Abstract
Background and Purpose
Renal fibrosis acts as the common pathway leading to the development of end‐stage renal disease. Previous studies have shown that resveratrol has anti‐fibrotic activity, but its potential molecular mechanisms of action are not well understood.
Experimental Approach
The anti‐fibrotic effects of resveratrol were assayed in a rat model of unilateral ureteral obstruction (UUO) in vivo and in fibroblasts and tubular epithelial cells (TECs) stimulated by TGF‐β1 in vitro. Gene and protein expression levels were analysed by PCR, Western blotting, and immunohistochemical staining.
Key Results
Resveratrol inhibits the myofibroblastic phenotype and fibrosis formation in UUO kidneys by targeting fibroblast–myofibroblast differentiation (FMD) and epithelial–mesenchymal transition (EMT). The anti‐fibrotic effects of resveratrol correlated with decreased proliferation of TECs in the interstitium and tubules, resulting in suppressed activity of the proliferation‐related signalling pathways, including that of the MAPK, PI3K/Akt, Wnt/β‐catenin, and JAK2/STAT3 pathways. Resveratrol treatment suppressed TGF‐β1‐induced FMD and the expression of the myofibroblastic phenotype in fibroblasts in vitro by antagonizing the activation of proliferation‐related signalling. Similarly, TGF‐β1‐mediated overactivation of the proliferation‐related signalling in TECs induced EMT, and the myofibroblastic phenotype was suppressed by resveratrol. The anti‐fibrotic and anti‐proliferative effects of resveratrol were associated with the inactivation of Smad2/3 signalling and resulted in a partial reversal of FMD and EMT and the inhibition of the myofibroblastic phenotype.
Conclusions and Implications
Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in vivo and in vitro via proliferation‐related pathways, making it a potential therapeutic agent for preventing renal fibrosis.
Abbreviations
- α‐SMA
α‐smooth muscle actin
- CKD
chronic kidney disease
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- FMD
fibroblast–myofibroblast differentiation
- TECs
tubular epithelial cells
- UUO
unilateral ureteral obstruction
What is already known
Resveratrol has anti‐fibrotic properties that limit fibroblast proliferation and myofibroblast differentiation.
What does this study add
Resveratrol inhibits the expression of myofibroblastic phenotype and attenuates fibrosis via the proliferation‐related signalling pathways.
What is the clinical significance
Resveratrol has potential as a therapeutic agent to prevent renal fibrosis.
1. INTRODUCTION
The incidence and prevalence of chronic kidney disease (CKD) is increasing worldwide. Tubulointerstitial fibrosis is considered the ultimate and most common pathway for all kinds of progressive CKDs to reach end‐stage renal failure (Nangaku, 2004). It is estimated that approximately 13% of the U.S. population has a CKD (Liu, 2011). Tubulointerstitial fibrosis is characterized by extracellular matrix (ECM) deposition in association with fibroblast accumulation and tubular epithelial cell loss (Ban & Twigg, 2008). Myofibroblasts expressing α‐smooth muscle actin (α‐SMA) are terminally differentiated cells and are responsible for the accumulation of ECM components such as type I and III collagen, which leads tubulointerstitial fibrosis in kidneys (Meran & Steadman, 2011). However, the origin and activation of myofibroblasts in the fibrotic kidney remain largely undefined and controversial (Loeffler & Wolf, 2015). In this regard, some studies have proposed that myofibroblasts may originate from tubular epithelial cells (TECs) that undergo the epithelial–mesenchymal transition (EMT; Barnes & Gorin, 2011; Baum & Duffy, 2011; Duffy, 2011; Kalluri & Weinberg, 2009). Others have suggested that resident interstitial fibroblasts may undergo fibroblast–myofibroblast differentiation (FMD) to contribute to the phenotypic appearance of myofibroblasts and fibrosis during kidney damage (LeBleu et al., 2013). Thus, new targets to inhibit the expression of the myofibroblastic phenotype and alleviate fibrosis through EMT and FMD are crucial in response to injury. During the fibrogenic phase, the major driving force behind FMD and EMT appears to be several profibrotic growth factors, including https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5060 (Kalluri & Weinberg, 2009; Zavadil & Bottinger, 2005). TGF‐β1 is the most ubiquitous profibrotic cytokine in progressive renal fibrosis, and its signals are transduced through Smad‐dependent and non‐Smad pathways, leading to multiple downstream biological effects (Kim & Choi, 2012; Lan & Chung, 2012). In kidney‐derived fibroblasts, TGF‐β1/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=303 signalling is triggered after injury and directly stimulates myofibroblasts to produce excessive ECM proteins (Liu et al., 2017). In TECs, the regulation of EMT via the TGF‐β1/Smad2/3 signal pathways may become a target for preventing progressive renal tubulointerstitial fibrosis (Lan, 2011; Meng et al., 2012). The number of interstitial myofibroblasts and fibroblasts is closely related to the severity of the fibrosis and the attendant decline of kidney function (Li & Wang, 2011; Strutz & Zeisberg, 2006). Taking into account all these factors, it is clear that understanding the mechanism of myofibroblast proliferation and activation is critical for developing novel treatments that suppress or halt the progression of CKD.
Previous studies have shown that the proliferation‐related signalling pathways, including https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285/https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=964/https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2048/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2994, and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519, are involved in myofibroblast transformation and fibrosis in various diseases (Gao et al., 2016; Liu et al., 2017; Tan, Zhou, Zhou, & Liu, 2014). For instance, the JAK2/STAT3 pathway, an important signal transduction cascade with a wide range of expressed cytokines and growth factors, is involved in cell cycle progression and proliferative cell transformation (Ni et al., 2014). The MAPK pathway has been shown to contribute to the TGF‐β1‐induced phenotypic transformation of human lung fibroblasts into myofibroblasts (Caraci et al., 2008). Thus, we hypothesized that aberrant activation of the proliferation‐related signalling pathways was likely to induce the expression of the myofibroblastic phenotype and renal fibrosis.
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741, a botanical compound derived mainly from the skins of red grapes, has been widely used in traditional medicine and dietary supplements (Di Pascoli et al., 2013). Recent studies have shown that resveratrol inhibited cellular proliferation and induced apoptosis in pancreatic cancer cells as well as in fibroblasts through proliferation‐related pathways, indicating that investigation into resveratrol as a potential anti‐fibrotic agent in humans is worthwhile (Di Pascoli et al., 2013; Ding & Adrian, 2002; Fagone et al., 2011; Mo et al., 2011). Furthermore, resveratrol also inhibited vascular smooth muscle cell remodelling and the growth and proliferation of cardiac fibroblasts (Olson, Naugle, Zhang, Bomser, & Meszaros, 2005). However, the underlying mechanism of the anti‐fibrotic effects of resveratrol in kidneys needs to be further clarified.
In the present study, a rat model of unilateral ureteral obstruction (UUO) in vivo and interstitial fibroblasts and TECs stimulated by TGF‐β1 in vitro were used to investigate whether resveratrol exerts anti‐fibrotic effects by antagonizing proliferation‐related pathways to reduce myofibroblast accumulation and the extent of tubulointerstitial fibrosis in the kidneys. Our results highlight the mechanism of resveratrol for the prevention of tubulointerstitial fibrosis, and resveratrol may become a therapeutic agent to prevent renal fibrosis.
2. METHODS
2.1. Animal care and experimental procedures
All animal care complied with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85‐23, revised 1996) and study protocols (wydw2016‐0030) were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology.
Forty‐eight 6‐ to 8‐week‐old male Sprague–Dawley rats weighing 200 ± 20 g were purchased from the Experimental Animal Center of Wenzhou Medical University (Wenzhou, China). The rats were housed in a temperature‐ (22–25°C), humidity‐ (40–60%), and light‐ (12‐hr dark/light) controlled environment and fed with standard rat chow and water. The rats were fasted the day before experiments were conducted. The rats were randomly assigned to a sham operation group after being treated with a vehicle (normal saline) or resveratrol (12 rats in each group) and a UUO group with a vehicle or resveratrol (12 rats in each group). The rats were anaesthetized with isoflurane (RWD Life Science, Shenzhen, China). UUO surgery was performed as previously described (Koike et al., 2014), and normal saline or resveratrol (20 mg·kg−1·day−1, Lot No. 20120330, Yuanye Biotechnology, Shanghai, China) was administered intragastrically for seven consecutive days according to the protocol of a previous study (Bai et al., 2014). We used 0.9% NaCl as the oral vehicle. Seven days after the resveratrol treatment, the rats in each group were anaesthetized with isoflurane and were killed by cervical dislocation.Renal tissues were collected for further tests.
2.1.1. Histopathological examination
Seven‐day obstructed kidney tissues were fixed in formalin and embedded in paraffin and then were cut into 4‐μm sections and stained with haematoxylin–eosin and Masson's trichrome (Yuanye). The slides were examined, and pictures were taken using a DM4000 B LED microscope system and a DFC420C 5M digital microscope camera, respectively (Leica Microsystems, Germany). The degree of interstitial collagen deposition was graded as previously described (Ai et al., 2015).
