Abstract
Background and Purpose
Praziquantel is a schistosomicide, which has been used for more than 30 years due to its efficiency, safety, and mild side effects. Previous studies showed that prolonged treatment with praziquantel suppressed the development of liver fibrosis in mice with schistosomiasis. In this study, we investigated the potential mechanisms underlying the antifibrotic effects of praziquantel.
Experimental Approach
To avoid the effect of schistosomicidal activity of praziquantel against liver fibrosis induced by Schistosoma japonicum infection, we established a mouse model of carbon tetrachloride (CCl4)‐induced liver fibrosis for in vivo studies and used TGF‐β1‐stimulated human hepatic stellate cell line (LX‐2) in addition to other fibroblast‐like cell line (MES13) and fibroblast cell line (NIH3T3) in vitro. Western blotting, immunohistochemistry, quantitative real‐time PCR, siRNA, and immunofluorescence staining were utilized to assess the expression of key molecules in liver fibrosis and the TGF‐β/Smad pathway.
Key Results
Praziquantel significantly attenuated CCl4‐induced liver fibrosis by inhibiting the activation of hepatic stellate cells (HSCs) and expression of collagen matrix via enhancement of Smad7 expression, which were confirmed in LX‐2, MES13, and NIH3T3 cells in vitro. In contrast, knockdown of Smad7 in LX‐2 cells prevented praziquantel‐mediated inhibition of LX‐2 cell activation and TGF‐β1‐mediated collagen type I α1 induction, revealing the critical role of Smad7 in the antifibrotic effect of praziquantel during liver fibrosis.
Conclusions and Implications
PZQ exhibited a strong efficacy against liver fibrosis by inhibiting activation of HSCs via Smad7 up‐regulation, suggesting potential broad utility in treatment of diseases characterized by liver fibrosis.
What is already known
Activation of hepatic stellate cells is a key process in hepatic fibrosis.
Such activation involves stimulation of the TGF‐β/Smad2/3 pathway.
What this study adds
Praziquantel inhibits liver fibrosis by blocking activation of hepatic stellate cells.
This blockade involves the up‐regulation of Smad7.
What is the clinical significance
Praziquantel could have clinical application in the treatment of fibrotic diseases of the liver.
Abbreviations
- Arg1
arginase 1
- COL1A1
collagen type I α1
- ECM
extracellular matrix
- HSCs
hepatic stellate cells
- miR‐21
microRNA‐21
- p‐Smad2/3
phosphorylated Smad2/3
- qRT‐PCR
quantitative real‐time PCR
- α‐SMA
α‐smooth muscle actin
1. INTRODUCTION
Liver fibrosis refers to a wound‐healing response to liver damage, which can be due to various aetiologies such as toxic damage, chronic viral infection, chronic alcohol abuse, immunological attack, and parasitic disease (Kisseleva, 2017; Trappoliere et al., 2009). Liver fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) and hepatic stellate cells (HSCs) that are undergoing myofibroblast transition, which can be identified by the expression of α‐smooth muscle actin (α‐SMA; Hernandez‐Gea & Friedman, 2011). The quiescent HSCs undergo a remarkable morphological and functional change, that is, become activated, in response to unresolved liver damage (Higashi, Friedman, & Hoshida, 2017). HSCs can be activated by numerous growth factors and inflammatory cytokines, including http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=5039 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=5060. Among these, TGF‐β1 is the most effective cytokine for the transformation and proliferation of HSCs (Derynck & Zhang, 2003). It is acknowledged that HSCs are the main collagen‐producing cells during liver fibrosis and their activation has been demonstrated in the pathogenesis of liver fibrosis (Duran et al., 2016). Considering the vital role of activated HSCs in the development of liver fibrosis, inhibition of HSC activation can potentially aid in ameliorating liver fibrosis (Higashi et al., 2017; Oh et al., 2016). Therefore, inhibition of the activation and modulation of the function of HSCs is a primary therapeutic goal for the treatment of liver fibrosis.
The TGF‐β/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=303 plays a key role in liver fibrosis (Dufton et al., 2017; Inagaki & Okazaki, 2007). It is now clear that TGF‐β1 activates the downstream signalling pathway, promotes phosphorylation of Smad2 and Smad3, and binds to Smad4 into the nucleus to mediate fibrosis, which is negatively regulated by Smad7 through the ubiquitin–proteasome degradation pathway (Fukasawa et al., 2004; Lei et al., 2016). Studies show that Smad3 contributes to the liver fibrosis pathogenesis because Smad3 knockout mice do not develop dimethylnitrosamine‐induced liver fibrosis (Latella et al., 2009). In contrast, Smad7 has a protective role as knockout of Smad7 promotes HSC activation and liver fibrosis in vivo and in vitro and Smad7 overexpression protects against liver fibrosis (Dooley et al., 2003; Dooley et al., 2008; Feng et al., 2015). In addition, Smad7 was identified as a target of microRNA‐21 (miR‐21) in a study (Yuan et al., 2017), which showed that up‐regulated miR‐21 promoted the activation of Smad proteins via inhibiting Smad7 in HSCs, thereby increasing the expression of collagens (He et al., 2015; Yang et al., 2017). A better understanding of the mechanisms underlying the activation of the TGF‐β/Smad signalling in diseases associated with fibrosis is a critical step towards the development of specific and novel antifibrosis drugs.
Praziquantel is a schistosomicide with a good safety profile that has been used for more than 30 years (Fenwick, Savioli, Engels, Robert Bergquist, & Todd, 2003; Trainor‐Moss & Mutapi, 2016). Praziquantel has low toxicity and only mild side effects in animals (Sayasone et al., 2017). No obvious long‐term safety issues were found in humans, and its safety was also confirmed in both pregnant women and children (Allen, Crompton, de Silva, LoVerde, & Olds, 2002; Friedman, Olveda, Mirochnick, Bustinduy, & Elliott, 2018). In addition to its proven antihelminthic activity against flukes and tapeworms, recent studies showed that praziquantel could also directly affect the tissues and the cells of the host (Pinlaor et al., 2006). Praziquantel treatment for 8 weeks could inhibit the development of pulmonary granulomas induced by intravenous injection of Schistosoma japonicum eggs, suggesting that praziquantel might be an effective antifibrotic agent (Huang et al., 2011). We previously demonstrated that prolonged treatment with praziquantel ameliorated liver fibrosis in mice infected with S. japonicum (Liang et al., 2011); however, the underlying mechanisms remain largely unknown.
Using TGF‐β1‐stimulated LX‐2 cells in addition to other myofibroblast‐like cell line and fibroblast cell line, MES13 and NIH3T3, in vitro as well as a mouse model of CCl4‐induced liver fibrosis in vivo, the present study was designed to test the hypothesis that praziquantel ameliorated liver fibrosis by inhibiting TGF‐β/Smad signalling via up‐regulating Smad7 in HSCs.
2. METHODS
2.1. Ethics statement
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University (Approval No. IACUC‐1601159). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
2.2. Animals and reagents
Female BALB/c mice (6–8 weeks old and weighing 18–22 g, RRID:IMSR_JAX:000651), which were purchased from the Animal Core Facility of Nanjing Medical University, were housed in groups of five mice per cage in the specific pathogen‐free animal centre of Nanjing Medical University. Animals were acclimatized for 1 week prior to the experiment under controlled temperature (25 ± 3°C) and a reverse 12‐hr light–dark cycle. Animals had ad libitum access to water and food (standard laboratory chow) throughout the study. Praziquantel (Sigma‐Aldrich, St. Louis, MO, USA) was suspended in 1% carboxymethyl cellulose solution for in vivo treatments and suspended in DMSO for in vitro studies.
2.3. Mouse model of liver fibrosis
Liver fibrosis in mice was induced by a single intraperitoneal injection of CCl4 (1 ml·kg−1, diluted to 25% with olive oil) twice per week for 4 weeks. Mice were randomly divided into four groups: (a) control group (n = 5), (b) PZQ group (n = 5), (c) CCl4 group (n = 5), and (d) CCl4 + PZQ group (n = 5). The mice were given praziquantel (300 mg·kg−1 per 12 hr) by gastric gavage for 4 weeks, either alone or after the CCl4 administration. Then the mice were deeply anaesthetized with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5480 by intraperitoneal injection (3%, 10 ml·kg−1). The depth of anaesthesia was monitored by observation of slow breathing, loss of muscular tone and no response to surgical manipulation; mice were killed by cervical dislocation. The serum, cells, or livers were collected for corresponding analyses.
2.4. Liver function tests
Blood was collected from the inferior vena cava in anaesthetized mice. Aspartate transaminase and alanine transaminase levels were detected by an Olympus AU5400 automatic biochemistry analyser.
