Abstract
Background
Critically ill patients who develop ARDS have substantial associated morbidity and mortality. Circulating mitochondrial DNA (mtDNA) released during critical illness causes endothelial dysfunction and lung injury in experimental models. This study hypothesized that elevated plasma mtDNA is associated with ARDS in critically ill patients with trauma and sepsis.
Methods
Plasma mtDNA concentrations were measured at ED presentation and approximately 48 h later in separate prospective cohorts of critically ill patients with trauma and sepsis. ARDS was classified according to the Berlin definition. The association of mtDNA with ARDS was tested by using multivariable logistic regression, adjusted for covariates previously shown to contribute to ARDS risk in each population.
Results
ARDS developed in 41 of 224 (18%) trauma patients and in 45 of 120 (38%) patients with sepsis. Forty-eight-hour mtDNA levels were significantly associated with ARDS (trauma: OR, 1.58/log copies/μL; 95% CI, 1.14-2.19 [P = .006]; sepsis: OR, 1.52/log copies/μL; 95% CI, 1.12-2.06 [P = .007]). Plasma mtDNA on presentation was not significantly associated with ARDS in either cohort. In patients with sepsis, 48-h mtDNA was more strongly associated with ARDS among those with a nonpulmonary infectious source (OR, 2.20/log copies/μL; 95% CI, 1.36-3.55 [P = .001], n = 69) than those with a pulmonary source (OR, 1.04/log copies/μL; 95% CI, 0.68-1.59 [P = .84], n = 51; P = .014 for interaction).
Conclusions
Plasma mtDNA levels were associated with incident ARDS in two critical illness populations. Given supportive preclinical data, our findings suggest a potential link between circulating mtDNA and lung injury and merit further investigation as a potentially targetable mediator of ARDS.
Key Words: ARDS, biomarkers, sepsis, trauma
Abbreviations: DAMP, damage-associated molecular pattern; ISS, Injury Severity Score; mtDNA, mitochondrial DNA
ARDS remains a common and morbid complication of critical illness. Trauma and sepsis contribute to 54% of ARDS cases,1, 2, 3 but the mechanisms by which nonpulmonary insults such as trauma or extrapulmonary sepsis propagate lung injury remain unclear. Identifying biomarkers with potential causal activity in trauma- and sepsis-associated ARDS may shed light on biological heterogeneity and inform efforts at targeted intervention.
Release of damage-associated molecular patterns (DAMPs) by stressed or dying cells has been proposed as a candidate mechanism linking distant injury to subsequent lung injury in critically ill patients.4 DAMPs ligate pattern recognition receptors that activate inflammatory pathways, amplifying damage from both sterile and infectious injuries. Mitochondrial DNA (mtDNA) is a prototypical DAMP capable of triggering innate immunity through multiple mechanisms.5 CpG DNA, a synthetic mtDNA analogue, caused pulmonary endothelial dysfunction and release of inflammatory mediators by activated leukocytes in in vitro studies, whereas in vivo models reported histologic lung injury following systemic administration of CpG or mitochondrial DAMPs.6, 7, 8, 9 These preclinical studies suggest that circulating mtDNA could represent a causal link between nonpulmonary insults and ARDS.
Multiple DAMPs have been detected in severely injured patients and may contribute to the persistent innate immune response observed following sterile injuries. Sepsis is also characterized by dysregulated inflammation, high levels of circulating DAMPs, and organ injury beyond the initial site of infection. These features make patients with trauma and sepsis useful populations in which to study the potential role of mtDNA in subsequent ARDS. In critically ill patients, mtDNA may be released into plasma by unregulated necrosis from direct cellular injury, programmed necrosis, or active ejection by leukocytes such as NETosis.10, 11, 12 mtDNA is also present in blood products and therefore may be introduced into circulation through transfusion, a potential mechanism of transfusion-associated organ dysfunction previously studied in surgical populations.13,14 In both populations, plasma mtDNA is elevated and associated with mortality,15, 16, 17, 18, 19 but clinical data linking mtDNA to ARDS are limited to one study in elderly patients with hip fractures and an unadjusted association of plasma mtDNA and ARDS in a mixed ICU population.18,20
Clarifying the role of circulating mtDNA in major populations at risk for ARDS is of particular clinical relevance, as it could predict response to mtDNA-targeted therapies. Inhibitors of a major mtDNA receptor, Toll-like receptor 9, are already in clinical trials for use in autoimmune disorders,21 and systemic administration of DNAse and Toll-like receptor 9 small interfering RNA reduced sepsis-induced organ dysfunction in preclinical and pilot human studies.22,23
The objective of the current study was to determine the association of circulating mtDNA with ARDS in two high-risk critical illness populations (trauma and sepsis), hypothesizing that higher plasma mtDNA levels would be associated with increased ARDS risk. We further hypothesized that this association would be strongest among patients with extrapulmonary injuries or sepsis sources. Secondary analyses were conducted to evaluate the impact of RBC transfusion on the mtDNA-ARDS relation and to determine the association of plasma mtDNA with mortality.