2.2. Cell culture and treatment
Normal rat kidney TECs (NRK‐52E, RRID:CVCL_0468) and fibroblasts (NRK‐49F, RRID:CVCL_2144) were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). NRK‐52E and NRK‐49F cells were maintained in DMEM (Invitrogen, CA, USA) supplemented with 10% FBS (Invitrogen), 100 U·ml−1 of penicillin, and 100 μg·ml−1 of streptomycin (Invitrogen). The NRK‐52E and NRK‐49F cells were seeded in complete medium containing 10% FBS until they reached approximately 70% confluence for use in the in vitro experiments. After 24 hr, the complete medium was replaced with serum‐free medium, in which the cells were incubated for 24 hr before treatment with recombinant TGF‐β1 (5 ng·ml−1; Lot No. 0312209‐1, PeproTech, NJ, USA) or resveratrol (10 and 100 μmol·L−1). The resveratrol used in cellular experiments was dissolved in DMSO (Sigma, Shanghai, China). In the control group, cells were treated with vehicle only.
2.3. Cell proliferation
In the experiments, 10% FBS served as the stimulator, and the cells were subjected to serum‐free medium for 24 hr before experimentation. Real‐time monitoring of cell proliferation was performed using an xCELLigence MP system (ACEA Biosciences, San Diego, USA) and an E‐plate 96, which is a single‐use 96‐well cell culture plate with the bottom surfaces covered with microelectrode sensors (0.2‐cm2 well surface area; 243 ± 5‐μl maximum volumes). Real‐time changes in electrical impedance were measured using gold microelectrodes and are expressed as “cell indices” defined as (Rn − Rb)/15, where Rb is the background impedance and Rn is the impedance of the well with the cells.
Before seeding cells into the E‐plate 96, the background impedance was measured after 100 μl of medium was incubated in the wells for 30 min at room temperature. Cell density was determined by using a haemocytometer after methylene blue staining. Following the seeding of the appropriate number of cells into each well, the plate was incubated at room temperature for 30 min to allow cell settling. Cell proliferation was monitored every 30 min for over 24 hr.
2.4. elisa
Rat kidney tissues (100 mg) were homogenized and centrifuged, and the supernatant was collected. Avidin–biotin complex–elisa was used according to the manufacturer's protocol to determine the TGF‐β1 level. elisa kits were purchased from Xitang Biotechnology (Shanghai, China).
2.5. Immunohistochemical staining
Immunohistochemical analysis was performed with 4‐μm‐thick kidney sections that had been dewaxed with xylene and hydrated using sequential ethanol volumes (100%, 95%, 85%, and 75%) and distilled water. Endogenous peroxidase was blocked with 3% hydrogen peroxide. Antigen retrieval was performed by heating sections in 0.1% sodium citrate buffer (pH 6.0). Using anti‐α‐SMA (1:200, Santa Cruz, CA, USA), anti‐glial fibrillary acidic protein (1:200, Proteintech), anti‐type III collagen (1:200, BioWorld), anti‐Ki67 (1:100, Santa Cruz), and anti‐E‐cadherin (1:100, Abcam, MA, USA) antibodies were detected by the immunochemical streptavidin–peroxidase method. Images were taken by microscopy (Leica). All samples were semi‐quantitatively or quantitatively assessed by two independent investigators in a blinded manner. The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology.
2.6. Immunofluorescence staining
Immunofluorescence staining was performed as previously described (Ding et al., 2012). Briefly, the cells were cultured on coverslips, and the 4‐μm‐thick kidney sections were fixed, permeabilized with 0.5% Triton X‐100, and incubated with the primary antibodies: anti‐α‐SMA (1:200, Santa Cruz), anti‐type I collagen (1:200, BioWorld), anti‐type III collagen (1:200, BioWorld), anti‐Ki67 (1:100, Santa Cruz), anti‐E‐cadherin (1:100, Abcam), and anti‐Smad2/3 (1:100, Abcam) overnight at 4°C, followed by incubation with secondary antibodies conjugated with Alexa Fluor 488 or 588 (Invitrogen). The cells were counterstained with 4′,6‐diamidino‐2‐phenylindole to visualize the nuclei. Images were taken by microscopy (Leica).
2.7. RT‐PCR
Total RNA was extracted from rat kidneys, the NRK‐49F and NRK‐52E cells were subjected to TRIzol reagent (Invitrogen), and reverse transcription was performed to generated cDNA templates using a ReverTra Ace qPCR RT kit (Takara, Tokyo, Japan). Quantitative RT‐PCR was performed using a SYBR Green Realtime PCR Master Mix Plus (Takara). The quality of the PCR products was analysed on agarose gels, and quantity was measured using a Varioskan Flash (Thermo Fisher Scientific, CA, USA). Sequence‐specific primers for TGF‐β1, TGF‐β1R, c‐Myc, CCND1, and type I and III collagen were synthesized by Invitrogen, and β‐actin was used as an endogenous reference gene (Table S1). Samples were analysed in triplicate using an Applied Biosystems 7500 Real‐Time PCR System (Life Technologies, CA, USA). The melting curve was examined to verify that a single product was amplified. For quantitative analysis, all samples were analysed using the ΔΔCT value method (Livak & Schmittgen, 2001).
2.8. Western blot analysis
Total protein in the renal tissues and NRK‐49F and NRK‐52E cells were extracted with RIPA. The concentration of total protein was measured according to BCA kit instructions (Beyotime, Shanghai, China). The protein (30–50 μg) was separated on 8–12% SDS‐PAGE and transferred to PVDF membranes (Millipore, MA, USA). The membranes were blocked in 5% skim milk in TBST (10 mmol·L−1 of Tris–HCl, 150 mmol·L−1 of NaCl, and 0.1% Tween 20) for 1 hr at room temperature. Then, the membranes were probed with a primary antibody overnight at 4°C and an HRP‐conjugated secondary antibody detection system. The signals were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). All primary antibodies were diluted to 1:1,000 (Table S2). The bands were quantified by measuring the intensity of the signals using Image‐Pro Plus (Version 6.0) and normalizing them to the signal for the GAPDH (1:8,000, BioWorld) antibody.
2.9. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Data are presented as means ± SEM. All statistical analyses were performed using the Statistical Package for Social Sciences (Version 16.0, SPSS Inc., Chicago, USA). A two‐sided Student's t test was used to analyse differences between two groups. One‐way ANOVA with Bonferroni's correction was used when more than two groups compared. A post hoc test was performed only if P < .05, and no significant inhomogeneity of variance was found. A P value of <.05 was considered statistically significant. The data analysis was conducted in a blinded manner.
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017).
3. RESULTS
3.1. Resveratrol inhibits the expression of the myofibroblastic phenotype and the formation of interstitial fibrosis in vivo
We initially investigated the effects of resveratrol on interstitial fibrosis in the kidneys in the UUO model, which is a typical model of renal fibrosis. (Song et al., 2014). As shown in Figure 1a,b, Masson's trichrome staining revealed that excessive deposition of total collagen in UUO kidneys was inhibited by resveratrol administration, and this suppression was accompanied by the down‐regulation of α‐SMA‐positive myofibroblasts, as determined by Western blotting and immunohistochemical staining (Figure 1c,d). As a result, resveratrol reduced the production of ECM components, including vimentin and type I and III collagen, thereby attenuating tubulointerstitial fibrosis in kidneys following injury (Figure 1c–e). Further study showed that resveratrol also significantly inhibited the up‐regulation of Rac1 and N‐cadherin and the down‐regulation of E‐cadherin in UUO rats at the protein level (Figure 1e,f,h), a finding that was consistent with previous reports (Choi et al., 2016; Wang, Wang, et al., 2016; Wang, Zhou, et al., 2016). EMT is characterized by the loss of epithelial cell polarity and cell‐to‐cell adhesion and increased mesenchymal cell migration, and it is associated with renal fibrosis (Qi, Chen, Poronnik, & Pollock, 2006; Strutz & Zeisberg, 2006). Thus, our findings indicated that partial reversal of the EMT process may be involved in the anti‐fibrotic effects of resveratrol. In addition, enhanced expression of glial fibrillary acidic protein, a surface marker mainly expressed in fibroblasts or stellate cells (Carotti et al., 2008) in the renal interstitium of the UUO rats, was also inhibited by resveratrol administration (Figure 1g,h), revealing that resveratrol reduces fibrosis formation by suppressing the FMD.
Figure 1.