2.5. Isolation of hepatic stellate cells
HSCs were isolated from mice by a modified version of a previously described method (Mederacke, Dapito, Affo, Uchinami, & Schwabe, 2015). Briefly, the livers in situ were perfused first with an EGTA solution for 5 min through the hepatic portal vein followed by perfusion with an EGTA solution containing 0.05% pronase (Roche, Rotkreuz, Switzerland) and 0.04% type IV collagenase (Invitrogen, Carlsbad, CA, USA) for 15 min. Next, whole liver was homogenized using a scalpel and digested with an EGTA solution containing 0.05% pronase, 10 U·ml−1 DNase I (Sigma‐Aldrich, St. Louis, MO, USA) and 0.08% type IV collagenase for 30 min at 37°C. After a 10% Optiprep (Axis‐Shield PoC AS, Oslo, Norway) solution was used for density gradient centrifugation to isolate HSCs, the CD45− cells were sorted using CD45 microbeads following the manufacturer's instructions (Miltenyi Biotec, San Diego, CA, USA).
2.6. Histological and immunohistochemical assays
Liver tissues were fixed in 10% neutral buffered formalin solution followed by paraffin embedding. Seven‐micron‐thick tissue sections were stained with haematoxylin–eosin to assess inflammation and necrosis through the Ishak index score, which is a scoring system commonly used in liver fibrosis (Ishak et al., 1995).
Immunohistochemistry was performed using paraffin sections, which were incubated with the following primary antibodies: α‐SMA (1:200 dilution, Abcam, ab124964, RRID:AB_11129103), http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4898 (1:200 dilution, Abcam, ab34710, RRID:AB_731684), phosphorylated Smad2/3 (p‐Smad2/3; 1:200 dilution, Cell Signaling Technology, #8828, RRID:AB_2631089), and Smad7 (1:100 dilution, R&D, #293039, RRID:AB_2193479). Then, the sections were incubated with HRP‐conjugated secondary antibodies (1:1,000 dilution, Cell Signaling Technology, #7074, RRID:AB_2099233). The percentage of stained positive areas was quantified by Image‐Pro Plus 6 (Media Cybernetics, USA, RRID:SCR_007369). The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
2.7. Cell culture
LX‐2 cells (RRID:CVCL_5792) were purchased from Xiangya Medical College (Changsha, China). The mouse glomerular mesangial cell line MES13 (RRID:CVCL_5368) and the mouse embryonic fibroblasts cell line NIH3T3 (RRID:CVCL_0594) were gifts from Wen Qiu and Shen Yue at Nanjing Medical University. All cell lines were cultured in DMEM (Gibco, Carlsbad, CA, USA) with 10% FBS (Gibco, Carlsbad, CA, USA) supplemented with 100 mg·ml−1 streptomycin and 100 U·ml−1 penicillin (Invitrogen, Carlsbad, CA, USA) at 37°C in the presence of 5% CO2 in a tissue culture incubator.
The LX‐2, MES13, and NIH3T3 cells plated into 96‐well plates were incubated with different concentrations of praziquantel for 24 hr. The dose‐dependent cytotoxicity and IC50 of praziquantel were measured using Cell Counting Kit‐8 (CCK‐8, Dojindo, Japan) and Cytotoxicity Detection Kit (LDH, Roche, Switzerland).
Smad7 knockdown in LX‐2 cells was performed by transfecting an siRNA targeting Smad7 into LX‐2 cells. The siRNA targeting human Smad7 (5′‐GGUUUCUCCAUCAAGGCUUTT‐3′) and negative control (5′‐UUCUCCGAACGUGUCACGUTT‐3′) were synthesized by GenePharma (Shanghai, China). Cells were cultured in six‐well plates in DMEM without antibiotics and FBS (2 ml per well) and transfected with 3 μl of 10‐μM siRNA per well by using 9‐μl lipofectamine RNAiMAX (Invitrogen, #13778). Medium was changed to DMEM with 10% FBS after 6 hr. Another 18 hr later, the cells were treated and then harvested for quantitative real‐time PCR (qRT‐PCR) and Western blot analyses.
2.8. Immunofluorescence staining
The LX‐2 cells were challenged with TGF‐β1 (2.5 ng·ml−1) for 12 hr followed by treatment with praziquantel (30 μg·ml−1) for 24 hr. After incubation, the cells were fixed in 4% paraformaldehyde in PBS for 15 min and permeabilized with 0.2% Triton X‐100 in PBS for 30 min at room temperature. Non‐specific binding sites were blocked by incubating the cells with 2% BSA for 1 hr. The fixed cells were incubated with a primary antibody against α‐SMA (1:200 dilution), COL1A1 (1:500 dilution), or Smad7 (1:100 dilution) overnight at 4°C, followed by incubation with a fluorescein‐labelled secondary antibody for 1 hr. The cellular nuclei were stained with DAPI for 10 min. All samples were imaged with a fluorescence microscope (Zeiss, Oberkochen, Germany) and the AxioVision Rel. 4.8 software (Zeiss, RRID:SCR_002677).
2.9. Western blotting
Total protein from isolated primary HSCs and the three cell lines were separated on 10% gels by SDS‐PAGE and transferred to PVDF membranes. The membranes were incubated with the following primary antibodies: α‐SMA (1:2,000 dilution, Abcam, ab124964), COL1A1 (1:1,000 dilution, Abcam, ab34710), p‐Smad2/3 (1:1,000 dilution, Cell Signaling Technology, #8828), Smad7 (1:1,000 dilution, R&D, #293039), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1244 (Arg1; 1:1,000 dilution, Abcam, ab133543), and GAPDH (1:2,000 dilution, Cell Signaling Technology, #5174, AB_10622025). The membranes were then incubated with an HRP‐conjugated secondary antibody (1:1,000 dilution, Cell Signaling Technology, #7074), developed by enhanced chemiluminescence (Merck Millipore). Images were acquired using ChemiDoc™ Touch Imaging System (Bio‐Rad, California, USA) and quantified by the Image Lab analysis software version 4.1 (Bio‐Rad, California, USA).
2.10. Quantitative real‐time PCR
RNA from the three cell lines was extracted using TRIzol® according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed into cDNA using PrimeScript RT Master Mix (Takara Bio, Japan, #RR036A). Primer sequences are listed in Tables 1 and 2. Gene expression was calculated using the ΔΔCT method normalized against β‐actin.
Table 1.
Human primers for quantitative real‐time PCR analysis
| Name | Sequence (5′ → 3′) | |
|---|---|---|
| β‐Actin | Forward primer | CATGTACGTTGCTATCCAGGC |
| Reverse primer | CTCCTTAATGTCACGCACGAT | |
| α‐SMA | Forward primer | CAGGGCTGTTTTCCCATCCAT |
| Reverse primer | GCCATGTTCTATCGGGTACTTC | |
| Col1α1 | Forward primer | GTCGAGGGCCAAGACGAAG |
| Reverse primer | CAGATCACGTCATCGCACAAC | |
| Arg1 | Forward primer | GTGGAAACTTGCATGGACAAC |
| Reverse primer | AATCCTGGCACATCGGGAATC | |
| Smad3 | Forward primer | CCATCTCCTACTACGAGCTGAA |
| Reverse primer | CACTGCTGCATTCCTGTTGAC | |
| Smad4 | Forward primer | CCACCAAGTAATCGTGCATCG |
| Reverse primer | TGGTAGCATTAGACTCAGATGGG | |
| Smad7 | Forward primer | GGACAGCTCAATTCGGACAAC |
| Reverse primer | GTACACCCACACACCATCCAC | |
Table 2.
Mouse primers for quantitative real‐time PCR analysis
| Name | Sequence (5′ → 3′) | |
|---|---|---|
| β‐Actin | Forward primer | TGGAAAGCTGTGGCGTGAT |
| Reverse primer | TGCTTCACCACCTTCTTGAT | |
| α‐SMA | Forward primer | TCAGCGCCTCCAGTTCCT |
| Reverse primer | AAAAAAAACCACGAGTAACAAATCAA | |
| Col1α1 | Forward primer | ACGTCCTGGTGAAGTTGGTC |
| Reverse primer | CAGGGAAGCCTCTTTCTCCT | |
| Arg1 | Forward primer | TGCTCACACTGACATCAACAC |
| Reverse primer | CCTGGTACATCTGGGAACTTTC | |
| Smad7 | Forward primer | GTGTTGCTGTGAATCTTACG |
| Reverse primer | AGAAGAAGTTGGGAATCTGA | |
2.11. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All data were presented as means ± SEM. GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA, RRID:SCR_002798) was used for statistical analysis of the data. Statistical analysis for multiple groups was performed by one‐way ANOVA followed by post hoc Tukey's tests if F achieved statistical significance (P < .05) and there was no significant variance in homogeneity. A value of P < .05 was considered significant. No data were excluded from any of the studies.
2.12. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. Praziquantel ameliorates CCl4‐induced functional and histological damage in the liver
To explore the role of praziquantel in the progression of liver fibrosis, we established a mouse model of liver fibrosis by administering CCl4 intraperitoneally to BALB/c mice twice per week for 4 weeks and collected tissues after the final intragastric praziquantel treatment at a dosage of 300 mg·kg−1 per 12 hr (Figure 1a). We observed that the liver surface in the CCl4 group was significantly rougher and more nodular compared with the control group, whereas the liver surface in CCl4‐administered mice treated with praziquantel was significantly improved (Figure 1b). In addition, praziquantel treatment had no effect on the appearance of the liver in healthy mice (Figure 1b). The CCl4 group exhibited severe liver fibrosis as demonstrated by the development of severe liver damage with thick fibrotic septa and pseudolobular formation as well as the significantly higher Ishak index score of inflammation compared with the control group (Figure 1c,d). Serum alanine transaminase and aspartate transaminase levels were also significantly higher in the CCl4 group compared with the control group (Figure 1e). In contrast, the praziquantel treatment resulted in a decrease in histological and functional liver damage in CCl4‐treated mice. Moreover, the results also showed that praziquantel had no effect on healthy mice.
