression. The EMBO Journal, 20, 7052–7059. 10.1093/emboj/20.24.7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faletti, C. J. , Perrotti, N. , Taylor, S. I. , & Blazer‐Yost, B. L. (2002). sgk: An essential convergence point for peptide and steroid hormone regulation of ENaC‐mediated Na+ transport. American Journal of Physiology. Cell Physiology, 282, C494–C500. 10.1152/ajpcell.00408.2001 [DOI] [PubMed] [Google Scholar]
- Falkenberg, K. J. , & Johnstone, R. W. (2014). Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nature Reviews. Drug Discovery, 13, 673–691. 10.1038/nrd4360 [DOI] [PubMed] [Google Scholar]
- Frindt, G. , Gravotta, D. , & Palmer, L. G. (2016). Regulation of ENaC trafficking in rat kidney. The Journal of General Physiology, 147, 217–227. 10.1085/jgp.201511533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeggeler, H. P. , Gonzalez‐Rodriguez, E. , Jaeger, N. F. , Loffing‐Cueni, D. , Norregaard, R. , Loffing, J. , … Rossier, B. C. (2005). Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone‐stimulated sodium transport in a novel renal cell line. Journal of the American Society of Nephrology : JASN, 16, 878–891. 10.1681/ASN.2004121110 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Rodriguez, E. , Gaeggeler, H. P. , & Rossier, B. C. (2007). IGF‐1 vs insulin: Respective roles in modulating sodium transport via the PI‐3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney International, 71, 116–125. 10.1038/sj.ki.5002018 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis, S. K. , Gallacher, M. , Brown, S. G. , McTavish, N. , Getty, J. , Husband, E. M. , … Wilson, S. M. (2009). SGK1 activity in Na+ absorbing airway epithelial cells monitored by assaying NDRG1‐Thr346/356/366 phosphorylation. Pflügers Archiv : European Journal of Physiology, 457, 1287–1301. 10.1007/s00424-008-0587-1 [DOI] [PubMed] [Google Scholar]
- Jimenez‐Canino, R. , Lorenzo‐Diaz, F. , Jaisser, F. , Farman, N. , Giraldez, T. , & de la Rosa, D. A. (2016). Histone deacetylase 6‐controlled Hsp90 acetylation significantly alters mineralocorticoid receptor subcellular dynamics but not its transcriptional activity. Endocrinology, 157, 2515–2532. 10.1210/en.2015-2055 [DOI] [PubMed] [Google Scholar]
- Kadiyala, V. , Patrick, N. M. , Mathieu, W. , Jaime‐Frias, R. , Pookhao, N. , An, L. L. , & Smith, C. L. (2013). Class I lysine deacetylases facilitate glucocorticoid‐induced transcription. The Journal of Biological Chemistry, 288, 28900–28912. 10.1074/jbc.M113.505115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T. , & Cohen, P. (1999). Activation of serum‐ and glucocorticoid‐regulated protein kinase by agonists that activate phosphatidylinositide 3‐kinase is mediated by 3‐phosphoinositide‐dependent protein kinase‐1 (PDK1) and PDK2. The Biochemical Journal, 339, 319–328. 10.1042/bj3390319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, F. , Artunc, F. , & Vallon, V. (2009). The physiological impact of the serum and glucocorticoid‐inducible kinase SGK1. Current Opinion in Nephrology and Hypertension, 18, 439–448. 10.1097/MNH.0b013e32832f125e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, F. , Böhmer, C. , Palmada, M. , Seebohm, G. , Strutz‐Seebohm, N. , & Vallon, V. (2006). (Patho)physiological significance of the serum‐ and glucocorticoid‐inducible kinase isoforms. Physiological Reviews, 86, 1151–1178. 10.1152/physrev.00050.2005 [DOI] [PubMed] [Google Scholar]
- Lee, H.‐A. , Lee, D.‐Y. , Cho, H.‐M. , Kim, S.‐Y. , Iwasaki, Y. , & Kim, I. K. (2013). Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circulation Research, 112, 1004–U1073. 10.1161/CIRCRESAHA.113.301071 [DOI] [PubMed] [Google Scholar]
- Loffing, J. , & Korbmacher, C. (2009). Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflügers Archiv : European Journal of Physiology, 458, 111–135. 10.1007/s00424-009-0656-0 [DOI] [PubMed] [Google Scholar]
- Mansell, S. A. , Barratt, C. R. , & Wilson, S. M. (2011). Voltage‐induced Na+ and K+ currents in human embryonic kidney epithelial (HEK293) cells. Proceedings of the Physiological Society, 24, PC29. [Google Scholar]
- Mansley, M. K. , Korbmacher, C. , & Bertog, M. (2018). Inhibitors of the proteasome stimulate the epithelial sodium channel (ENaC) through SGK1 and mimic the effect of aldosterone. Pflügers Archiv : European Journal of Physiology, 470, 295–304. 10.1007/s00424-017-2060-5 [DOI] [PubMed] [Google Scholar]
- Mansley, M. K. , Neuhuber, W. , Korbmacher, C. , & Bertog, M. (2015). Norepinephrine stimulates the epithelial Na+ channel in cortical collecting duct cells via α2‐adrenoceptors. American Journal of Physiology. Renal Physiology, 308, F450–F458. 10.1152/ajprenal.00548.2014 [DOI] [PubMed] [Google Scholar]