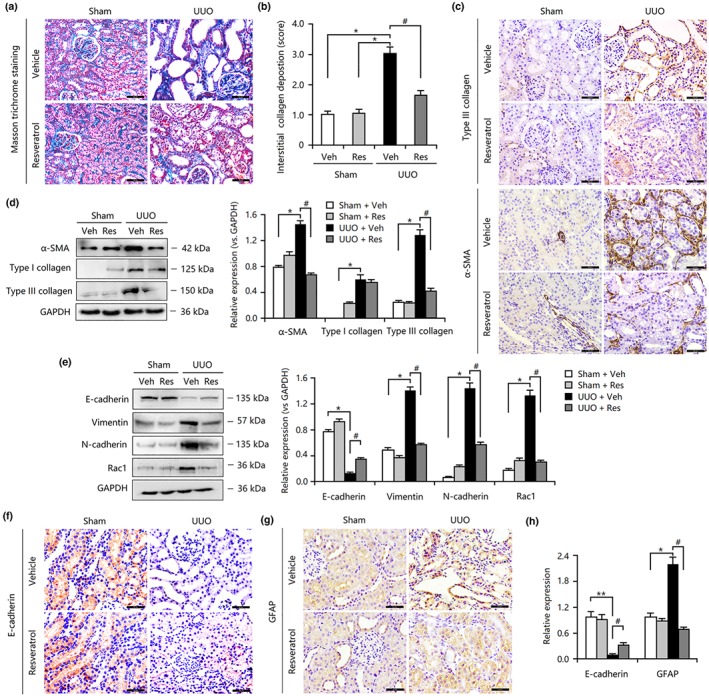
Resveratrol (Res) treatment attenuated renal fibrosis in the obstructed kidneys by inhibiting epithelial–mesenchymal transition and fibroblast–myofibroblast differentiation. (a) Masson trichrome staining showed that Res significantly reduced renal tubulointerstitial damage and total collagen deposition in unilateral ureteral obstruction (UUO) rat models. Bar = 50 μm. (b) The scores based on Masson's staining indicate that interstitial collagen deposition in the resveratrol‐treated UUO group is lower than that in the UUO group. (c) Resveratrol reduced the expression of α‐smooth muscle actin (α‐SMA) and type III collagen in UUO kidneys. (d) Resveratrol inhibited the overexpression of α‐SMA and type I and type III collagen in UUO kidneys. (e) Resveratrol down‐regulated the expression of vimentin, N‐cadherin, and Rac1 but up‐regulated the expression of E‐cadherin in UUO kidneys. (f) Resveratrol increased E‐cadherin expression in UUO kidneys. (g) Resveratrol decreased glial fibrillary acidic protein (GFAP) expression in UUO kidneys. (h) Results from the quantification of E‐cadherin and GFAP expression based on immunohistochemical staining. Data are expressed as means ± SEM for six rats per group. *P < .05, significantly different from the sham group; # P < .05, significantly different from the UUO group. Veh, vehicle
Changes concerning myofibroblast transdifferentiation via EMT or FMD may be associated with abnormal proliferation of various cells in the kidney. We also further investigated whether resveratrol affected cell proliferation, apoptosis, and the cell cycle in fibrotic models. Immunohistochemical analysis revealed that the ratio of Ki67‐positive cells to total cells was significantly decreased in resveratrol‐treated UUO rats compared with that in the UUO rats (Figure 2a,b). Notably, Ki67‐positive cells were primarily distributed in the tubules and the interstitium of the kidney, suggesting that TECs and interstitial fibroblasts may be the originators of myofibroblasts (Figure 2c). Additionally, the expression of proliferative protein c‐Myc, anti‐apoptotic protein https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2844, and cell cycle regulator cyclin D1 was increased significantly compared with the levels of these proteins in the sham group (Figure 2d), a finding that was also supported by previous findings indicating that the aberrant cell proliferation and suppressed apoptosis were involved in determining myofibroblast activation in kidneys (Fagone et al., 2011). Administration of resveratrol suppressed the expression of these proliferation‐related proteins in response to kidney injury (Figure 2d). Thus, the aberrant proliferation of myofibroblasts may play an important role in the resveratrol‐mediated inhibition of interstitial fibrosis in vivo.
Figure 2.
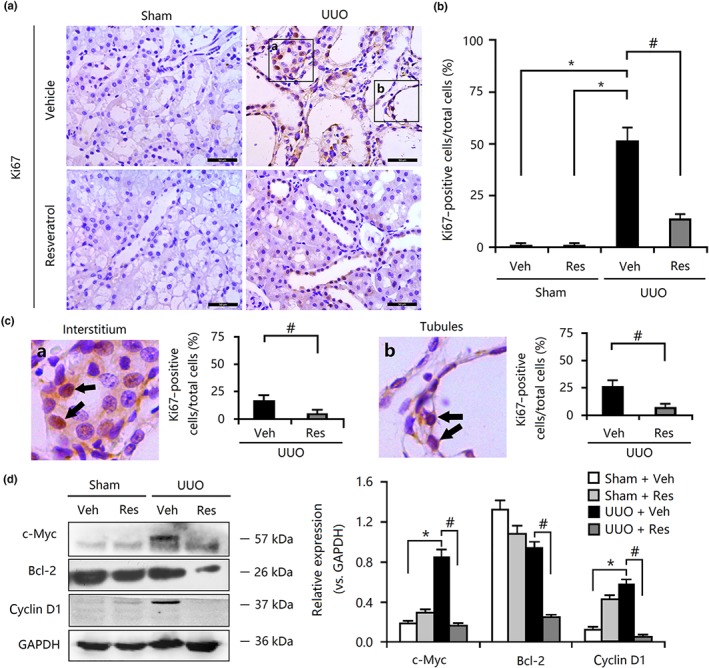
Resveratrol (Res) inhibited cellular proliferation in unilateral ureteral obstruction (UUO) kidneys. (a) Effect of resveratrol on Ki67‐positive cells in UUO kidney tissues, as determined by immunohistochemical staining. Bar = 50 μm. (b) Resveratrol reduced the ratio of Ki67‐positive cells to total cells in UUO kidneys, according to the results of immunohistochemical staining. (c) Resveratrol reduced the ratio of Ki67‐positive cells both in the interstitium and in the tubules. (d) The enhanced expression of c‐Myc, Bcl‐2, and cyclin D1 in UUO kidneys was abolished by resveratrol treatment. Veh, vehicle. Data are expressed as means ± SEM for six rats per group. *P < .05, significantly different from the sham group; # P < .05, significantly different from the UUO group
3.2. Resveratrol inhibits the activation of the proliferation‐related pathways in vivo
Given the vital role of myofibroblast proliferation in resveratrol‐mediated anti‐fibrosis, we speculated that proliferation‐related pathways likely participate in fibrogenesis. Previous studies have revealed that the MAPK, PI3K/Akt, JAK2/STAT3, and Wnt/β‐catenin signalling pathways are involved in renal fibrosis (Ai et al., 2015; Gao et al., 2016; Lin et al., 2017; Wang, Wang, et al., 2016; Wang, Zhou, et al., 2016). In this study, the activities of MAPK (Figure 3a), PI3K/Akt (Figure 3b), JAK2/STAT3 (Figure 3c), and Wnt/β‐catenin (Figure 3d) in the UUO group were enhanced compared with those in the sham‐operated group, indicating that activated signal transduction was involved in the proliferation and transdifferentiation of myofibroblasts. Treatment with resveratrol inhibited excessive activation of the above mentioned pathways (Figure 3a–d). To further explore the molecular mechanism of these pathways in myofibroblast transdifferentiation and fibrosis, the levels of TGF‐β1, a key inducer of myofibroblastic phenotype expression, were determined in UUO kidneys. Evidence from the elisa and Western blotting analysis revealed a significant increase in the levels of TGF‐β1 and the expression of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1788 in fibrotic kidneys compared with the levels of each in the sham group, but the levels were reduced in the resveratrol‐treated group (Figure 3e,f). Taken together, these results showed that the proliferation‐related pathways, as well as TGF‐β1, may be involved in the anti‐fibrotic action mediated by resveratrol.
Figure 3.