Figure 1.
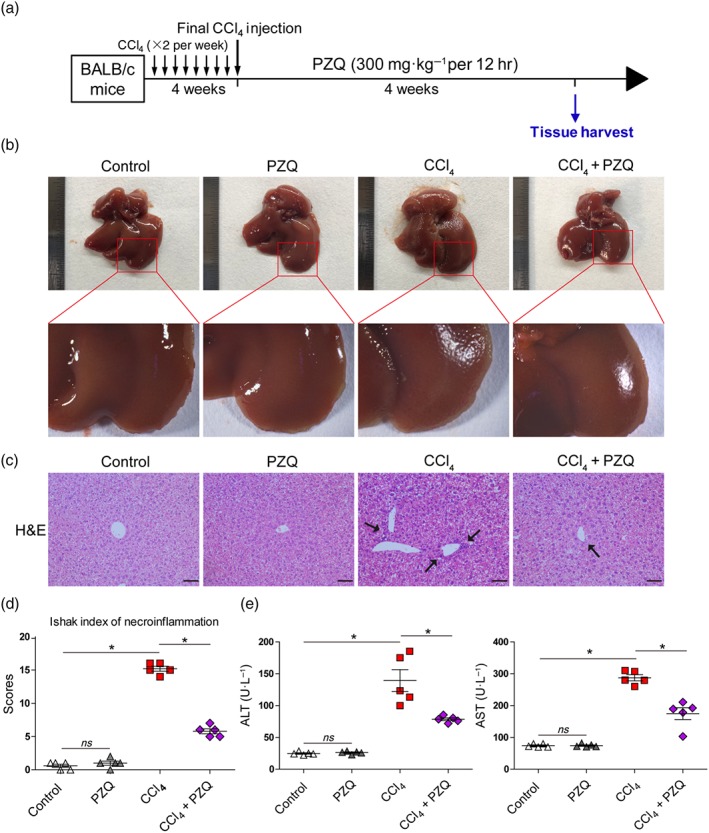
Praziquantel (PZQ) ameliorates functional and histological damage in CCl4‐induced liver injury. (a) Schematic representation of the liver fibrosis model in BALB/c mice induced by intraperitoneal CCl4 (1 ml·kg−1, diluted to 25% with olive oil) that was administered twice a week for 4 weeks, which was followed by intragastric administration of praziquantel (300 mg·kg−1 per 12 hr) for 4 weeks. The livers were harvested after the final administration. (b) Gross examination of the liver in mice with liver fibrosis that did or did not receive praziquantel. The ruler is on the left side of the diagram (n = 5). (c) Representative images of the liver sections stained with haematoxylin and eosin (H&E; arrows show examples of lobular inflammation). Scale bar, 100 μm. (d) Quantification of inflammatory changes according to the Ishak index scoring (n = 5). (e) Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels after the final praziquantel administration (n = 5). All data are presented as means ± SEM. *P < .05, significantly different as indicated; ns, non‐significant
3.2. Praziquantel reduces CCl4‐induced liver fibrosis by targeting HSCs in vivo
To further clarify the protective effect of praziquantel in the mouse model of liver fibrosis, we analysed the expression of COL1A1 and α‐SMA by immunohistochemistry. As shown in Figure 2, we found that the deposition of intrahepatic COL1A1 was increased in the mice with liver fibrosis after the administration of CCl4 for 4 weeks, whereas treatment with praziquantel attenuated collagen accumulation in the liver as compared with the CCl4 group (Figure 2a). This finding was further confirmed by quantification of the COL1A1‐immunopositive areas (Figure 2b), indicating that mice with liver fibrosis treated with praziquantel exhibited significantly reduced collagen deposition in the liver compared with the CCl4 group. We also assessed the expression levels of the HSCs activation marker α‐SMA and found that the addition of praziquantel was capable of blocking α‐SMA‐positive cell accumulation along the fibrotic septa compared with the CCl4 group (Figure 2a), which was confirmed by quantification of α‐SMA‐positive cell area (Figure 2b). Meanwhile, praziquantel had no effect on the expression of COL1A1 and α‐SMA in the liver in healthy mice (Figure 2a,b).
Figure 2.
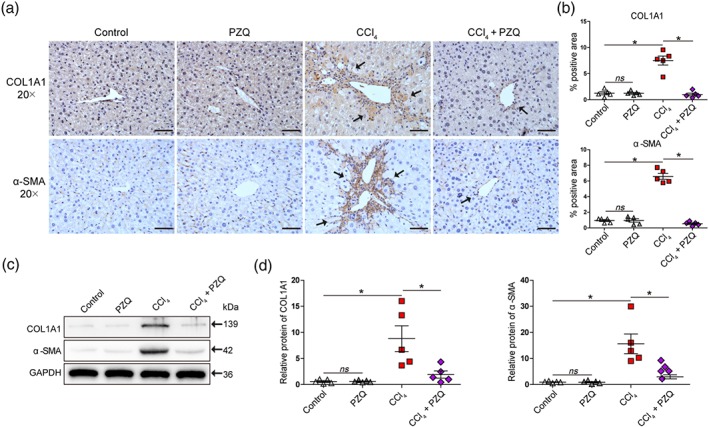
Praziquantel (PZQ) ameliorates CCl4‐induced liver fibrosis by targeting hepatic stellate cells (HSCs) in vivo. (a) Histological characterization of liver fibrosis and myofibroblast activation by immunohistochemistry for collagen type I α1 (COL1A1) and α‐smooth muscle actin (α‐SMA). Scale bar, 100 μm. (b) Quantification of histological changes in different treatment groups with positive area using image analysis software (n = 5). (c) HSCs were purified from the mice with liver fibrosis and with or without praziquantel treatment. Western blot analysis for COL1A1, α‐SMA, and a loading control (GAPDH) protein levels in HSCs. (d) Quantitative analysis of COL1A1, α‐SMA, and GAPDH expression (n = 5). All data are presented as means ± SEM. *P < .05; significantly different as indicated; ns, non‐significant
Liver fibrosis is accompanied by the activation of HSCs (Tsuchida & Friedman, 2017), which lead to increased extracellular matrix production and decreased degradation and remodelling. Therefore, we isolated primary HSCs to further explore the mechanism of praziquantel‐mediated suppression of liver fibrosis. First, we analysed HSCs markers of GFAP and desmin to guarantee the specific isolations of HSCs by flow cytometry (Ichikawa, Mucida, Tyznik, Kronenberg, & Cheroutre, 2011). It was showed that the GFAP+ and desmin+ cells were about 96% (Figure S1a,b). Then, the HSCs purified from the livers of healthy mice were incubated with different concentrations of praziquantel for 24 hr. As shown in Figure S2a, praziquantel at dose under 40 μg·ml−1 did not cause significant cytotoxicity as determined by CCK‐8 assay and increasing LDH release. Last, we analysed apoptosis and cell cycle in HSCs isolated from healthy mice with or without praziquantel treatment using flow cytometry and determined that praziquantel at the dose of 300 mg·kg−1 per 12 hr did not increase apoptosis or suppress cell cycle in HSCs. As shown in Figure 2c,d, Western blot analysis showed that the CCl4‐induced up‐regulation of COL1A1 and α‐SMA protein levels was significantly reduced in the CCl4 mice treated with PZQ. Moreover, the results also showed that praziquantel had no effect on HSCs in healthy mice. These results indicated that PZQ‐mediated inhibition of HSCs activation might be the potential mechanism underlying its liver fibrosis‐mitigating effect in vivo.
3.3. Praziquantel suppresses HSCs activation by inhibiting TGF‐β/Smad signalling in vivo
As TGF‐β/Smad signalling plays a key contributory role in HSCs activation and ECM production that determine liver fibrosis (Yang et al., 2013), we next explored the mechanisms by which praziquantel inhibited HSCs activation by investigating the TGF‐β/Smad signalling pathway. Indeed, by immunohistochemistry, we found that the expression of p‐Smad2/3 was significantly higher and the expression of Smad7 was significantly lower in the livers of CCl4‐treated mice than that in the untreated animals, which were reversed in the livers of CCl4‐treated mice treated with praziquantel (Figure 3a). The results were confirmed by quantification of the areas positive for p‐Smad2/3 and Smad7 (Figure 3b). Furthermore, we detected the inhibitory effect of praziquantel on the TGF‐β/Smad signalling pathway in primary HSCs. As shown in Figure 3c,d, Western blot analysis revealed that the CCl4‐induced up‐regulation of p‐Smad2/3 and down‐regulation of Smad7 at protein levels were reversed in the CCl4‐treated mice that were treated with praziquantel. Together, these results suggested that praziquantel reduced the phosphorylation of Smad2/3 and increased the Smad7 expression, thereby inhibiting the TGF‐β/Smad signalling pathway to suppress the HSCs activation.