Patients and Methods
The full methods are presented in e-Appendix 1 and are summarized here.
Patient Population
This study included patients enrolled in two prospective cohorts, the Penn Trauma Organ Dysfunction Study (PETROS) and the Molecular Epidemiology of Sepsis in the ICU (MESSI), who had plasma samples available from both ED presentation and approximately 48 h later. The Institutional Review Board of the University of Pennsylvania approved these studies (approval numbers 802428 and 808542.)
mtDNA Measurement
We quantified mtDNA levels from blood samples drawn at ED presentation and 48 h by using polymerase chain reaction for the mitochondrial ND1 gene, measured in triplicate, as reported previously; log-transformed copy number per microliter was used to ensure normality.24
Primary and Secondary Outcomes
ARDS was defined as previously described applying the Berlin criteria, utilizing data over the first 6 days following presentation.25 All chest radiographs were assessed by two trained reviewers (J. P. R. and M. G. S. S.). Mortality was assessed at 30 days following presentation.
Statistical Analysis
Differences in clinical characteristics according to ARDS status and plasma mtDNA level were tested for by using the Student t test and the χ2 test. We assessed whether mtDNA concentration differed according to ARDS status by using the Student t test. Multivariable logistic regression models were used to adjust for confounders associated with ARDS in these populations (detailed in e-Appendix 1). Adjusted ARDS risk was determined across the range of plasma mtDNA levels by using postestimation marginal analysis. We assessed for interactions between mtDNA and pulmonary contusion in trauma patients and pulmonary source of infection in patients with sepsis using likelihood ratio testing.
Secondary analyses evaluated the impact of RBC transfusions and determined the association of plasma mtDNA concentrations with mortality. Logistic regression models for both cohorts were adjusted for RBC transfusion, and the Spearman correlation coefficient was used to test associations between mtDNA levels and transfusions. The association of plasma mtDNA concentrations with mortality, adjusted for confounders, was determined with logistic regression. Stata version 15 (StataCorp LLC) was used for all analyses, and P values < .05 were considered significant.
Results
Cohort Characteristics
Between April 2012 and July 2016, a total of 225 trauma patients met PETROS enrollment criteria, and between May 2012 and October 2014, a total of 120 patients with sepsis met MESSI enrollment criteria. One trauma subject was excluded for an indeterminate 48-h plasma mtDNA measurement. Patient characteristics are shown in Table 1.
Table 1.
Cohort Characteristics
| Characteristic | Cohort |
|
|---|---|---|
| PETROS (n = 224) | MESSI (n = 120) | |
| Demographic characteristics | ||
| Race | ||
| Black | 115 (51.3%) | 51 (42.5%) |
| White | 94 (42.0%) | 63 (52.5%) |
| Asian | 10 (4.4%) | 1 (0.8%) |
| Other/unknown | 5 (2.2%) | 5 (4.2%) |
| Age, ya | 33 (25-60) | 61 (50-68) |
| Male sex | 180 (80.0%) | 49 (40.8%) |
| Illness and injury severity | ||
| APACHE II | 18 (12-24) | 32.5 (27-36) |
| Pulmonary infection source | N/A | 51 (42.5%) |
| ISSa | 25 (19-30) | NA |
| Blunt injury mechanism | 161 (71.9%) | NA |
| Pre-ICU shockb | 106 (47.3%) | 93 (77.5%) |
| Medical history | ||
| Diabetes | 15 (6.7%) | 45 (37.5%) |
| HTN | 56 (24.6%) | 70 (58.3%) |
| CHF | 8 (3.6%) | 21 (17.5%) |
| CKD | 6 (2.7%) | 20 (16.7%) |
| Packed RBC transfusions | ||
| Patients transfused | 117 (52.5%) | 44 (36.7%) |
| Units transfuseda,c | 6 (2-8) | 0 (0-1) |
| Outcomes | ||
| ARDS | 41 (18.3%) | 45 (37.5%) |
| 30-Day mortality | 17 (7.6%) | 50 (41.7%) |
APACHE II = Acute Physiology and Chronic Health Evaluation II; CHF = congestive heart failure; CKD = chronic kidney disease; HTN = hypertension; ISS = Injury Severity Score; MESSI = Molecular Epidemiology of Sepsis in the ICU; NA = not applicable; PETROS = Penn Trauma Organ Dysfunction Study.