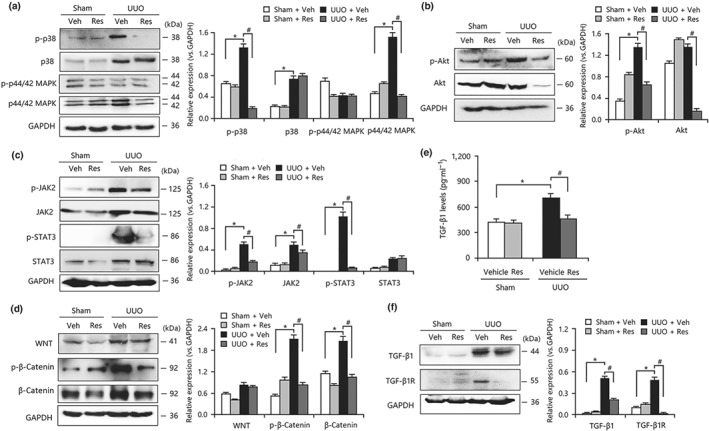
Resveratrol (Res) inhibited the activation of proliferation‐related signalling pathways in unilateral ureteral obstruction (UUO) kidneys. (a) Resveratrol suppressed the activation of MAPK signalling in UUO kidneys. (b) Resveratrol inhibited the expression and phosphorylation of Akt in UUO kidneys. (c) Resveratrol inhibited the activities of JAK2/STAT3 signalling in UUO kidneys. (d) The overactivation of WNT/β‐catenin signalling in the obstructed kidneys was decreased by resveratrol treatment. (e) UUO‐induced increase in TGF‐β1 levels in kidneys was inhibited by resveratrol administration, as determined by elisa. (f) Resveratrol reduced the mRNA expression of TGF‐β1 and TGF‐β1 receptors in the obstructed kidneys. Veh, vehicle. Data are expressed as means ± SEM for six rats per group. *P < .05, significantly different from the sham group; # P < .05, significantly different from the UUO group
3.3. Resveratrol inhibits TGF‐β1‐mediated phenotypic transformation and ECM deposition in vitro
Although our in vivo experiments demonstrated that resveratrol had renal anti‐fibrotic effects, as indicated through inhibited proliferation‐related pathway activities and transdifferentiation of myofibroblasts, it needed to be tested and confirmed by in vitro experiments. As explained above, myofibroblasts may emerge from interstitial fibroblasts and TECs due to the abnormal proliferative cells mainly in the interstitium and tubules of the kidney (Lin et al., 2005). Thus, in this study, interstitial fibroblasts (NRK‐49F) and TECs (NRK‐52E) were used to investigate the anti‐fibrotic mechanism of resveratrol. First, the real‐time cell analysis assay indicated that resveratrol markedly attenuated the cell viability of the fibroblasts in a dose‐dependent manner (Figure 4a) and markedly reduced the ratio of the Ki67‐positive cells in the TGF‐β1‐treated fibroblasts (Figure 4b). In addition, the expression of the proliferation‐related genes c‐Myc and CCND1 (cyclin D1) was strongly down‐regulated in resveratrol‐treated fibroblasts (Figure 4c). Moreover, resveratrol inhibited the expression of cyclin D1 and Bcl‐2 and enhanced the expression of Bax (Figure 4d), which are involved in the induction of cell cycle arrest and the inhibition of cellular proliferation (Thomasova & Anders, 2015). As a result, resveratrol treatment inhibited TGF‐β1‐mediated FMD and ECM deposition in fibroblasts, as indicated by the decreased levels of α‐SMA, fibronectin, and type I and III collagen mRNA and protein expression (Figure 4e–g).
Figure 4.
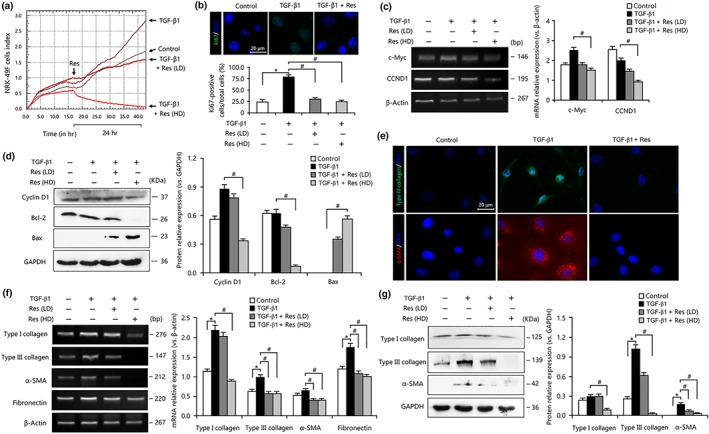
Resveratrol (Res) inhibited cell proliferation, suppressed fibroblast–myofibroblast differentiation, and ameliorated extracellular matrix accumulation in TGF‐β1‐treated fibroblasts. (a) Real‐time cell analysis assay revealed that resveratrol suppressed the TGF‐β1‐mediated proliferation of NRK‐49F cells. (b) The TGF‐β1‐mediated proliferation of NRK‐49F cells was inhibited by resveratrol. Bar = 20 μm. Data are expressed as means ± SEM of 20 independent sections. (c) Resveratrol reduced the mRNA expression of c‐Myc and CCND1 in TGF‐β1‐treated NRK‐49F cells. (d) Resveratrol down‐regulated the expression of cyclin D1 and Bcl‐2 and up‐regulated the expression of bax in TGF‐β1‐treated NRK‐49F cells. (e) Resveratrol inhibited the expression of type III collagen and α‐smooth muscle actin (α‐SMA) in TGF‐β1‐treated NRK‐49F cells. Bar = 20 μm. (f) Resveratrol inhibited the mRNA expression of fibronectin, α‐SMA, and type I and III collagen in TGF‐β1‐treated NRK‐49F cells. (g) Resveratrol inhibited the expression of α‐SMA and type I and III collagen in TGF‐β1‐treated NRK‐49F cells. Res (HD), Res (100 μmol·L−1); Res (LD), Res (10 μmol·L−1). Data are expressed as means ± SEM in quintuplicate for the cell line experiment. *P < .05, significantly different from the control group; # P < .05, significantly different from the TGF‐β1‐treated group
Similarly, in TGF‐β1‐treated TECs, resveratrol reduced the expression levels of c‐Myc, cyclin D1, and Bcl‐2 proteins, which resulted in a decreased ratio of Ki67‐positive cells and inhibited proliferation (Figure 5a–d). Thus, resveratrol treatment abolished TGF‐β1‐induced EMT and ECM deposition in TECs, as indicated by the decreased expression of α‐SMA, vimentin, and type I and III collagen and the increased expression of E‐cadherin (Figure 5d,e).
Figure 5.
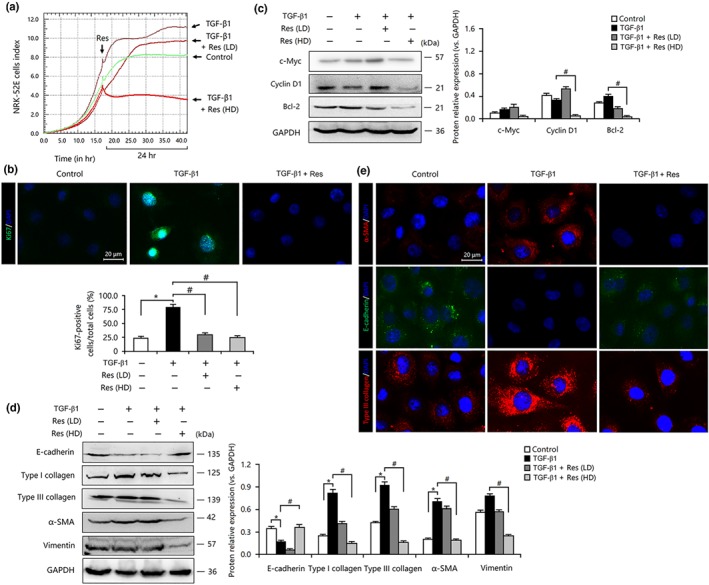
Resveratrol (Res) inhibited cell proliferation, suppressed epithelial–mesenchymal transition, and prevented extracellular matrix accumulation in TGF‐β1‐treated tubular epithelial cells. (a) TGF‐β1‐induced proliferation of NRK‐52E cells was inhibited by Res treatment. (b) The TGF‐β1‐mediated proliferation of NRK‐52E cells was inhibited by Res. Bar = 20 μm. (c) Res down‐regulated the expression of c‐Myc, cyclin D1, and Bcl‐2 in TGF‐β1‐treated NRK‐52E cells. (d) Res inhibited the expression of α‐smooth muscle actin (α‐SMA), vimentin, and type I and III collagen but induced the expression of E‐cadherin in TGF‐β1‐treated NRK‐52E cells. (e) Res reduced the expression of α‐SMA and type III collagen but increased the expression of E‐cadherin in TGF‐β1‐treated NRK‐52E cells. Bar = 20 μm. Res (HD), resveratrol (100 μmol·L−1); Res (LD), resveratrol (10 μmol·L−1). Data are expressed as means ± SEM in quintuplicate for the cell line experiments. *P < .05, compared with the control group; # P < .05, compared with the TGF‐β1‐treated group
In summary, our in vitro experiments indicated that resveratrol treatment reduced the proliferation of fibroblasts and TECs, thereby inhibiting TGF‐β1‐mediated phenotypic transformation and ECM accumulation.