Figure 3.
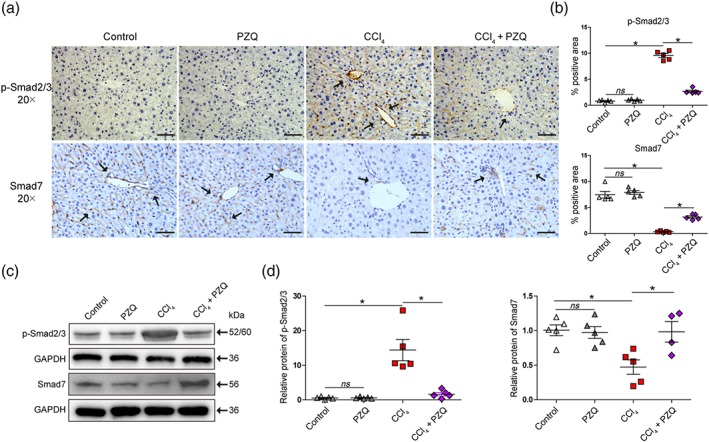
Praziquantel (PZQ) suppresses hepatic stellate cells (HSCs) activation by inhibiting TGF‐β/Smad signalling pathway in vivo. (a) Immunohistochemistry for phosphorylated Smad2/3 (p‐Smad2/3) and Smad7 in liver sections. Scale bar, 100 μm. (b) Quantification of p‐Smad2/3 and Smad7 expression in different treatment groups with positive area by image analysis software (n = 5). (c) HSCs were purified from the mice of liver fibrosis that did or did not receive praziquantel treatment. Western blot analysis for p‐Smad2/3, Smad7, and a loading control (GAPDH) protein levels in HSCs. (d) Quantitative analysis of p‐Smad2/3, Smad7, and GAPDH expression (n = 5). All data are presented as means ± SEM. *P < .05; significantly different as indicated; ns, non‐significant
3.4. TGF‐β/Smad signalling mediates the inhibitory effect of HSCs activation by praziquantel in vitro
To further investigate whether the inhibitory effect of praziquantel on fibrogenesis was due to the suppression of HSCs activation through the arrest of the TGF‐β/Smad signalling pathway, we examined the effect of praziquantel on the LX‐2 cell line in vitro. We first determined the dose of praziquantel that did not cause cytotoxicity in LX‐2 cells in vitro. As shown in Figure S3a, praziquantel at doses over 40 μg·ml−1 caused significant cytotoxicity by inhibiting LX‐2 cell proliferation as determined by the CCK‐8 assay and increasing LDH release. In contrast, there was no detectable cytotoxicity at praziquantel doses at and below 30 μg·ml−1. Thus, praziquantel at 20 and 30 μg·ml−1 was used to examine its inhibitory effect on TGF‐β1‐induced HSCs activation and ECM production in vitro. To further clarify whether praziquantel at 30 μg·ml−1 could reduce the number of HSCs and prevent the development of liver fibrosis, we analysed apoptosis and cell cycle in PZQ‐treated HSCs using flow cytometry and determined that praziquantel at the safe dose of 30 μg·ml−1 did not increase apoptosis or suppress cell cycle in LX‐2 cells (Figure S4a–d).
As shown in Figure 4, qRT‐PCR detections showed that the expression of COL1A1 and α‐SMA genes associated with activation of HSCs was significantly up‐regulated in TGF‐β1‐treated LX‐2 cells, and this effect was reversed after praziquantel treatment (Figure 4a). Meanwhile, praziquantel had no effect on the expression of COL1A1 and α‐SMA genes in LX‐2 cells (Figure 4a). Furthermore, Western blot analysis demonstrated that praziquantel presented the inhibitory effects on the TGF‐β1‐induced expression of COL1A1 and α‐SMA proteins in LX‐2 cells (Figure 4b,c). Similar results were found at the protein level as demonstrated by immunostaining with anti‐COL1A1 and anti‐α‐SMA antibodies of the LX‐2 cells (Figure 4d).
Figure 4.
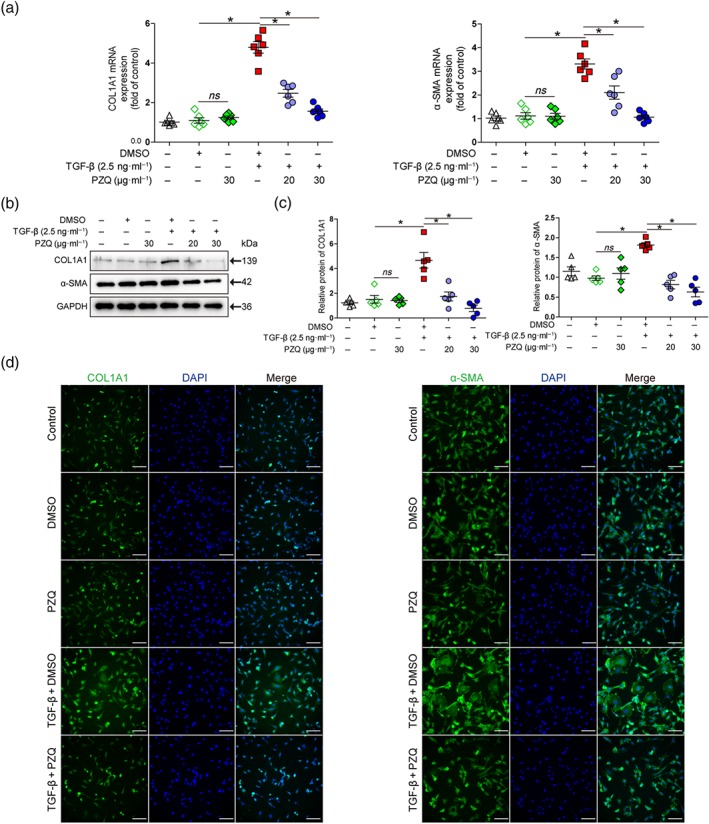
Praziquantel (PZQ) inhibits TGF‐β1‐induced hepatic stellate cells activation in vitro. (a) Quantitative real‐time PCR analysis of collagen type I α1 (COL1A1) and α‐smooth muscle actin (α‐SMA) mRNA expression in LX‐2 cells. The ΔΔCt method was used to quantify relative changes (n = 6). (b) Western blot for COL1A1, α‐SMA, and a loading control (GAPDH) protein levels in LX‐2 cells. (c) Quantitative analysis of COL1A1, α‐SMA, and GAPDH expression (n = 5). (d) Immunofluorescence staining of LX‐2 cells with antibodies against COL1A1 and α‐SMA. Images are captured with confocal microscopy (n = 5). Scale bar, 100 μm. All data are presented as means ± SEM. *P < .05, significantly different as indicated; ns, non‐significant
We next explored the mechanism underlying PZQ‐mediated inhibition of LX‐2 cells activation by blocking the TGF‐β/Smad signalling pathway in vitro. As shown in Figure 5, qRT‐PCR analysis revealed that praziquantel significantly reduced the mRNA level of Smad3 and increased that of Smad7 compared with the TGF‐β1‐induced LX‐2 cells, while no significant effect of praziquantel was observed on levels of Smad4 (Figure 5a). In addition, we observed that the protein level of p‐Smad2/3 was reduced and that of Smad7 was increased in LX‐2 cells when cotreated with praziquantel and TGF‐β1 compared with the TGF‐β1‐induced LX‐2 cells by Western blotting (Figure 5b,c). Furthermore, we found that incubation of the TGF‐β1‐induced LX‐2 cells with 30 μg·ml−1 praziquantel significantly induced the Smad7 expression as shown by immunostaining (Figure 5d).
Figure 5.