Median (interquartile range).
Defined as mean arterial pressure < 65 mm Hg or use of vasopressors prior to ICU admission.
Within 6 h of presentation (PETROS); within first 2 days of ICU stay (MESSI); units transfused are totaled for patients who received at least one transfusion.
Forty-one trauma patients (18.3%) and 45 patients with sepsis (37.5%) developed ARDS. Sources of infection are noted in e-Table 1. In both cohorts, patients with ARDS had higher illness and injury severity scores and a greater likelihood of shock; they also received more RBC units vs non-ARDS patients (e-Table 2). Thirty-day mortality was increased 10-fold in trauma patients and twofold in patients with sepsis and ARDS compared with patients without ARDS.
Plasma mtDNA and ARDS Risk
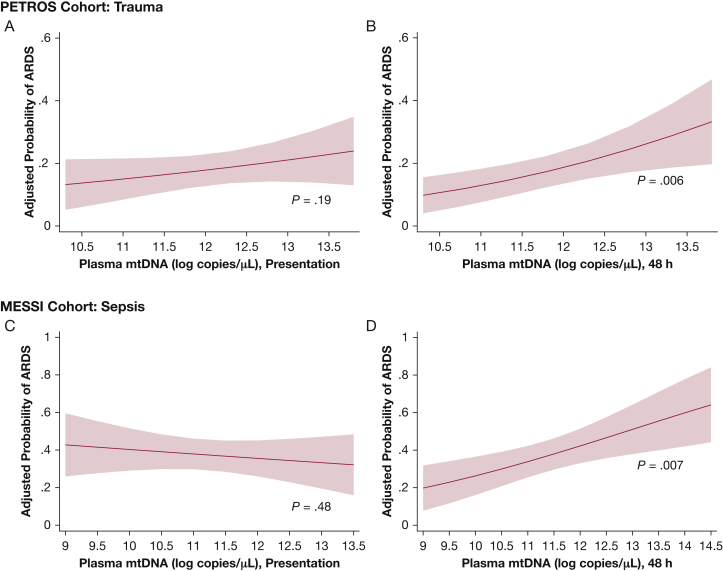
In the PETROS cohort, 48-h plasma mtDNA levels were significantly associated with ARDS (Table 2, e-Table 3), an association that persisted after adjustment for Injury Severity Score (ISS), shock, and mechanism of injury (Model 1, Table 3). The adjusted ARDS risk rose from approximately 10% to 30% between the fifth and 95th percentiles of 48-h plasma mtDNA values (Fig 1). In contrast, presentation mtDNA and change in mtDNA from presentation to 48 h were not associated with ARDS in unadjusted and adjusted analyses (e-Fig 1, Fig 1, Table 2, e-Table 3).
Table 2.
Plasma mtDNA in PETROS and MESSI Cohorts by ARDS
| Cohort | Outcome | Plasma [mtDNA],a Presentation | P Value | Plasma [mtDNA],a 48 h | P Value |
|---|---|---|---|---|---|
| PETROS (n = 224) | ARDS (n = 41) | 12.28 ± 1.07 | .184 | 12.22 ± 1.37 | .009 |
| No ARDS (n = 183) | 12.04 ± 1.01 | 11.74 ± 1.00 | |||
| Dead (n = 17) | 12.86 ± 0.83 | .001 | 12.30 ± 1.26 | .060 | |
| Alive (n = 207) | 12.02 ± 1.02 | 11.78 ± 1.07 | |||
| MESSI (n = 120) | ARDS (n = 45) | 11.06 ± 1.31 | .431 | 11.86 ± 1.62 | .003 |
| No ARDS (n = 75) | 11.25 ± 1.20 | 11.06 ± 1.26 | |||
| Dead (n = 50) | 11.09 ± 1.23 | .496 | 11.64 ± 1.59 | .073 | |
| Alive (n = 70) | 11.25 ± 1.26 | 11.16 ± 1.32 |
Unadjusted associations of plasma mitochondrial DNA (mtDNA) with ARDS and mortality according to cohort are shown. See Table 1 legend for expansion of other abbreviations.
Plasma level is given in log copies per microliter; mean ± SD.
Table 3.