3.4. Resveratrol abolishes TGF‐β1‐mediated activation of proliferation‐related pathways in vitro
As explained above, resveratrol inhibited FMD‐ and EMT‐induced myofibroblastic phenotype expression by suppressing the proliferation and activation of proliferation‐related pathways in vivo. We next investigated whether a similar mechanism could be confirmed in vitro. As shown in Figure 6a–d, the activities of canonical MAPK, PI3K/Akt, Wnt/β‐catenin, and JAK2/STAT3 signalling in fibroblasts were enhanced after TGF‐β1 stimulation, but these changes were abolished by resveratrol treatment. Similarly, resveratrol also inhibited the activities of MAPK, PI3K/Akt, Wnt/β‐catenin, and JAK2/STAT3 signalling in TGF‐β1‐treated TECs (Figure 7a–d). Consequently, these results showed that there was a similar effect on fibroblasts and TECs that aligned with that in the in vivo results.
Figure 6.
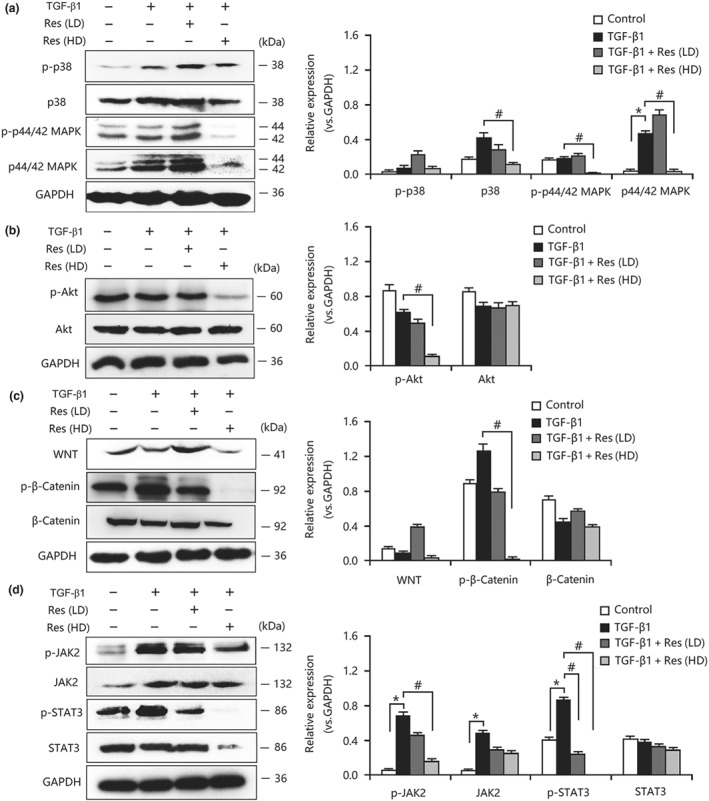
Resveratrol (Res) inhibited the activation of proliferation‐related signalling pathways in TGF‐β1‐treated fibroblasts. (a) Resveratrol suppressed the activation of MAPK signalling in TGF‐β1‐treated NRK‐49F cells. (b) Resveratrol inhibited the expression and phosphorylation of AKT in TGF‐β1‐treated NRK‐49F cells. (c) Resveratrol inhibited the activities of WNT/β‐catenin signalling in TGF‐β1‐treated NRK‐49F cells. (d) The overactivation of JAK2/STAT3 signalling in TGF‐β1‐treated NRK‐49F cells was decreased by resveratrol treatment. Res (HD), resveratrol (100 μmol·L−1); Res (LD), resveratrol (10 μmol·L−1). Data are expressed as means ± SEM in quintuplicate for the cell line experiments. *P < .05, significantly different from the control group; # P < .05, significantly different from the TGF‐β1‐treated group
Figure 7.
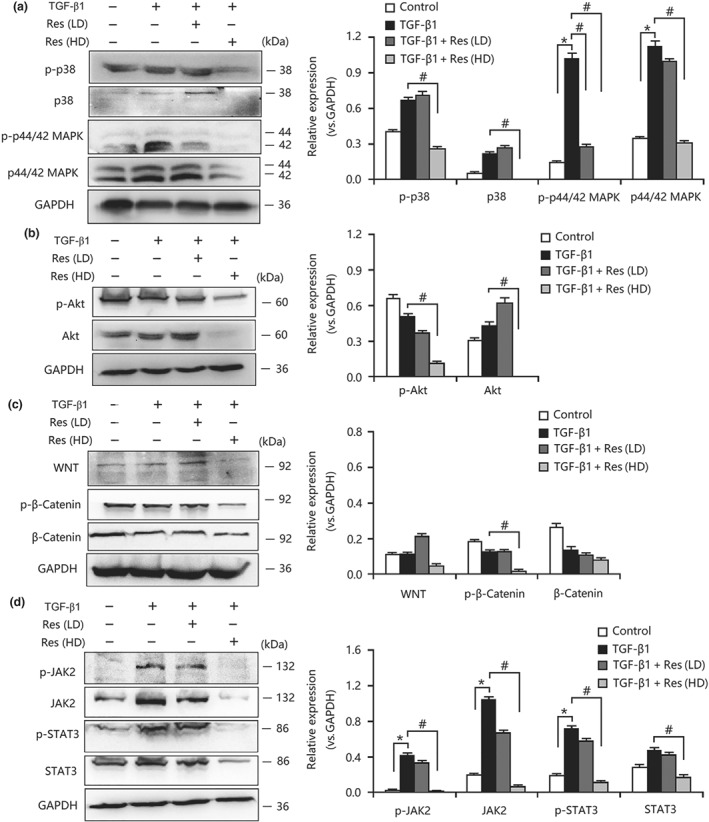
Resveratrol (Res) inhibited the activation of proliferation‐related signalling pathways in TGF‐β1‐treated tubular epithelial cells. (a) Resveratrol suppressed the activation of MAPK signalling in TGF‐β1‐treated NRK‐52E cells. (b) Resveratrol inhibited the expression and phosphorylation of AKT in TGF‐β1‐treated NRK‐52E cells. (c) Resveratrol inhibited the activities of WNT/β‐catenin signalling in TGF‐β1‐treated NRK‐52E cells. (d) The overactivation of JAK2/STAT3 signalling in TGF‐β1‐treated NRK‐52E cells was decreased by resveratrol treatment. Res (HD), resveratrol (100 μmol·L−1); Res (LD), resveratrol (10 μmol·L−1). Data are expressed as means ± SEM in quintuplicate for the cell line experiments. *P < .05, significantly different from the control group; # P < .05, significantly different from the TGF‐β1‐treated group
3.5. Resveratrol reduces the activities of downstream Smad2/3 in vivo and in vitro
As Smad2/3 is a well‐known downstream mediator that responds TGF‐β1 stimulation, as indicated by translocation of phosphorylated Smad2/3 to the nucleus for gene regulation (Javelaud & Mauviel, 2004), we investigated whether resveratrol inhibited TGF‐β1‐downstream Smad2/3 in vivo and in vitro. As presented in Figure 8a,b, the nuclear localization of Smad2/3 in TECs and fibroblasts was induced by TGF‐β1 stimulation but inhibited by resveratrol treatment. Additionally, TGF‐β1‐induced up‐regulation of Smad2/3 phosphorylation in both types of cells was suppressed by resveratrol in a dose‐dependent manner (Figure 8c,d). Furthermore, resveratrol administration also abolished TGF‐β1‐mediated localization and phosphorylation of Smad2/3 in the nucleus of TECs and fibroblasts (Figure 8e,f). Thus, these data showed that resveratrol negatively affected TGF‐β‐mediated transcription and expression of profibrotic factors, at least in part, by targeting Smad2/3 activity in vivo and in vitro.
Figure 8.
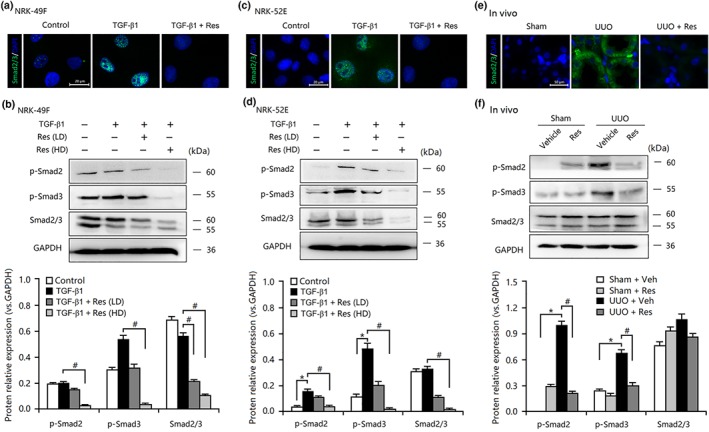
Resveratrol (Res) inhibited the activities of Smad2/3 in vivo and in vitro. Immunofluorescence staining revealed that resveratrol inhibited the expression and nuclear localization of Smad2/3 in TGF‐β1‐treated (a) NRK‐49F cells and (c) NRK‐52E cells and in (e) unilateral ureteral obstruction (UUO) kidneys. Bar = 20 μm in vitro; Bar = 50 μm in vivo. Resveratrol inhibited the expression and phosphorylation of Smad2/3 in TGF‐β1‐treated (b) NRK‐49F cells and (d) NRK‐52E cells and in (f) UUO kidneys. Res (HD), resveratrol (100 μmol·L−1); Res (LD), resveratrol (10 μmol·L−1); Veh, vehicle. Data are expressed as means ± SEM in quintuplicate for the cell line experiments. *P < .05, significantly different from the control group; # P < .05, significantly different from the TGF‐β1‐treated group
4. DISCUSSION
Renal fibrosis is widely accepted as being closely associated with the deterioration of the renal function that is characterized by 90% of the kidney volume being composed of tubule interstitium. Therefore, the progression of kidney disease can be halted through the prevention of interstitial fibrosis. In the present study, we have provided evidence that resveratrol inhibited the expression of the myofibroblastic phenotype and attenuated fibrosis in vitro and in vivo. Resveratrol inhibited TGF‐β1‐mediated the EMT in TECs and FMD in the fibroblasts by antagonizing proliferation‐related processes. As a result, resveratrol reduced TGF‐β1‐downstream Smad2/3 activity, suppressed myofibroblast accumulation, and attenuated tubulointerstitial fibrosis in the kidney.