Praziquantel (PZQ) inhibits TGF‐β/Smad signalling in LX‐2 cells in vitro. (a) Quantitative real‐time PCR analysis of Smad3, Smad4, and Smad7 mRNA expression in LX‐2 cells. The ΔΔCt method was used to quantify relative changes (n = 6). (b) Western blot for phosphorylated Smad2/3 (p‐Smad2/3), Smad7, and a loading control (GAPDH) protein levels in LX‐2 cells. (c) Quantitative analysis of p‐Smad2/3, Smad7, and GAPDH expression (n = 5). (d) Immunofluorescence staining of LX‐2 cells with an antibody against Smad7, captured using confocal microscopy (n = 5). Scale bar, 100 μm. All data are presented as means ± SEM. *P < .05, significantly different as indicated; ns, non‐significant. ns P > .05
3.5. Praziquantel suppresses the activation of fibroblasts by inhibiting the TGF‐β/Smad signalling pathway
We further determined whether our findings showing that praziquantel could suppress HSCs activation via the inhibition of the TGF‐β/Smad signalling pathway did occur in myofibroblast‐like cells and fibroblasts. To this end, we used MES13 and NIH3T3 cells. We first determined the dose of praziquantel that did not cause cytotoxicity in MES13 and NIH3T3 cells in vitro. As shown in Figure S3b,c, praziquantel only at doses over 160 μg·ml−1 caused significant cytotoxicity by inhibiting MES13 and NIH3T3 cell proliferation as determined by CCK‐8 assay and increasing LDH release. Thus, praziquantel at 20 and 30 μg·ml−1 was used to examine its inhibitory effect on TGF‐β1‐induced MES13 and NIH3T3 cells. As shown in Figure 6, addition of praziquantel at doses of 20–30 μg·ml−1 significantly inhibited the TGF‐β1‐induced the COL1A1 and α‐SMA mRNA levels and increased the Smad7 mRNA expression by qRT‐PCR analysis in MES13 and NIH3T3 cells (Figure 6a,d). Similar changes were observed at the protein levels of COL1A1 and α‐SMA by Western blot assay (Figure 6b,c,e,f). In addition, based on recent studies providing strong evidence that Arg1 plays a critical role in collagen synthesis in fibroblasts, which is regulated by TGF‐β/Smad signalling (Ganji, Roshan, Varasteh, Moghadam, & Sankian, 2015; Kitowska et al., 2008), we investigated the effect of praziquantel on Arg1 and Smad7 expression to further verify the inhibition of the TGF‐β/Smad signalling pathway by PZQ. We detected that treatment of LX‐2, MES13, and NIH3T3 cells with praziquantel at 30 μg·ml−1 led to a significant reduction in Arg1 expression and a significant increase in Smad7 expression by both qRT‐PCR and Western blot assay (Figure S5a,b). Altogether, these results suggested that the effect of praziquantel on HSCs was also present in other fibroblast‐like cells and fibroblasts.
Figure 6.
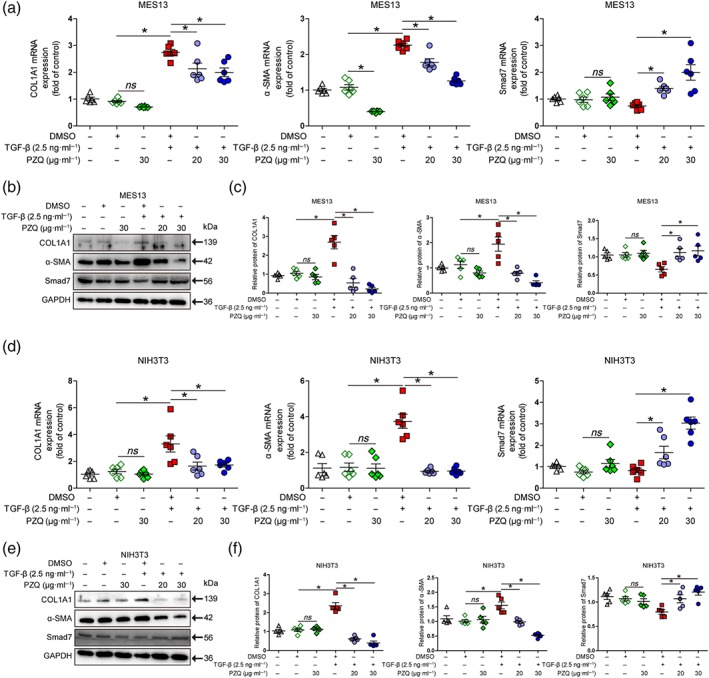
Praziquantel (PZQ) suppresses the activation of other fibroblast‐like cell or fibroblast by inhibiting the TGF‐β/Smad signalling pathway. (a, d) Quantitative real‐time PCR analysis of collagen type I α1 (COL1A1), α‐smooth muscle actin (α‐SMA), and Smad7 mRNA expression in MES13 and NIH3T3 cells. The ΔΔCt method was used to quantify relative changes (n = 6). (b, e) Western blot for COL1A1, α‐SMA, Smad7, and a loading control (GAPDH) protein levels in MES13 and NIH3T3 cells. (c, f) Quantitative analysis of COL1A1, α‐SMA, Smad7, and GAPDH expression (n = 5). All data are presented as means ± SEM. *P < .05, significantly different as indicated; ns, non‐significant
3.6. Smad7 is a key target of praziquantel in the inhibition of the TGF‐β/Smad signalling pathway in HSCs
Smad7 is a well‐known antagonist of the TGF‐β/Smad signalling pathway (Dooley et al., 2008). Thus, we investigated the praziquantel‐induced up‐regulation of Smad7 during the suppression of HSCs activation in vitro by siRNA‐mediated knockdown of Smad7 in LX‐2 cells. To elucidate the detailed mechanism, we analysed the effect of Smad7 depletion on the inhibitory effect of praziquantel on TGF‐β1‐mediated induction of COL1A1 and α‐SMA expression. Western blot and qRT‐PCR analysis showed that the efficiency of Smad7 mRNA and protein knockdown by siRNA was about 70% in LX‐2 cells compared with that in normal cells and scrambled siRNA‐infected cells (Figure 7a–c). Next, we detected that depletion of Smad7 was able to prevent the inhibitory effect of praziquantel on TGF‐β1‐mediated elevation of COL1A1, α‐SMA, and p‐Smad2/3 expression at both the mRNA level by qRT‐PCR and the protein level by Western blot assay (Figure 7e–g). In addition, we evaluated whether praziquantel induced Smad7 expression by inhibiting miR‐21 production. We found that praziquantel did not change miR‐21 expression in LX‐2 cells cotreated with TGF‐β1 compared with the LX‐2 cells treated only with TGF‐β1 by qRT‐PCR at different time points (Figure 7d). Collectively, these results suggested that Smad7 was a direct target of praziquantel for the inhibition of HSCs activation via the TGF‐β/Smad signalling pathway.
Figure 7.
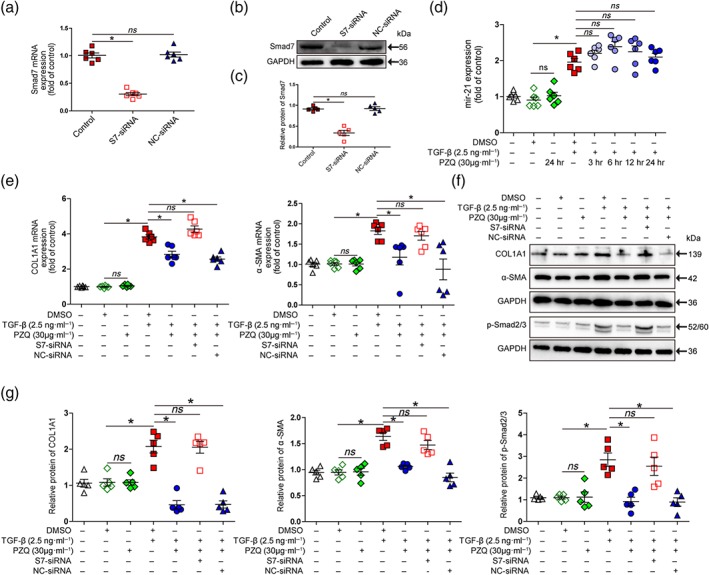
Praziquantel (PZQ) inhibits TGF‐β/Smad signalling pathway by up‐regulating Smad7 expression in hepatic stellate cells. (a) Quantitative real‐time PCR (qRT‐PCR) analysis to show the siRNA‐mediated reduction in Smad7 mRNA levels in LX‐2 cells (n = 6). (b, c) Western blot analysis to show the siRNA‐mediated reduction in Smad7 protein levels in LX‐2 cells (n = 5). (e) qRT‐PCR analysis of collagen type I α1 (COL1A1) and α‐smooth muscle actin (α‐SMA) mRNA expression in LX‐2 cells. The ΔΔCt method was used to quantify relative changes (n = 6). (f) Western blot for COL1A1, α‐SMA, phosphorylated Smad2/3 (p‐Smad2/3), and a loading control (GAPDH) protein levels in LX‐2 cells. (g) Quantitative analysis of COL1A1, α‐SMA, p‐Smad2/3, and GAPDH expression (n = 5). (d) qRT‐PCR analysis of miR‐21 expression in LX‐2 cells after treatment with praziquantel (30 μg·ml−1) at different time points. The ΔΔCt method was used to quantify relative changes (n = 6). All data are presented as means ± SEM. *P < .05, significantly different as indicated; ns, non‐significant
4. DISCUSSION
In recent years, drug repurposing has gained increased attention based on the efforts to shorten the development cycle, reduce risks, and improve the success rate during new drug development. Drug repurposing defines new indications or new uses of previously developed drugs. Due to the detailed information that is available on the pharmacokinetics and safety profiles of the approved drugs, their analysis and development for new purposes can be effectively and swiftly performed by phase II clinical trials (Cha et al., 2018). Approximately 40% of the research and development costs can be saved, and the development cycle can be shortened to 3 to 12 years (Chong & Sullivan, 2007; Sharlow, 2016). Therefore, drug repurposing is deemed to be one of the fastest and most effective strategies in drug discovery (Mercorelli, Palu, & Loregian, 2018; Panchapakesan & Pollock, 2018).