Adjusted Association of 48-h Plasma mtDNA and ARDS in the PETROS Cohort
| Model | ARDS Risk Factor | OR (95% CI) | P Value |
|---|---|---|---|
| 1 | 48-h plasma [mtDNA] | 1.58 (1.14-2.19) | .006 |
| ISS | 1.03 (1.00-2.19) | .086 | |
| Blunt mechanism | 0.70 (0.29-1.68) | .420 | |
| Pre-ICU shock | 2.17 (1.03-4.56) | .041 | |
| 2 | 48-h plasma [mtDNA] | 1.37 (0.97-1.94) | .077 |
| ISS | 1.02 (0.98-1.06) | .257 | |
| Blunt mechanism | 0.31 (0.10-0.95) | .041 | |
| Pre-ICU shock | 1.50 (0.68-3.30) | .316 | |
| Packed RBC transfusions | 1.14 (1.04-1.25) | .006 |
Model 1 includes adjustment for ISS, blunt mechanism, and shock prior to ICU admission. Model 2 includes the additional covariate of packed RBC transfusions in the first 6 h following presentation to the ED. Two models were constructed because plasma mtDNA may be in a causal pathway between blood products, which contain mtDNA, and ARDS. See Table 1 and 2 legends for expansion of abbreviations.
Figure 1.
Association of mtDNA and ARDS in the first 6 days of hospitalization. Adjusted risk of ARDS as a function of presentation and 48-h plasma mtDNA is presented for the PETROS (A = Presentation, B = 48-h) and MESSI (C = Presentation, D = 48-h) cohorts. Forty-eight-hour plasma mtDNA was associated with ARDS in the PETROS and MESSI cohorts. mtDNA is presented in log copies per microliter of plasma. MESSI = Molecular Epidemiology of SepsiS in the ICU; mtDNA = mitochondrial DNA; PETROS = Penn TRauma Organ Dysfunction Study.
Findings in the MESSI cohort were highly consistent with those of the PETROS cohort. Patients with ARDS exhibited significantly higher 48-h mtDNA levels than non-ARDS patients, whereas presentation mtDNA levels did not differ significantly (Table 2). Following adjustment for presence of shock, pulmonary source of infection, and age, 48-h mtDNA remained significantly associated with ARDS risk (Table 4), with an increase in ARDS risk from approximately 20% to 60% from the fifth to 95th percentiles of mtDNA (Fig 1). Change in mtDNA at 48 h was highly associated with ARDS (e-Appendix 1, e-Fig 1).
Table 4.
Adjusted Association of Plasma mtDNA and ARDS in the MESSI Cohort
| Variable | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Overall MESSI cohorta | ||||
| ARDS risk factor | ||||
| 48-h plasma [mtDNA]b | 1.52 | 1.12-2.06 | .007 | |
| Age, per year | 0.99 | 0.97-1.02 | .520 | |
| Lung source of infection | 5.17 | 2.14-12.59 | < .001 | |
| Pre-ICU shock | 2.98 | 0.98-9.08 | .055 | |
| Stratified by infection sourcec | ||||
| Infection source | ARDS risk factor | |||
| Extrapulmonary (n = 69) | 48-h plasma [mtDNA]b | 2.20 | 1.36-3.55 | .001 |
| Age, per year | 1.02 | 0.98-1.06 | .453 | |
| Pre-ICU shock | 3.44 | 0.388-31.24 | .273 | |
| Pulmonary (n = 51) | 48-h plasma [mtDNA]b | 1.04 | 0.68-1.59 | .840 |
| Age, per year | 0.96 | 0.92-1.00 | .083 | |
| Pre-ICU shock | 3.00 | 0.79-11.45 | .108 | |
Adjusted association of 48-h plasma mtDNA concentration and ARDS in the MESSI cohort, adjusted for lung source of sepsis, shock, and age.
Per log copies per microliter.
Adjusted association of 48-h plasma mtDNA concentration in the MESSI cohort, stratified according to source of infection and adjusted for age and pre-ICU shock.
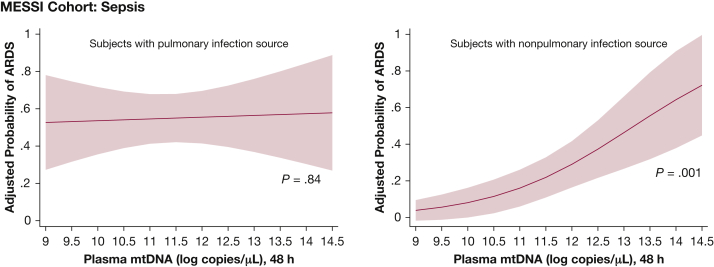
Effect of Extrapulmonary Injury or Infectious Source on mtDNA-ARDS Association
In the MESSI cohort, a significant interaction (P = .014) was detected between source of infection and 48-h mtDNA in the multivariable model predicting ARDS (Fig 2). Patients with extrapulmonary infection source (n = 69) exhibited a strong association of mtDNA with ARDS (OR, 2.20 per log copies/μL; 95% CI, 1.36-3.55; P = .001) (Table 4), whereas no association was seen in patients with a pulmonary infection source (n = 51; OR, 1.04 per log copies/μL; 95% CI, 0.68-1.59; P = .840). In PETROS, lung contusion (n = 62; 27.5% of subjects) did not interact with mtDNA-associated ARDS risk (P = .95).