Traditionally, UUO has been a well‐established animal model used to feature tubulointerstitial injury and progressive fibrosis (Song et al., 2014). Some studies have demonstrated that kidney injuries often induce interstitial myofibroblast proliferation, ECM accumulation in the interstitium, and the transdifferentiation of fibroblasts and epithelial cells are the major sources of myofibroblasts (Grande & Lopez‐Novoa, 2009). Additionally, fibroblasts in the renal interstitium are considered to be the principal sources of the fibrillar matrix (collagens). Thus, tubulointerstitial fibrosis is inevitably associated with a robust accumulation of fibroblasts and the activation of myofibroblasts, increasing intrinsic cell proliferation (Liu, 2011). The proliferation of myofibroblasts in the renal tubulointerstitium also plays a key role in the progression of renal fibrosis. The activation of resident UUO kidney fibroblasts can lead to a massive increase in the number of expressed myofibroblasts during the early stage of obstructive nephropathy (Meng et al., 2011). Thus, this observation led us to hypothesize that resveratrol exerted anti‐fibrotic effects in TGF‐β1‐treated fibroblasts and TECs as well as in the UUO model. The initial pathological experiments confirmed that the accumulation of ECM components in fibrotic kidneys can be effectively abolished by resveratrol treatment. Subsequently, resveratrol markedly reduced the overexpression of TGF‐β1‐stimulated α‐SMA both in vivo and in vitro. Therefore, in addition to protecting tubular interstitial integrity, resveratrol might be able to hinder myofibroblast activation by antagonizing TGF‐β1‐induced EMT and FMD.
Increasing evidence indicates that abnormal cell proliferation and differentiation are important inducers of EMT and FMD (Grande & Lopez‐Novoa, 2009). Ki67 is often associated with the activation of proliferation‐related signal transduction (Bijkerk et al., 2016). We found that enhanced expression of Ki67 in the tubules and interstitium of the kidney may indicate that proliferative TECs and interstitial fibroblasts may be the origin of myofibroblasts. In addition, c‐Myc, cyclin D1, and Bcl‐2 are important factors that participate in regulating cellular proliferation, G1 phase transition and apoptosis, respectively (Lashinger et al., 2005; Yang, Besschetnova, Brooks, Shah, & Bonventre, 2010). Overexpression of these proteins in TGF‐β1‐treated TECs and fibroblasts, as well as in injured kidneys, was identified with the abnormal proliferation of TECs and fibroblasts in the induction of EMT and FMD. This proliferative behaviour of TECs and fibroblasts in fibrotic kidneys can be reduced by resveratrol administration, thereby inhibiting the expression of the myofibroblastic phenotype. However, in contrast to the previous study showing that resveratrol attenuated kidney fibrosis by inhibiting death receptor‐mediated apoptosis (Hao et al., 2016), our current findings suggest that resveratrol exerts an anti‐proliferative role against UUO‐induced tubulointerstitial fibrosis. The anti‐proliferative activities of resveratrol in a variety of cell lines have been reported through observations of suppressed proliferation‐related signalling (Wang, Wang, et al., 2016; Wang, Zhou, et al., 2016; Wu et al., 2016). Targeting proliferation‐related signalling to inhibit the expression of the myofibroblastic phenotype and reduce renal fibrosis formation may be promising.
Although pharmacological studies have demonstrated the therapeutic effect of anti‐fibrotic action induced through some pathways signalling, such as those of AMPK/NOX4/ROS or ERK1/2 and Smad2/3, these studies lacked sufficient evidence of proliferation‐induced signalling (Bai et al., 2014; Chen et al., 2011; He et al., 2015; He et al., 2016). Based on these findings, we next investigated the effects of resveratrol on the activities of proliferation‐related signalling pathways, including those of MAPK, PI3K/Akt, Wnt/β‐catenin, and JAK2/STAT3. For instance, the JAK2/STAT3 signalling pathway is an important signal transduction cascade involving a wide variety of growth factors and cytokines that induce cellular activation, proliferation, and differentiation. This pathway is activated in a variety of renal diseases and has been implicated in the pathophysiology of renal fibrosis (Koike et al., 2014). Similarly, the pathways such as MAPK, PI3K/Akt, and Wnt/β‐catenin have also reportedly been involved in the development of renal fibrosis (Gao et al., 2016; Liu et al., 2017; Tan et al., 2014). Our results were consistent with the conclusion that the activities of proliferation‐related pathways in injured kidneys were enhanced and contributed to the expression of the myofibroblastic phenotype and suppression of fibrosis formation. Treatment with resveratrol significantly inhibited the activities of proliferation‐related signalling and thereby led to the reduction of myofibroblast accumulation. Our findings thus demonstrated a crucial role of proliferation‐related signalling in the protection of resveratrol‐mediated anti‐fibrotic effects.
The enhanced activity of TGF‐β1/Smads2/3 is essential for tubulointerstitial fibrosis. TGF‐β1 can initiate a cellular response by binding to its receptor, which leads to Smad2/3 phosphorylation and translocation into the nucleus where profibrotic genes are transcribed (Lan & Chung, 2012; Leask & Abraham, 2004). Considering the imperative role of Smad2/3 phosphorylation in TGF‐β1 signal transduction, it is predictable that inhibiting Smad2/3 phosphorylation by resveratrol may contribute to the preservation of tubular interstitial integrity. Many mediators have been created to target TGF‐β1/Smad2/3 signalling, including decorin, antibodies that neutralize TGF‐β1 action and low MW inhibitors of TGF‐β1 receptors, and alleviate renal fibrosis (Mori et al., 2004; Sharma, Jin, Guo, & Ziyadeh, 1996; Yamaguchi, Mann, & Ruoslahti, 1990). Thus, we investigated whether resveratrol dramatically suppressed TGF‐β1‐induced Smad2/3 phosphorylation in both a cell‐based assay and a UUO model. We found that the treatment of the UUO kidney model with resveratrol significantly inhibited the nuclear translocation of the Smad complex and the expression of Smad2/3‐driven genes, including a‐SMA and type I and III collagen, which was consistent with previous studies (Lee et al., 2013; Li, Qu, Ricardo, Bertram, & Nikolic‐Paterson, 2010). In addition, resveratrol also lowered the mRNA and protein expression of TGF‐β1 and TGF‐β1 receptors, which are associated with the induction of EMT and FMD. In TGF‐β1‐treated TECs, resveratrol inhibited nuclear expression and phosphorylation of Smad2/3 and thereby partly reversed the EMT process. Similarly, resveratrol ameliorated FMD in TGF‐β1‐treated fibroblasts by reducing Smad2/3 activity. Thus, resveratrol can protect tubular interstitial integrity and reduce excessive ECM deposition by suppressing TGF‐β1/Smads signalling and, as a consequence, EMT and FMD.
It should be noted that there were some limitations in this study. For example, a genetic approach and an in vivo experiment involved in the association between proliferation‐related pathways and TGF‐β1 signalling during EMT or FMD needs to be presented. Additionally, several factors and pathways may contribute to fibrosis progression (independent of TGF‐β1) and this possibility should be clarified. Thus, further studies are required to investigate other potential pathways that may be targeted by resveratrol and to test its effects as an intervention therapy.
In conclusion, we have proposed a new anti‐fibrotic mechanism of resveratrol in kidneys following injury. Resveratrol suppresses the expression of the myofibroblastic phenotype by partly reversing EMT in TECs and FMD in fibroblasts. The inhibition of EMT and FMD is associated with the suppressed activity of TGF‐β/Smad2/3, which results in the reduction of ECM accumulation and tubulointerstitial fibrosis. Moreover, the inactivation of the proliferation‐related pathways may be involved in regulating the activities of TGF‐β/Smads. Therefore, these findings indicated that resveratrol may become a therapeutic agent to prevent renal fibrosis.