Praziquantel is a broad‐spectrum antihelminthic drug developed in the 1970s (Fenwick et al., 2003), which is not only effective for five human schistosomiasis types but also has favourable features of a good curative effect, minimal adverse reactions, and convenience of use. Therefore, praziquantel is recommended by the World Health Organization as the drug of choice for treating human schistosomiasis (1993). Since the development of praziquantel, several hypotheses on its mechanism of action have been proposed but the exact mechanism has not yet been fully elucidated. The most prominent theory suggests that praziquantel leads to an increase in calcium ion levels in schistosomes, which causes damage to the tegument and death of the worms (Chan et al., 2017). In addition, the schistosomicidal effect of praziquantel is associated with immune dependence and immune synergy (Ribeiro, Mello, Tavares, Kusel, & Coelho, 2004). When used as an adjuvant to the surface antigen of the hepatitis B virus DNA vaccine pcD‐S2, praziquantel could trigger T‐cell proliferation, surface antigen of the hepatitis B virus‐specific antibody response, and antigen‐specific cytotoxic T‐cell‐mediated immune response. The inhibition of TGF‐β expression and the TGF‐β/Smad signalling pathway suggests that praziquantel can enhance humoral and cellular immunity of the pcD‐S2 vaccine by inhibiting the TGF‐β/Smad signalling pathway (Zou et al., 2010). These studies revealed that the schistosomicidal effect of praziquantel depended on the host immunity. More importantly, schistosomiasis‐induced liver fibrosis was effectively ameliorated by praziquantel treatment aimed at killing the schistosomes (Berhe, Myrvang, & Gundersen, 2008; el‐Badrawy et al., 1988; Singh, Gerard, Hudson, & Boros, 2004). However, the antifibrotic effect of praziquantel was attributed to the self‐repair after the death of the schistosomes. We previously demonstrated that, in addition to its antihelmintic activity, prolonged treatment with praziquantel could suppress the development of liver fibrosis in mice infected with S. japonicum (Liang et al., 2011). In the current study, we elucidated the exact mechanism of the praziquantel‐mediated amelioration of liver fibrosis and focused on the effects of praziquantel on the TGF‐β/Smad signalling pathway in HSCs. Given that worm‐derived factors are the main drivers of liver fibrosis in schistosomiasis, we established the mouse model of CCl4‐induced liver fibrosis to avoid theeffects of the helminthicidal activity of praziquantel itself on liver fibrosis induced by S. japonicum infection. Thus, the current study provides a theoretical basis for the elucidation of a novel application of praziquantel as a drug for the treatment of clinical liver fibrosis.
Progression of liver fibrosis due to different aetiologies involves a common mechanism. Continuous activation of HSCs plays a crucial role in the development of liver fibrosis: Activated HSCs participate in the formation of liver fibrosis and the reconstruction of intrahepatic structures by proliferating and secreting ECM. Conversely, the intrahepatic sinus pressure is increased by cell contraction. These two changes are the ultimate pathological basis of liver fibrosis and portal hypertension (Thabut & Shah, 2010; Tsuchida & Friedman, 2017). In the present study, we detected that praziquantel administration at a safe dose significantly inhibited the activation of HSCs induced by CCl4 and decreased liver fibrosis in mice. The safe dose of praziquantel, 30 μg·ml−1, did not ameliorate liver fibrosis by increasing apoptosis or suppressing the cell cycle, as determined in LX‐2 cells (Figure S4a–d). In addition, praziquantel treatment was able to inhibit TGF‐β1‐induced HSCs activation as determined by α‐SMA and COL1A1 expression in LX‐2 cells. More importantly, up‐regulating Smad7, thereby blocking the TGF‐β/Smad pathway, was an underlying mechanism by which praziquantel ameliorated CCl4‐induced liver fibrosis in vivo and TGF‐β1‐induced HSCs activation in vitro. Moreover, we demonstrated that this mechanism of action of praziquantel, which was independent of miR‐21 expression, was observed in LX‐2 cells. Furthermore, in agreement with recent studies presenting strong evidence for Arg1 as a critical player in collagen synthesis in fibroblasts that is regulated by TGF‐β/Smad signalling (Ganji et al., 2015; Kitowska et al., 2008), we found that praziquantel decreased Arg1 expression by improving Smad7 expression, further confirming that praziquantel inhibited HSCs activation by up‐regulating Smad7. All together, these findings demonstrated a new pharmacological action of praziquantel on regulating TGF‐β/Smad signalling pathway.
The elucidation of Smad7 up‐regulation by praziquantel that blocked the TGF‐β/Smad to inhibit HSCs was an important finding of the current study. Although the mechanism of TGF‐β/Smad‐mediated liver fibrosis is well known, the development of therapeutic drugs that target this pathway directly should be explored further. The present study demonstrated that treatment with praziquantel could up‐regulate Smad7 expression in HSCs, thereby blocking TGF‐β/Smad pathway in CCl4‐induced liver fibrosis in vivo and in TGF‐β1‐activated HSCs in vitro, indicating that up‐regulation of Smad7, thereby restoring the balance in TGF‐β/Smad signalling, might be a critical mechanism by which praziquantel ameliorates liver fibrosis, which was indeed confirmed by knocking down Smad7 that blocked PZQ‐mediated attenuation of TGF‐β1‐induced activation and fibrosis in HSCs in vitro.
In conclusion, we propose a mechanism for the involvement of the TGF‐β/Smad signalling pathway in the inhibition of HSCs activation by praziquantel in vivo and in vitro (Figure 8). Briefly, praziquantel can induce the expression of Smad7 in HSCs, thereby inhibiting the activation of TGF‐β/Smad signalling, which is an underlying mechanism of praziquantel ‐mediated protection against liver fibrosis. Since the activation and collagen production of HSCs is the common mechanism of hepatic fibrosis derived from various causes, the present study also indicates a potential prospect of clinical application of praziquantel in the treatment of chronic fibrotic diseases of liver.
Figure 8.
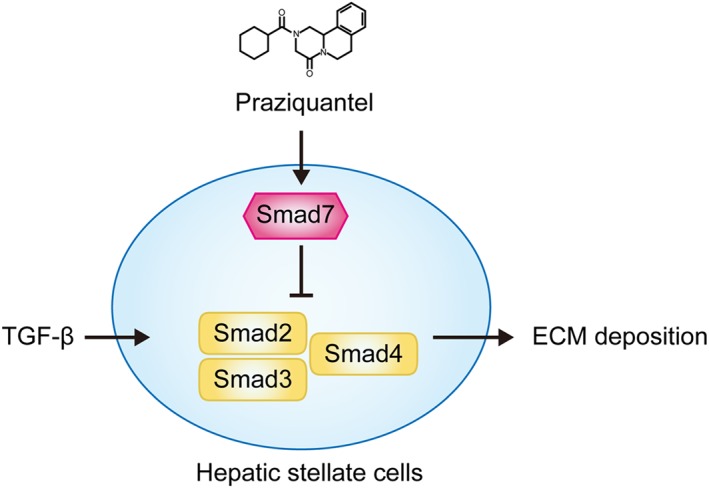
Schematic diagram of the antifibrotic effect of praziquantel in inhibition of hepatic stellate cells activation. Praziquantel increased the expression of Smad7 that inhibited Smad2/3 phosphorylation to inhibit the TGF‐β/Smad signalling. This can further result in the inhibition of HSCs activation and extracellular matrix (ECM) production
AUTHOR CONTRIBUTIONS
J.L. conceived and performed the experiments, analysed the results, and wrote the manuscript. D.K., C.Z., Y.X., and Z.L. performed the experiments. J.Q., X.L., and R.Z. assisted with experimental design and data interpretation. Y.W. was awarded funding to support the study and conceived and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206 and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1. Detection of HSCs
(A, B) HSCs were stained for GFAP and intracellular desmin and analysed by flow cytometry.
Figure S2. Cytotoxicity of PZQ in HSCs isolated from healthy mice
(A) Dose‐dependent cytotoxicity of PZQ in HSCs isolated from healthy mice by cell counting kit‐8 (CCK‐8) and lactate dehydrogenase (LDH) release assays (n = 6). (B) HSCs were purified from the healthy mice with or without PZQ (300 mg·kg−1·12 hours−1) treatment for 4 weeks. The rate of apoptosis was evaluated by flow cytometry. The cells in early apoptosis (Annexin+7/AAD−) are in the lower right quadrant. (C) Quantification of apoptotic rate in HSCs (n = 6). (D) The cell cycle distribution of HSCs was assessed by flow cytometry. (E) Quantification of the cell cycle distribution in HSCs (n = 6). All data are presented means ± SEM. * P < 0.05, ns P > 0.05.
Figure S3. Dose curve to determine the safe dose of PZQ in LX‐2, MES13 and NIH3T3 cells.
(A, B, C) Dose‐dependent cytotoxicity of PZQ in LX‐2, MES13 and NIH3T3 cells by cell counting kit‐8 (CCK‐8) and lactate dehydrogenase (LDH) release assays (n = 6). All data are presented as means ± SEM. * P < 0.05.