Figure 2.
Association of 48-h mtDNA and ARDS stratified according to infection source in the MESSI cohort. Adjusted risk of ARDS as a function of 48-h plasma mtDNA in patients with a nonpulmonary infection source compared with patients with pneumonia. Forty-eight-hour mtDNA was strongly associated with ARDS in patients with nonpulmonary infectious sources but not in patients with pneumonia. mtDNA is presented in log copies per microliter of plasma. See Figure 1 legend for expansion of abbreviations.
Plasma mtDNA and RBC Transfusion
More than 50% of trauma patients in the PETROS cohort received an RBC transfusion within 6 h of presentation (Table 1). The number of RBC units received was significantly correlated with 48-h plasma mtDNA (rho = 0.21; P = .002) but not presentation mtDNA (rho = 0.09; P = .192), suggesting a possible contribution of transfusion to plasma mtDNA levels. Addition of 6-h RBC transfusions to the multivariable model predicting ARDS attenuated the association of 48-h plasma mtDNA with ARDS (Model 2, Table 3).
In MESSI, 27% of patients with sepsis were transfused with RBCs by 48 h; 78% of transfused patients received ≤ 2 units of RBCs. Plasma mtDNA at 48 h was not associated with transfusion. Transfusion was associated with ARDS (OR, 1.47 per log copies/μL; 95% CI, 1.01-2.12; P = .043) but did not attenuate the association of 48-h mtDNA and ARDS (OR, 1.50 per log copies/μL; 95% CI, 1.10-2.04; P = .011) following adjustment for RBC transfusion.
Plasma mtDNA and Mortality
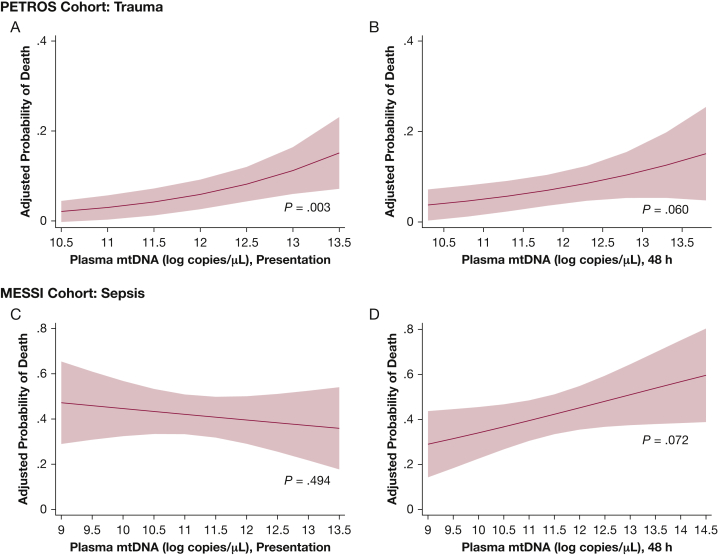
Presentation plasma mtDNA was significantly different in PETROS survivors vs nonsurvivors (Table 2) and remained highly associated with 30-day mortality following adjustment for ISS (OR, 2.08 per log copies/μL; 95% CI, 1.29-3.36; P = .003) (e-Table 4, Fig 3). Adjustment for RBC transfusion did not substantially affect this association (OR, 2.05 per log copies/μL; 95% CI, 1.27-3.32; P = .003). Plasma mtDNA levels at 48 h were higher in nonsurvivors but did not reach statistical significance. Change in mtDNA from presentation to 48 h was not associated with mortality in the PETROS cohort (P = .31).
Figure 3.
Association of mtDNA and mortality. Plasma mtDNA at presentation (A) was associated with mortality in the PETROS cohort. B = adjusted risk of mortality as a function of mtDNA at 48-h. C = adjusted risk of mortality as a function of presentation (C) and 48-h (D) in the MESSI cohort. mtDNA is presented in log copies per microliter of plasma. See Figure 1 legend for expansion of abbreviations.