AUTHOR CONTRIBUTIONS
Y.B. designed the research. X.Z., Y.X., H.Z., and H.L. performed the experiments, analysed the data, and drafted the manuscript. S.X., C.W., Y.G., S.Z., and Y.Z. performed the experiments and collected the data for the revision. Y.B. edited the manuscript. X.Z., S.X., C.W., and Y.Z. contributed to the discussion and review of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
5. DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208 and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1
Two‐step real‐time RT‐PCR primers for analysis
Table S2
Primary antibodies for proteins
ACKNOWLEDGEMENTS
This study was sponsored by the National Natural Science Foundation of China (Grant 81772264), the Natural Science Foundation of Zhejiang Province, China (Grant LY17H050005), and the Wenzhou Municipal Science and Technology Plan Project (Y20190124). We thank Dr Tongke Chen (Experimental Animal Center of Wenzhou Medical University, Wenzhou, China) for technical assistance with the animal experiments.
Zhang X, Lu H, Xie S, et al. Resveratrol suppresses the myofibroblastic phenotype and fibrosis formation in kidneys via proliferation‐related signalling pathways. Br J Pharmacol. 2019;176:4745–4759. 10.1111/bph.14842
Xing Zhang and Hong Lu are the first two authors that contributed equally to this work.
REFERENCES
- Ai, J. , Nie, J. , He, J. , Guo, Q. , Li, M. , Lei, Y. , … Hou, F. F. (2015). GQ5 hinders renal fibrosis in obstructive nephropathy by selectively inhibiting TGF‐β‐induced Smad3 phosphorylation. Journal of the American Society of Nephrology, 26, 1827–1838. 10.1681/ASN.2014040363 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Lu, H. , Wu, C. , Liang, Y. , Wang, S. , Lin, C. , … Xia, P. (2014). Resveratrol inhibits epithelial–mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochemical Pharmacology, 92, 484–493. [DOI] [PubMed] [Google Scholar]
- Ban, C. R. , & Twigg, S. M. (2008). Fibrosis in diabetes complications: Pathogenic mechanisms and circulating and urinary markers. Vascular Health and Risk Management, 4, 575–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, J. L. , & Gorin, Y. (2011). Myofibroblast differentiation during fibrosis: Role of NAD(P)H oxidases. Kidney International, 79, 944–956. 10.1038/ki.2010.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, J. , & Duffy, H. S. (2011). Fibroblasts and myofibroblasts: What are we talking about? Journal of Cardiovascular Pharmacology, 57, 376–379. 10.1097/FJC.0b013e3182116e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijkerk, R. , de Bruin, R. G. , van Solingen, C. , van Gils, J. M. , Duijs, J. M. , van der Veer, E. P. , … van Zonneveld, A. J. (2016). Silencing of microRNA‐132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney International, 89, 1268–1280. 10.1016/j.kint.2016.01.029 [DOI] [PubMed] [Google Scholar]
- Caraci, F. , Gili, E. , Calafiore, M. , Failla, M. , La Rosa, C. , Crimi, N. , … Vancheri, C. (2008). TGF‐β1 targets the GSK‐3β/β‐catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacological Research, 57, 274–282. 10.1016/j.phrs.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Carotti, S. , Morini, S. , Corradini, S. G. , Burza, M. A. , Molinaro, A. , Carpino, G. , … Gaudio, E. (2008). Glial fibrillary acidic protein as an early marker of hepatic stellate cell activation in chronic and posttransplant recurrent hepatitis C. Liver Transplantation, 14, 806–814. 10.1002/lt.21436 [DOI] [PubMed] [Google Scholar]
- Chen, K. H. , Hung, C. C. , Hsu, H. H. , Jing, Y. H. , Yang, C. W. , & Chen, J. K. (2011). Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF‐β/smad and ERK1/2 signaling in streptozotocin‐induced diabetic rats. Chemico‐Biological Interactions, 190, 45–53. 10.1016/j.cbi.2011.01.033 [DOI] [PubMed] [Google Scholar]
- Choi, S. Y. , Piao, Z. H. , Jin, L. , Kim, J. H. , Kim, G. R. , Ryu, Y. , … Jeong, M. H. (2016). Piceatannol attenuates renal fibrosis induced by unilateral ureteral obstruction via downregulation of histone deacetylase 4/5 or p38‐MAPK signaling. PLoS ONE, 11, e0167340 10.1371/journal.pone.0167340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pascoli, M. , Divi, M. , Rodriguez‐Vilarrupla, A. , Rosado, E. , Gracia‐Sancho, J. , Vilaseca, M. , … García‐Pagán, J. C. (2013). Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. Journal of Hepatology, 58, 904–910. 10.1016/j.jhep.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Ding, H. , Zhou, D. , Hao, S. , Zhou, L. , He, W. , Nie, J. , … Liu, Y. (2012). Sonic hedgehog signaling mediates epithelial–mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol, 23, 801–813. 10.1681/ASN.2011060614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X. Z. , & Adrian, T. E. (2002). Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Pancreas, 25, e71–e76. 10.1097/00006676-200211000-00024 [DOI] [PubMed] [Google Scholar]
- Duffy, H. S. (2011). Fibroblasts, myofibroblasts, and fibrosis: Fact, fiction, and the future. Journal of Cardiovascular Pharmacology, 57, 373–375. 10.1097/FJC.0b013e3182155a38 [DOI] [PubMed] [Google Scholar]
- Fagone, E. , Conte, E. , Gili, E. , Fruciano, M. , Pistorio, M. P. , Lo Furno, D. , … Vancheri, C. (2011). Resveratrol inhibits transforming growth factor‐β‐induced proliferation and differentiation of ex vivo human lung fibroblasts into myofibroblasts through ERK/Akt inhibition and PTEN restoration. Experimental Lung Research, 37, 162–174. 10.3109/01902148.2010.524722 [DOI] [PubMed] [Google Scholar]
- Gao, F. , Wang, Y. , Li, S. , Wang, Z. , Liu, C. , & Sun, D. (2016). Inhibition of p38 mitogen‐activated protein kinases attenuates renal interstitial fibrosis in a murine unilateral ureteral occlusion model. Life Sciences, 167, 78–84. 10.1016/j.lfs.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Grande, M. T. , & Lopez‐Novoa, J. M. (2009). Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nature Reviews. Nephrology, 5, 319–328. 10.1038/nrneph.2009.74 [DOI] [PubMed] [Google Scholar]
- Hao, Q. , Xiao, X. , Zhen, J. , Feng, J. , Song, C. , Jiang, B. , & Hu, Z. (2016). Resveratrol attenuates acute kidney injury by inhibiting death receptormediated apoptotic pathways in a cisplatininduced rat model. Molecular Medicine Reports, 14, 3683–3689. 10.3892/mmr.2016.5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res., 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T. , Guan, X. , Wang, S. , Xiao, T. , Yang, K. , Xu, X. , … Zhao, J. (2015). Resveratrol prevents high glucose‐induced epithelial–mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Molecular and Cellular Endocrinology, 402, 13–20. 10.1016/j.mce.2014.12.010 [DOI] [PubMed] [Google Scholar]
- He, T. , Xiong, J. , Nie, L. , Yu, Y. , Guan, X. , Xu, X. , … Zhao, J. (2016). Resveratrol inhibits renal interstitial fibrosis in diabetic nephropathy by regulating AMPK/NOX4/ROS pathway. Journal of Molecular Medicine (Berlin, Germany), 94, 1359–1371. 10.1007/s00109-016-1451-y [DOI] [PubMed] [Google Scholar]
- Javelaud, D. , & Mauviel, A. (2004). Mammalian transforming growth factor‐βs: Smad signaling and physio‐pathological roles. The International Journal of Biochemistry & Cell Biology, 36, 1161–1165. 10.1016/S1357-2725(03)00255-3 [DOI] [PubMed] [Google Scholar]
- Kalluri, R. , & Weinberg, R. A. (2009). The basics of epithelial–mesenchymal transition. The Journal of Clinical Investigation, 119, 1420–1428. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. J. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology, 8, e1000412 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. I. , & Choi, M. E. (2012). TGF‐β‐activated kinase‐1: New insights into the mechanism of TGF‐β signaling and kidney disease. Kidney Research and Clinical Practice, 31, 94–105. 10.1016/j.krcp.2012.04.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, K. , Ueda, S. , Yamagishi, S. , Yasukawa, H. , Kaida, Y. , Yokoro, M. , … Okuda, S. (2014). Protective role of JAK/STAT signaling against renal fibrosis in mice with unilateral ureteral obstruction. Clinical Immunology, 150, 78–87. 10.1016/j.clim.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Lan, H. Y. (2011). Diverse roles of TGF‐β/Smads in renal fibrosis and inflammation. International Journal of Biological Sciences, 7, 1056–1067. 10.7150/ijbs.7.1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, H. Y. , & Chung, A. C. (2012). TGF‐β/Smad signaling in kidney disease. Seminars in Nephrology, 32, 236–243. 10.1016/j.semnephrol.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Lashinger, L. M. , Zhu, K. , Williams, S. A. , Shrader, M. , Dinney, C. P. , & McConkey, D. J. (2005). Bortezomib abolishes tumor necrosis factor‐related apoptosis‐inducing ligand resistance via a p21‐dependent mechanism in human bladder and prostate cancer cells. Cancer Research, 65, 4902–4908. 10.1158/0008-5472.CAN-04-3701 [DOI] [PubMed] [Google Scholar]