Figure S4. PZQ does not induce apoptosis or inhibit cell cycle in LX‐2 cells.
(A) LX‐2 cells were treated with PZQ (30 μg·ml−1) for 24 h. The rate of apoptosis was evaluated by flow cytometry. The cells in early apoptosis (Annexin+7/AAD−) are in the lower right quadrant. (C) Quantification of apoptotic rate in LX‐2 cells (n = 6). (B) The cell cycle distribution of LX‐2 cells was assessed by flow cytometry. (D) Quantification of the cell cycle distribution in LX‐2 cells (n = 6). All data are presented means ± SEM. ns P > 0.05.
Figure S5. PZQ inhibits Arg1 expression in fibroblast‐like cells or fibroblast.
(A) qRT‐PCR analysis of Arg1 mRNA expression in LX‐2, MES13 and NIH3T3 cells. The ΔΔCt method was used to quantify relative changes (n = 5). (B) Western blot for arginase 1 (Arg1), Smad7 and a loading control (GAPDH) protein levels in LX‐2, MES13 and NIH3T3 cells (n = 5). All data are presented as means ± SEM. * P < 0.05.
ACKNOWLEDGEMENTS
We are grateful to Dr Wen Qiu and Dr Shen Yue (Nanjing Medical University) for providing MES13 and NIH3T3 cell lines. This work was supported by the National Natural Science Foundation of China (81273234 and 81471573) and the Natural Science Foundation of Higher Education of Jiangsu Province (No. 11KJA330003) to Y.W.
Liu J, Kong D, Qiu J, et al. Praziquantel ameliorates CCl4‐induced liver fibrosis in mice by inhibiting TGF‐β/Smad signalling via up‐regulating Smad7 in hepatic stellate cells. Br J Pharmacol. 2019;176:4666–4680. 10.1111/bph.14831
REFERENCES
- (1993). Public health impact of schistosomiasis: Disease and mortality. WHO Expert Committee on the Control of Schistosomiasis. Bulletin of the World Health Organization, 71(6), 657–662. [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, H. E. , Crompton, D. W. , de Silva, N. , LoVerde, P. T. , & Olds, G. R. (2002). New policies for using anthelmintics in high risk groups. Trends in Parasitology, 18(9), 381–382. 10.1016/S1471-4922(02)02386-3 [DOI] [PubMed] [Google Scholar]
- Berhe, N. , Myrvang, B. , & Gundersen, S. G. (2008). Reversibility of schistosomal periportal thickening/fibrosis after praziquantel therapy: A twenty‐six month follow‐up study in Ethiopia. The American Journal of Tropical Medicine and Hygiene, 78(2), 228–234. 10.4269/ajtmh.2008.78.228 [DOI] [PubMed] [Google Scholar]
- Cha, Y. , Erez, T. , Reynolds, I. J. , Kumar, D. , Ross, J. , Koytiger, G. , … Laifenfeld, D. (2018). Drug repurposing from the perspective of pharmaceutical companies. British Journal of Pharmacology, 175(2), 168–180. 10.1111/bph.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. D. , Cupit, P. M. , Gunaratne, G. S. , McCorvy, J. D. , Yang, Y. , Stoltz, K. , … Marchant, J. S. (2017). The anthelmintic praziquantel is a human serotoninergic G‐protein‐coupled receptor ligand. Nature Communications, 8(1), 1910 10.1038/s41467-017-02084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, C. R. , & Sullivan, D. J. Jr. (2007). New uses for old drugs. Nature, 448(7154), 645–646. 10.1038/448645a [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck, R. , & Zhang, Y. E. (2003). Smad‐dependent and Smad‐independent pathways in TGF‐β family signalling. Nature, 425(6958), 577–584. 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- Dooley, S. , Hamzavi, J. , Breitkopf, K. , Wiercinska, E. , Said, H. M. , Lorenzen, J. , … Gressner, A. M. (2003). Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology, 125(1), 178–191. 10.1016/S0016-5085(03)00666-8 [DOI] [PubMed] [Google Scholar]
- Dooley, S. , Hamzavi, J. , Ciuclan, L. , Godoy, P. , Ilkavets, I. , Ehnert, S. , … Mertens, P. R. (2008). Hepatocyte‐specific Smad7 expression attenuates TGF‐β‐mediated fibrogenesis and protects against liver damage. Gastroenterology, 135(2), 642–659. 10.1053/j.gastro.2008.04.038 [DOI] [PubMed] [Google Scholar]
- Dufton, N. P. , Peghaire, C. R. , Osuna‐Almagro, L. , Raimondi, C. , Kalna, V. , Chuahan, A. , … Randi, A. M. (2017). Dynamic regulation of canonical TGFβ signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nature Communications, 8(1), 895 10.1038/s41467-017-01169-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran, A. , Hernandez, E. D. , Reina‐Campos, M. , Castilla, E. A. , Subramaniam, S. , Raghunandan, S. , … Moscat, J. (2016). p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell, 30(4), 595–609. 10.1016/j.ccell.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el‐Badrawy, N. M. , Hassanein, H. I. , Botros, S. S. , Nagy, F. M. , Abdallah, N. M. , & Herbage, D. (1988). Effect of praziquantel on hepatic fibrosis in experimental Schistosomiasis mansoni . Experimental and Molecular Pathology, 49(2), 151–160. 10.1016/0014-4800(88)90029-9 [DOI] [PubMed] [Google Scholar]
- Feng, T. , Dzieran, J. , Gu, X. , Marhenke, S. , Vogel, A. , Machida, K. , … Meindl‐Beinker, N. M. (2015). Smad7 regulates compensatory hepatocyte proliferation in damaged mouse liver and positively relates to better clinical outcome in human hepatocellular carcinoma. Clinical Science, 128(11), 761–774. 10.1042/CS20140606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick, A. , Savioli, L. , Engels, D. , Robert Bergquist, N. , & Todd, M. H. (2003). Drugs for the control of parasitic diseases: Current status and development in schistosomiasis. Trends in Parasitology, 19(11), 509–515. 10.1016/j.pt.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Friedman, J. F. , Olveda, R. M. , Mirochnick, M. H. , Bustinduy, A. L. , & Elliott, A. M. (2018). Praziquantel for the treatment of schistosomiasis during human pregnancy. Bulletin of the World Health Organization, 96(1), 59–65. 10.2471/BLT.17.198879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa, H. , Yamamoto, T. , Togawa, A. , Ohashi, N. , Fujigaki, Y. , Oda, T. , … Hishida, A. (2004). Down‐regulation of Smad7 expression by ubiquitin‐dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proceedings of the National Academy of Sciences of the United States of America, 101(23), 8687–8692. 10.1073/pnas.0400035101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji, A. , Roshan, H. M. , Varasteh, A. , Moghadam, M. , & Sankian, M. (2015). The effects of WW2/WW3 domains of Smurf2 molecule on TGF‐β signaling and arginase I gene expression. Cell Biology International, 39(6), 690–695. 10.1002/cbin.10446 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Xie, J. , Zhang, D. , Su, Q. , Sai, X. , Bai, R. , … Pan, W. (2015). Recombinant adeno‐associated virus‐mediated inhibition of microRNA‐21 protects mice against the lethal schistosome infection by repressing both IL‐13 and transforming growth factor beta 1 pathways. Hepatology, 61(6), 2008–2017. 10.1002/hep.27671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Gea, V. , & Friedman, S. L. (2011). Pathogenesis of liver fibrosis. Annual Review of Pathology, 6, 425–456. 10.1146/annurev-pathol-011110-130246 [DOI] [PubMed] [Google Scholar]
- Higashi, T. , Friedman, S. L. , & Hoshida, Y. (2017). Hepatic stellate cells as key target in liver fibrosis. Advanced Drug Delivery Reviews, 121, 27–42. 10.1016/j.addr.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. X. , Xu, Y. L. , Yu, C. X. , Li, H. J. , Yin, X. R. , Wang, T. S. , … Liang, Y. S. (2011). Effect of praziquantel prolonged administration on granuloma formation around Schistosoma japonicum eggs in lung of sensitized mice. Parasitology Research, 109(5), 1453–1459. 10.1007/s00436-011-2485-2 [DOI] [PubMed] [Google Scholar]
- Ichikawa, S. , Mucida, D. , Tyznik, A. J. , Kronenberg, M. , & Cheroutre, H. (2011). Hepatic stellate cells function as regulatory bystanders. Journal of Immunology, 186(10), 5549–5555. 10.4049/jimmunol.1003917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, Y. , & Okazaki, I. (2007). Emerging insights into transforming growth factor β Smad signal in hepatic fibrogenesis. Gut, 56(2), 284–292. 10.1136/gut.2005.088690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak, K. , Baptista, A. , Bianchi, L. , Callea, F. , De Groote, J. , Gudat, F. , … Thaler, H. (1995). Histological grading and staging of chronic hepatitis. Journal of Hepatology, 22(6), 696–699. 10.1016/0168-8278(95)80226-6 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. J. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Journal of Pharmacology & Pharmacotherapeutics, 1(2), 94–99. 10.4103/0976-500X.72351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva, T. (2017). The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology, 65(3), 1039–1043. 10.1002/hep.28948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitowska, K. , Zakrzewicz, D. , Konigshoff, M. , Chrobak, I. , Grimminger, F. , Seeger, W. , … Eickelberg, O. (2008). Functional role and species‐specific contribution of arginases in pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology, 294(1), L34–L45. 10.1152/ajplung.00007.2007 [DOI] [PubMed] [Google Scholar]