- Mansley, M. K. , Watt, G. B. , Francis, S. L. , Walker, D. J. , Land, S. C. , Bailey, M. A. , & Wilson, S. M. (2016). Dexamethasone and insulin activate serum and glucocorticoid‐inducible kinase 1 (SGK1) via different molecular mechanisms in cortical collecting duct cells. Physiological Reports, 4, e12792 10.14814/phy2.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansley, M. K. , & Wilson, S. M. (2010a). Dysregulation of epithelial Na+ absorption induced by inhibition of the kinases TORC1 and TORC2. British Journal of Pharmacology, 161, 1778–1792. 10.1111/j.1476-5381.2010.01003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansley, M. K. , & Wilson, S. M. (2010b). Effects of nominally selective inhibitors of the kinases PI3K, SGK1 and PKB on the insulin‐dependent control of epithelial Na+ absorption. British Journal of Pharmacology, 161, 571–588. 10.1111/j.1476-5381.2010.00898.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, O. , Nizzari, M. , & Conti, F. (2000). Endogenous expression of the β1A sodium channel subunit in HEK‐293 cells. FEBS Letters, 473, 132–134. 10.1016/S0014-5793(00)01518-0 [DOI] [PubMed] [Google Scholar]
- Murray, J. T. , Campbell, D. G. , Morrice, N. , Auld, G. C. , Shpiro, N. , Marquez, R. , … Cohen, P. (2004). Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. The Biochemical Journal, 384, 477–488. 10.1042/BJ20041057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Leong, M. L. L. , Buse, P. , Maiyar, A. C. , Firestone, G. L. , & Hemmings, B. A. (1999). Serum and glucocorticoid‐inducible kinase (SGK) is a target of the PI 3‐kinase‐stimulated signaling pathway. The EMBO Journal, 18, 3024–3033. 10.1093/emboj/18.11.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, D. , & Kleyman, T. R. (2007). Salt, sodium channels, and SGK1. The Journal of Clinical Investigation, 117, 592–595. 10.1172/JCI31538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record, R. D. , Froelich, L. L. , Vlahos, C. J. , & Blazer‐Yost, B. L. (1998). Phosphatidylinositol 3‐kinase activation is required for insulin‐stimulated sodium transport in A6 cells. The American Journal of Physiology ‐ Endocrinol Metabol, 274, C531–C536. [DOI] [PubMed] [Google Scholar]
- Rueden, C. T. , Schindelin, J. , Hiner, M. C. , DeZonia, B. E. , Walter, A. E. , Arena, E. T. , & Eliceiri, K. W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics, 18, 529 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, L. , Taylor, G. W. , Aboagye, E. O. , Alao, J. P. , Latigo, J. R. , Coombes, R. C. , & Vigushin, D. M. (2004). Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin A after intraperitoneal administration to mice. Drug Metabolism and Disposition, 32, 1132–1138. 10.1124/dmd.104.000638 [DOI] [PubMed] [Google Scholar]
- Snyder, P. M. (2005). Minireview: Regulation of epithelial Na+ channel trafficking. Endocrinology, 146, 5079–5085. 10.1210/en.2005-0894 [DOI] [PubMed] [Google Scholar]
- Snyder, P. M. , Olson, D. R. , Kabra, R. , Zhou, R. , & Steines, J. C. (2004). cAMP and serum and glucocorticoid‐inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4‐2. The Journal of Biological Chemistry, 279, 45753–45758. 10.1074/jbc.M407858200 [DOI] [PubMed] [Google Scholar]
- Soundararajan, R. , Melters, D. , Shih, I. C. , Wang, J. , & Pearce, D. (2009). Epithelial sodium channel regulated by differential composition of a signaling complex. Proceedings of the National Academy of Sciences of the United States of America, 106, 7804–7809. 10.1073/pnas.0809892106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan, R. , Pearce, D. , & Ziera, T. (2012). The role of the ENaC‐regulatory complex in aldosterone‐mediated sodium transport. Molecular and Cellular Endocrinology, 350, 242–247. 10.1016/j.mce.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan, R. , Wang, J. , Melters, D. , & Pearce, D. (2007). Differential activities of glucocorticoid‐induced leucine zipper protein isoforms. The Journal of Biological Chemistry, 282, 36303–36313. 10.1074/jbc.M707287200 [DOI] [PubMed] [Google Scholar]
- Soundararajan, R. , Zhang, T. T. , Wang, J. , Vandewalle, A. , & Pearce, D. (2005). A novel role for glucocorticoid‐induced leucine zipper protein in epithelial sodium channel‐mediated sodium transport. The Journal of Biological Chemistry, 280, 39970–39981. 10.1074/jbc.M508658200 [DOI] [PubMed] [Google Scholar]
- Vigushin, D. M. , Ali, S. , Pace, P. E. , Mirsaidi, N. , Ito, K. , Adcock, I. , & Coombes, R. C. (2001). Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clinical Cancer Research, 7, 971–976. [PubMed] [Google Scholar]
- Wang, J. , Barbry, P. , Maiyar, A. C. , Rozansky, D. J. , Bhargava, A. , Leong, M. , … Pearce, D. (2001). SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. American Journal of Physiology. Renal Physiology, 280, F303–F313. 10.1152/ajprenal.2001.280.2.F303 [DOI] [PubMed] [Google Scholar]
- Winkler, R. , Benz, V. , Clemenz, M. , Bloch, M. , Foryst‐Ludwig, A. , Wardat, S. , … Kintscher, U. (2012). Histone deacetylase 6 (HDAC6) is an essential modifier of glucocorticoid‐induced hepatic gluconeogenesis. Diabetes, 61, 513–523. 10.2337/db11-0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, M. , Horinouchi, S. , & Beppu, T. (1995). Trichostatin‐A and trapoxin—Novel chemical probes for the role of histone acetylation in chromatin structure and function. BioEssays, 17, 423–430. 10.1002/bies.950170510 [DOI] [PubMed] [Google Scholar]
- Yoshida, M. , Kijima, M. , Akita, M. , & Beppu, T. (1990). Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. The Journal of Biological Chemistry, 265, 17174–17179. [PubMed] [Google Scholar]