Unlike in PETROS, there was no association of presentation mtDNA and mortality in MESSI (e-Table 4, Table 2). Similar to the PETROS cohort, however, 48-h mtDNA in MESSI cohort patients was numerically but nonsignificantly higher in nonsurvivors (OR, 1.27 per log copies/μL; 95% CI, 0.98-1.66; P = .072). Change in mtDNA from presentation to 48 h was highly associated with mortality in the MESSI cohort (P = .007 in unadjusted analysis; OR, 0.66 per decrease in log copies/μL; 95% CI, 0.48-0.91; P = .01). Stratification according to infection source did not affect the association of plasma mtDNA with mortality.
Discussion
In critically ill patients with trauma and sepsis, we found a significant association of circulating mtDNA with ARDS evident at 48 h following ED presentation, robust to adjustment for multiple ARDS risk factors specific to each population. Presentation levels of mtDNA were not associated with ARDS. ARDS risk increased nearly threefold in both cohorts among those in the 95th percentile compared with the fifth percentile of 48-h plasma mtDNA. In patients with sepsis, the association was strongest among those with a nonpulmonary infectious source. Taken in the context of published preclinical studies, these findings suggest that circulating mtDNA may contribute to ARDS pathophysiology in critically ill populations, potentially illuminating a mechanism by which nonpulmonary insults precipitate lung injury.6, 7, 8
Animal studies convincingly show that systemic mtDNA administration, a useful model of nonpulmonary systemic inflammation, causes lung injury.7, 8, 9 Previous human studies of mtDNA in ARDS are limited to studies by Zhang et al20 in 156 elderly hip fracture patients and Nakahira et al18 in a mixed ICU population, both of which reported testing at single time points. Our findings in nearly 350 patients add further data in two key populations, sepsis and trauma, that contribute to > 50% of ARDS cases.1, 2, 3 We showed that the mtDNA-ARDS association was robust to adjustment for multiple confounders but did not become evident until following presentation. Furthermore, the interaction of mtDNA with sepsis source corroborates mechanisms seen in animal models whereby remote inflammatory insults may be propagated to the lung by mtDNA. Although a reverse causal relationship, with ARDS resulting in elevated plasma mtDNA, is possible, the lack of any mtDNA-ARDS association among patients with pulmonary sepsis argues against this explanation. Stratifying patients with trauma based on presence of pulmonary contusion did not show a similar interaction; however, this may reflect the common co-occurrence of nonpulmonary injury in patients with lung contusion. Plasma mtDNA levels were substantially higher in trauma patients compared with patients with ARDS. Both passive release from cell death and active ejection by stressed cells may contribute to plasma mtDNA levels in critically ill patients,10,26 but abrupt and profound cell death due to injury may explain higher levels in trauma relative to sepsis. With pharmacologic inhibitors of mtDNA signaling pathways in development, our findings suggest this pathway may be relevant to ARDS prevention and treatment efforts.8,16, 17, 18,27, 28, 29, 30, 31, 32
RBC transfusion was associated with ARDS in both cohorts, and adjustment for RBC transfusion mitigated the association of mtDNA and ARDS in trauma patients. Blood products, which contain substantial amounts of mtDNA,14,33 may directly increase circulating mtDNA levels. mtDNA quantity in transfused blood products is associated with subsequent ARDS, suggesting that mtDNA may partially mediate the relationship of transfusion with ARDS.13 We found a significant correlation between 48-h mtDNA and transfusion, and therefore some proportion of circulating mtDNA may originate from transfusion. The associations of both RBC transfusion and 48-h plasma mtDNA with ARDS were independent of ISS, supporting the idea that transfusions and mtDNA are not simply surrogates for injury severity but may in fact have a mechanistic relationship with ARDS. Because transfusion is a potentially modifiable risk factor for ARDS, the relation between mtDNA and transfusion merits further investigation.
We showed a strong association of mtDNA with mortality in trauma patients, consistent with several previous studies in smaller cohorts.8,16,19,27,28,31 Although the association of plasma mtDNA with mortality was not statistically significant in the MESSI cohort, we were likely underpowered to detect such an association compared with previous studies.18,19,29,34,35 Furthermore, timing of blood draws differed between our study and those that detected associations of mtDNA and mortality, potentially accounting for the variable findings.18,19,32,34
Our study has notable strengths. We used two cohorts with distinct but significant ARDS risk, substantial mtDNA release, and in which circulating factors may be particularly important in connecting distant tissue damage to lung injury. We clarified differences in plasma mtDNA effect at two time points, including the earliest possible time point of ED presentation, and used detailed ARDS phenotyping. To the best of our knowledge, this analysis is the largest cohort of trauma patients to date to determine the association of plasma mtDNA with ARDS and mortality.