- Leask, A. , & Abraham, D. J. (2004). TGF‐β signaling and the fibrotic response. The FASEB Journal, 18, 816–827. 10.1096/fj.03-1273rev [DOI] [PubMed] [Google Scholar]
- LeBleu, V. S. , Taduri, G. , O'Connell, J. , Teng, Y. , Cooke, V. G. , Woda, C. , … Kalluri, R. (2013). Origin and function of myofibroblasts in kidney fibrosis. Nature Medicine, 19, 1047–1053. 10.1038/nm.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Hwang, I. , Lee, J. H. , Lee, H. W. , Jeong, L. S. , & Ha, H. (2013). The selective A3AR antagonist LJ‐1888 ameliorates UUO‐induced tubulointerstitial fibrosis. The American Journal of Pathology, 183, 1488–1497. 10.1016/j.ajpath.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Li, B. , & Wang, J. H. (2011). Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. Journal of Tissue Viability, 20, 108–120. 10.1016/j.jtv.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Qu, X. , Ricardo, S. D. , Bertram, J. F. , & Nikolic‐Paterson, D. J. (2010). Resveratrol inhibits renal fibrosis in the obstructed kidney: Potential role in deacetylation of Smad3. The American Journal of Pathology, 177, 1065–1071. 10.2353/ajpath.2010.090923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. L. , Chen, R. H. , Chen, Y. M. , Chiang, W. C. , Lai, C. F. , Wu, K. D. , & Tsai, T. J. (2005). Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4‐activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol, 16, 2702–2713. 10.1681/ASN.2005040435 [DOI] [PubMed] [Google Scholar]
- Lin, X. , Zha, Y. , Zeng, X. Z. , Dong, R. , Wang, Q. H. , & Wang, D. T. (2017). Role of the Wnt/β‐catenin signaling pathway in inducing apoptosis and renal fibrosis in 5/6‐nephrectomized rats. Molecular Medicine Reports, 15, 3575–3582. 10.3892/mmr.2017.6461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. , Ning, X. , Li, R. , Yang, Z. , Yang, X. , Sun, S. , & Qian, Q. (2017). Signalling pathways involved in hypoxia‐induced renal fibrosis. Journal of Cellular and Molecular Medicine, 21, 1248–1259. 10.1111/jcmm.13060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. (2011). Cellular and molecular mechanisms of renal fibrosis. Nature Reviews. Nephrology, 7, 684–696. 10.1038/nrneph.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Loeffler, I. , & Wolf, G. (2015). Epithelial‐to‐mesenchymal transition in diabetic nephropathy: Fact or fiction? Cell, 4, 631–652. 10.3390/cells4040631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, L. Q. , Tang, J. W. , Wang, Y. , Zhao, J. R. , Shang, M. Y. , Zhang, M. , … Li, X. M. (2011). Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. British Journal of Pharmacology, 162, 1805–1818. 10.1111/j.1476-5381.2011.01206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, X. M. , Huang, X. R. , Xiao, J. , Chen, H. Y. , Zhong, X. , Chung, A. C. , & Lan, H. Y. (2012). Diverse roles of TGF‐β receptor II in renal fibrosis and inflammation in vivo and in vitro. The Journal of Pathology, 227, 175–188. 10.1002/path.3976 [DOI] [PubMed] [Google Scholar]
- Meran, S. , & Steadman, R. (2011). Fibroblasts and myofibroblasts in renal fibrosis. International Journal of Experimental Pathology, 92, 158–167. 10.1111/j.1365-2613.2011.00764.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, W. , Xu, X. , Xu, L. , Wang, F. , Ke, A. , Wang, X. , & Guo, C. (2011). Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology, 11, 601–609. 10.1159/000333542 [DOI] [PubMed] [Google Scholar]
- Mori, Y. , Ishida, W. , Bhattacharyya, S. , Li, Y. , Platanias, L. C. , & Varga, J. (2004). Selective inhibition of activin receptor‐like kinase 5 signaling blocks profibrotic transforming growth factor β responses in skin fibroblasts. Arthritis and Rheumatism, 50, 4008–4021. 10.1002/art.20658 [DOI] [PubMed] [Google Scholar]
- Nangaku, M. (2004). Mechanisms of tubulointerstitial injury in the kidney: Final common pathways to end‐stage renal failure. Internal Medicine, 43, 9–17. 10.2169/internalmedicine.43.9 [DOI] [PubMed] [Google Scholar]
- Ni, J. , Shen, Y. , Wang, Z. , Shao, D. C. , Liu, J. , Fu, L. J. , … Lu, L. M. (2014). Inhibition of STAT3 acetylation is associated with angiotesin renal fibrosis in the obstructed kidney. Acta Pharmacologica Sinica, 35, 1045–1054. 10.1038/aps.2014.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E. R. , Naugle, J. E. , Zhang, X. , Bomser, J. A. , & Meszaros, J. G. (2005). Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. American Journal of Physiology. Heart and Circulatory Physiology, 288, H1131–H1138. 10.1152/ajpheart.00763.2004 [DOI] [PubMed] [Google Scholar]
- Qi, W. , Chen, X. , Poronnik, P. , & Pollock, C. A. (2006). The renal cortical fibroblast in renal tubulointerstitial fibrosis. The International Journal of Biochemistry & Cell Biology, 38, 1–5. 10.1016/j.biocel.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Sharma, K. , Jin, Y. , Guo, J. , & Ziyadeh, F. N. (1996). Neutralization of TGF‐β by anti‐TGF‐β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ‐induced diabetic mice. Diabetes, 45, 522–530. 10.2337/diab.45.4.522 [DOI] [PubMed] [Google Scholar]
- Song, K. , Wang, F. , Li, Q. , Shi, Y. B. , Zheng, H. F. , Peng, H. , … Hu, L. F. (2014). Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney International, 85, 1318–1329. 10.1038/ki.2013.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz, F. , & Zeisberg, M. (2006). Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol, 17, 2992–2998. 10.1681/ASN.2006050420 [DOI] [PubMed] [Google Scholar]
- Tan, R. J. , Zhou, D. , Zhou, L. , & Liu, Y. (2014). Wnt/β‐catenin signaling and kidney fibrosis. Kidney International. Supplement (2011), 4, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasova, D. , & Anders, H. J. (2015). Cell cycle control in the kidney. Nephrology, Dialysis, Transplantation, 30, 1622–1630. 10.1093/ndt/gfu395 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wang, C. , Jia, Y. , Liu, Z. , Shu, X. , & Liu, K. (2016). Resveratrol increases anti‐proliferative activity of bestatin through downregulating P‐glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. Journal of Cellular Biochemistry, 117, 1233–1239. 10.1002/jcb.25407 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Zhou, P. H. , Xu, C. G. , Zhou, X. J. , Hu, W. , & Zhang, J. (2016). Baicalein ameliorates renal interstitial fibrosis by inducing myofibroblast apoptosis in vivo and in vitro. BJU International, 118, 145–152. 10.1111/bju.13219 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Li, G. N. , Xie, J. , Li, R. , Chen, Q. H. , Chen, J. Z. , … Xu, B. (2016). Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF‐β/periostin pathway in STZ‐induced diabetic mice. BMC Cardiovascular Disorders, 16, 5 10.1186/s12872-015-0169-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, Y. , Mann, D. M. , & Ruoslahti, E. (1990). Negative regulation of transforming growth factor‐β by the proteoglycan decorin. Nature, 346, 281–284. 10.1038/346281a0 [DOI] [PubMed] [Google Scholar]
- Yang, L. , Besschetnova, T. Y. , Brooks, C. R. , Shah, J. V. , & Bonventre, J. V. (2010). Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature Medicine, 16, 535–543. 531p following 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil, J. , & Bottinger, E. P. (2005). TGF‐β and epithelial‐to‐mesenchymal transitions. Oncogene, 24, 5764–5774. 10.1038/sj.onc.1208927 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Two‐step real‐time RT‐PCR primers for analysis
Table S2
Primary antibodies for proteins