- Latella, G. , Vetuschi, A. , Sferra, R. , Catitti, V. , D'Angelo, A. , Zanninelli, G. , … Gaudio, E. (2009). Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine‐induced hepatic fibrosis in mice. Liver International: Official Journal of the International Association for the Study of the Liver, 29(7), 997–1009. 10.1111/j.1478-3231.2009.02011.x [DOI] [PubMed] [Google Scholar]
- Lei, X. F. , Fu, W. , Kim‐Kaneyama, J. R. , Omoto, T. , Miyazaki, T. , Li, B. , & Miyazaki, A. (2016). Hic‐5 deficiency attenuates the activation of hepatic stellate cells and liver fibrosis through upregulation of Smad7 in mice. Journal of Hepatology, 64(1), 110–117. 10.1016/j.jhep.2015.08.026 [DOI] [PubMed] [Google Scholar]
- Liang, Y. J. , Luo, J. , Yuan, Q. , Zheng, D. , Liu, Y. P. , Shi, L. , … Zhang, Z. S. (2011). New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum . PLoS ONE, 6(5), e20247 10.1371/journal.pone.0020247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederacke, I. , Dapito, D. H. , Affo, S. , Uchinami, H. , & Schwabe, R. F. (2015). High‐yield and high‐purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nature Protocols, 10(2), 305–315. 10.1038/nprot.2015.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercorelli, B. , Palu, G. , & Loregian, A. (2018). Drug repurposing for viral infectious diseases: How far are we? Trends in Microbiology., 26, 865–876. 10.1016/j.tim.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, Y. , Park, O. , Swierczewska, M. , Hamilton, J. P. , Park, J. S. , Kim, T. H. , … Lee, S. (2016). Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology, 64(1), 209–223. 10.1002/hep.28432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchapakesan, U. , & Pollock, C. (2018). Drug repurposing in kidney disease. Kidney International, 94(1), 40–48. 10.1016/j.kint.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Pinlaor, S. , Hiraku, Y. , Yongvanit, P. , Tada‐Oikawa, S. , Ma, N. , Pinlaor, P. , … Kawanishi, S. (2006). iNOS‐dependent DNA damage via NF‐κB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. International Journal of Cancer, 119(5), 1067–1072. 10.1002/ijc.21893 [DOI] [PubMed] [Google Scholar]
- Ribeiro, F. , Mello, R. T. , Tavares, C. A. , Kusel, J. R. , & Coelho, P. M. (2004). Synergistic action of praziquantel and host specific immune response against Schistosoma mansoni at different phases of infection. Revista Do Instituto de Medicina Tropical de Sao Paulo, 46(4), 231–233. 10.1590/S0036-46652004000400010 [DOI] [PubMed] [Google Scholar]
- Sayasone, S. , Meister, I. , Andrews, J. R. , Odermatt, P. , Vonghachack, Y. , Xayavong, S. , … Keiser, J. (2017). Efficacy and safety of praziquantel against light infections of Opisthorchis viverrini: A randomized parallel single‐blind dose‐ranging trial. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 64(4), 451–458. 10.1093/cid/ciw785 [DOI] [PubMed] [Google Scholar]
- Sharlow, E. R. (2016). Revisiting repurposing. Assay and Drug Development Technologies, 14(10), 554–556. 10.1089/adt.2016.766 [DOI] [PubMed] [Google Scholar]
- Singh, K. P. , Gerard, H. C. , Hudson, A. P. , & Boros, D. L. (2004). Expression of matrix metalloproteinases and their inhibitors during the resorption of schistosome egg‐induced fibrosis in praziquantel‐treated mice. Immunology, 111(3), 343–352. 10.1111/j.0019-2805.2004.01817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabut, D. , & Shah, V. (2010). Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: New targets for the treatment of portal hypertension? Journal of Hepatology, 53(5), 976–980. 10.1016/j.jhep.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Trainor‐Moss, S. , & Mutapi, F. (2016). Schistosomiasis therapeutics: Whats in the pipeline? Expert Review of Clinical Pharmacology, 9(2), 157–160. 10.1586/17512433.2015.1102051 [DOI] [PubMed] [Google Scholar]
- Trappoliere, M. , Caligiuri, A. , Schmid, M. , Bertolani, C. , Failli, P. , Vizzutti, F. , … Pinzani, M. (2009). Silybin, a component of sylimarin, exerts anti‐inflammatory and anti‐fibrogenic effects on human hepatic stellate cells. Journal of Hepatology, 50(6), 1102–1111. 10.1016/j.jhep.2009.02.023 [DOI] [PubMed] [Google Scholar]
- Tsuchida, T. , & Friedman, S. L. (2017). Mechanisms of hepatic stellate cell activation. Nature Reviews. Gastroenterology & Hepatology, 14(7), 397–411. 10.1038/nrgastro.2017.38 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Luo, L. , Zhu, Z. D. , Zhou, X. , Wang, Y. , Xue, J. , … Zhao, L. (2017). Chlorogenic acid inhibits liver fibrosis by blocking the miR‐21‐regulated TGF‐β1/Smad7 signaling pathway in vitro and in vivo. Frontiers in Pharmacology, 8, 929 10.3389/fphar.2017.00929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Inokuchi, S. , Roh, Y. S. , Song, J. , Loomba, R. , Park, E. J. , & Seki, E. (2013). Transforming growth factor‐β signaling in hepatocytes promotes hepatic fibrosis and carcinogenesis in mice with hepatocyte‐specific deletion of TAK1. Gastroenterology, 144(5), 1042–1054 e1044. 10.1053/j.gastro.2013.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, J. , Chen, H. , Ge, D. , Xu, Y. , Xu, H. , Yang, Y. , … Zhao, Y. (2017). Mir‐21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 42(6), 2207–2219. 10.1159/000479995 [DOI] [PubMed] [Google Scholar]
- Zou, Q. , Zhong, Y. , Su, H. , Kang, Y. , Jin, J. , Liu, Q. , … Wang, B. (2010). Enhancement of humoral and cellular responses to HBsAg DNA vaccination by immunization with praziquantel through inhibition TGF‐β/Smad2,3 signaling. Vaccine, 28(8), 2032–2038. 10.1016/j.vaccine.2009.10.101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Detection of HSCs
(A, B) HSCs were stained for GFAP and intracellular desmin and analysed by flow cytometry.
Figure S2. Cytotoxicity of PZQ in HSCs isolated from healthy mice
(A) Dose‐dependent cytotoxicity of PZQ in HSCs isolated from healthy mice by cell counting kit‐8 (CCK‐8) and lactate dehydrogenase (LDH) release assays (n = 6). (B) HSCs were purified from the healthy mice with or without PZQ (300 mg·kg−1·12 hours−1) treatment for 4 weeks. The rate of apoptosis was evaluated by flow cytometry. The cells in early apoptosis (Annexin+7/AAD−) are in the lower right quadrant. (C) Quantification of apoptotic rate in HSCs (n = 6). (D) The cell cycle distribution of HSCs was assessed by flow cytometry. (E) Quantification of the cell cycle distribution in HSCs (n = 6). All data are presented means ± SEM. * P < 0.05, ns P > 0.05.
Figure S3. Dose curve to determine the safe dose of PZQ in LX‐2, MES13 and NIH3T3 cells.
(A, B, C) Dose‐dependent cytotoxicity of PZQ in LX‐2, MES13 and NIH3T3 cells by cell counting kit‐8 (CCK‐8) and lactate dehydrogenase (LDH) release assays (n = 6). All data are presented as means ± SEM. * P < 0.05.
Figure S4. PZQ does not induce apoptosis or inhibit cell cycle in LX‐2 cells.
(A) LX‐2 cells were treated with PZQ (30 μg·ml−1) for 24 h. The rate of apoptosis was evaluated by flow cytometry. The cells in early apoptosis (Annexin+7/AAD−) are in the lower right quadrant. (C) Quantification of apoptotic rate in LX‐2 cells (n = 6). (B) The cell cycle distribution of LX‐2 cells was assessed by flow cytometry. (D) Quantification of the cell cycle distribution in LX‐2 cells (n = 6). All data are presented means ± SEM. ns P > 0.05.
Figure S5. PZQ inhibits Arg1 expression in fibroblast‐like cells or fibroblast.
(A) qRT‐PCR analysis of Arg1 mRNA expression in LX‐2, MES13 and NIH3T3 cells. The ΔΔCt method was used to quantify relative changes (n = 5). (B) Western blot for arginase 1 (Arg1), Smad7 and a loading control (GAPDH) protein levels in LX‐2, MES13 and NIH3T3 cells (n = 5). All data are presented as means ± SEM. * P < 0.05.