There are several important limitations to the current study, however. Despite utilizing two cohorts, this was a single-center study, which may limit generalizability. The sample size limited adjustments to known confounders of large effect and reduced power for subgroup analyses. This study does not prove that circulating mtDNA causes ARDS; in fact, lung injury could result in elevated circulating mtDNA at 48 h, although our finding that patients with sepsis and a pulmonary infection source had no mtDNA-ARDS association argues against this reverse causal effect, as does the substantial preclinical evidence from animal models.7,8,24,36,37 Our ability to identify whether mtDNA is elevated prior to ARDS incidence was limited by the lack of samples available between presentation and 48 h. Further research detailing early plasma mtDNA dynamics during critical illness will help to identify the earliest association and infer a causal relationship between circulating mtDNA and ARDS, a necessary step to inform the potential value of mtDNA pathway-specific therapies. Nevertheless, this study provides compelling evidence linking mtDNA and ARDS.
Conclusions
This study identified an association between plasma mtDNA levels 48 h after presentation and ARDS in critically ill patients with trauma and sepsis. In patients with sepsis, this relation was strongest in those with a nonpulmonary infection source. Our results suggest that plasma mtDNA could identify a mechanistic endotype of ARDS risk in the setting of distant injury. Because ARDS currently lacks specific pharmacologic preventive or therapeutic strategies, the identification of markers that inform the likelihood of patient-level treatment response may accelerate development of effective therapies. With agents targeting mtDNA signaling already in trials for noncritically ill patients, such precision therapy is within reach. This pathway merits further research to validate and potentially exploit its therapeutic potential.
Acknowledgments
Author contributions: H. E. F. and M. G. S. S. are guarantors of the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. H. E. F, M. G. S. S., N. J. M., and N. S. M. conceived the hypothesis and designed the study. H. E. F. analyzed the data and drafted the initial manuscript. C. A. G. I., P. Z., B. A. W., and C. M. F. were involved in data collection and processing. All authors participated in manuscript editing for critical intellectual content and approved the final manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the patients who consented to be included in the study, and the physicians, nurses, and staff of the ICUs for their care of the patients.
Additional information: The e-Appendix, e-Tables, and e-Figure can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [T32HL007775 to H. E. F.], [K23HL125723 to J. P. R.], [K23HL140482 to B. J. A.], [K08HL131995 to D. N. H.], [K24HL115354 to J. D. C.], [R01HL137006 to N. J. M.], and the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award No. W81XWH-15-1-0363 (N. S. M.) and the National Institutes of Health [grant R01DK111638 to M. G. S. S.].
Supplementary Data
References
- 1.Bellani G., Laffey J.G., Pham T. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary M.P., Keeley J.A., Yule A. Clinical predictors of early acute respiratory distress syndrome in trauma patients. Am J Surg. 2016;212(6):1096–1100. doi: 10.1016/j.amjsurg.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Eworuke E., Major J.M., Gilbert McClain L.I. National incidence rates for acute respiratory distress syndrome (ARDS) and ARDS cause-specific factors in the United States (2006-2014) J Crit Care. 2018;47:192–197. doi: 10.1016/j.jcrc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Kaczmarek A., Vandenabeele P., Krysko D.V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.West A.P., Shadel G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17(6):363–375. doi: 10.1038/nri.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun S., Sursal T., Adibnia Y. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Deng S., Zhao S. Intra-peritoneal administration of mitochondrial DNA provokes acute lung injury and systemic inflammation via Toll-like receptor 9. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q., Raoof M., Chen Y. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knuefermann P., Baumgarten G., Koch A. CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir Res. 2007;8:72. doi: 10.1186/1465-9921-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefi S., Gold J.A., Andina N. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 11.Thurairajah K., Briggs G.D., Balogh Z.J. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg. 2018;44(3):325–334. doi: 10.1007/s00068-018-0954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sureshbabu A., Patino E., Ma K.C. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018;3(11) doi: 10.1172/jci.insight.98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons J.D., Lee Y.L., Pastukh V.M. Potential contribution of mitochondrial DNA damage associated molecular patterns in transfusion products to the development of acute respiratory distress syndrome after multiple transfusions. J Trauma Acute Care Surg. 2017;82(6):1023–1029. doi: 10.1097/TA.0000000000001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y.L., King M.B., Gonzalez R.P. Blood transfusion products contain mitochondrial DNA damage-associated molecular patterns: a potential effector of transfusion-related acute lung injury. J Surg Res. 2014;191(2):286–289. doi: 10.1016/j.jss.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Caro V., Walko T.D., III, Bola R.A. Plasma mitochondrial DNA—a novel DAMP in pediatric sepsis. Shock. 2016;45(5):506–511. doi: 10.1097/SHK.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons J.D., Lee Y.L., Mulekar S. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013;258(4):591–596. doi: 10.1097/SLA.0b013e3182a4ea46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu X., Yao Y., Wu G., Lv T., Luo L., Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakahira K., Kyung S.Y., Rogers A.J. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10(12) doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanouchi S., Kudo D., Yamada M., Miyagawa N., Furukawa H., Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care. 2013;28(6):1027–1031. doi: 10.1016/j.jcrc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J.Z., Wang J., Qu W.C. Plasma mitochondrial DNA levels were independently associated with lung injury in elderly hip fracture patients. Injury. 2017;48(2):454–459. doi: 10.1016/j.injury.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Balak D.M., van Doorn M.B., Arbeit R.D. IMO-8400, a toll-like receptor 7, 8, and 9 antagonist, demonstrates clinical activity in a phase 2a, randomized, placebo-controlled trial in patients with moderate-to-severe plaque psoriasis. Clin Immunol. 2017;174:63–72. doi: 10.1016/j.clim.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji N., Tsuji T., Ohashi N., Kato A., Fujigaki Y., Yasuda H. Role of mitochondrial DNA in septic AKI via Toll-like receptor 9. J Am Soc Nephrol. 2016;27(7):2009–2020. doi: 10.1681/ASN.2015040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L., Li Y., Hu Z. Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron Exp Nephrol. 2012;122(1-2):51–61. doi: 10.1159/000346953. [DOI] [PubMed] [Google Scholar]
- 24.Hotz M.J., Qing D., Shashaty M.G.S. Red blood cells homeostatically bind mitochondrial DNA through TLR9 to maintain quiescence and to prevent lung injury. Am J Respir Crit Care Med. 2018;197(4):470–480. doi: 10.1164/rccm.201706-1161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson N.D., Fan E., Camporota L. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 26.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 27.McIlroy D.J., Bigland M., White A.E. Cell necrosis-independent sustained mitochondrial and nuclear DNA release following trauma surgery. J Trauma Acute Care Surg. 2015;78(2):282–288. doi: 10.1097/TA.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam N.Y., Rainer T.H., Chan L.Y., Joynt G.M., Lo Y.M. Time course of early and late changes in plasma DNA in trauma patients. Clin Chem. 2003;49(8):1286–1291. doi: 10.1373/49.8.1286. [DOI] [PubMed] [Google Scholar]
- 29.Krychtiuk K.A., Ruhittel S., Hohensinner P.J. Mitochondrial DNA and Toll-like receptor-9 are associated with mortality in critically ill patients. Crit Care Med. 2015;43(12):2633–2641. doi: 10.1097/CCM.0000000000001311. [DOI] [PubMed] [Google Scholar]
- 30.Timmermans K., Kox M., Scheffer G.J., Pickkers P. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. 2016;45(6):607–612. doi: 10.1097/SHK.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 31.Lam N.Y., Rainer T.H., Chiu R.W., Joynt G.M., Lo Y.M. Plasma mitochondrial DNA concentrations after trauma. Clin Chem. 2004;50(1):213–216. doi: 10.1373/clinchem.2003.025783. [DOI] [PubMed] [Google Scholar]
- 32.Schafer S.T., Franken L., Adamzik M. Mitochondrial DNA: an endogenous trigger for immune paralysis. Anesthesiology. 2016;124(4):923–933. doi: 10.1097/ALN.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 33.Bakkour S., Acker J.P., Chafets D.M. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016;111(1):22–32. doi: 10.1111/vox.12390. [DOI] [PubMed] [Google Scholar]
- 34.Puskarich M.A., Shapiro N.I., Trzeciak S., Kline J.A., Jones A.E. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock. 2012;38(4):337–340. doi: 10.1097/SHK.0b013e318266a169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kung C.T., Hsiao S.Y., Tsai T.C. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med. 2012;10:130. doi: 10.1186/1479-5876-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gan L., Chen X., Sun T. Significance of serum mtDNA concentration in lung injury induced by hip fracture. Shock. 2015;44(1):52–57. doi: 10.1097/SHK.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 37.Kuck J.L., Obiako B.O., Gorodnya O.M. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol. 2015;308(10):L1078–L1085. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.