Abstract
Background and Purpose
General anaesthetics can act on synaptic GABAA receptors by binding to one of three classes of general anaesthetic sites. Canonical drugs that bind selectively to only one class of site are etomidate, alphaxalone, and the mephobarbital derivative, R‐mTFD‐MPAB. We tested the hypothesis that the general anaesthetic potencies of mixtures of such site‐selective agents binding to the same or to different sites would combine additively or synergistically respectively.
Experimental Approach
The potency of general anaesthetics individually or in combinations to cause loss of righting reflexes in tadpoles was determined, and the results were analysed using isobolographic methods.
Key Results
The potencies of combinations of two or three site‐selective anaesthetics that all acted on a single class of site were strictly additive, regardless of which single site was involved. Combinations of two or three site‐selective anaesthetics that all bound selectively to different sites always interacted synergistically. The strength of the synergy increased with the number of separate sites involved such that the percentage of each agent's EC50 required to cause anaesthesia was just 35% and 14% for two or three sites respectively. Propofol, which binds non‐selectively to the etomidate and R‐mTFD‐MPAB sites, interacted synergistically with each of these agents.
Conclusions and Implications
The established pharmacology of the three anaesthetic binding sites on synaptic GABAA receptors was sufficient to predict whether a mixture of anaesthetics interacted additively or synergistically to cause loss of righting reflexes in vivo. The principles established here have implications for clinical practice.
What is already known
Mixtures of general anaesthetics are known to sometimes interact additively and other times synergistically.
There are three distinct transmembrane‐domain intra‐subunit binding sites for general anaesthetics on synaptic GABAA receptors.
What this study adds
Combinations of anaesthetics that act on just one of the three known sites interact additively.
Combinations of anaesthetics that each act on a different site interact synergistically.
What is the clinical significance
Combining agents at 15% of their EC50s that selectively target three different sites causes anaesthesia.
Such a strategy may be generalizable to other ligand‐gated ion channels and other multi‐subunit targets.
Abbreviations
- 3α5βP
5β‐pregnan‐3α‐ol‐20‐one (pregnanolone)
- ECD
extracellular domain
- LoRR
loss of righting reflexes
- R‐mCF3‐MPAB
5‐allyl‐1‐methyl‐5‐(3‐trifluoromethylphenyl)‐pyrimidine‐2,4,6‐trione
- R‐miPr‐MPAB
5‐allyl‐5‐(3‐isopropylphenyl)‐1‐methyl‐pyrimidine‐2,4,6‐trione
- R‐mTFD‐MPAB
allyl m‐trifluoromethyldiazirine mephobarbital
- THDOC
tetrahydrodeoxycorticosterone
- TMD
transmembrane domain
1. INTRODUCTION
Although early work linked the action of general anaesthetics with the GABAA receptors (Leeb‐Lundberg, Snowman, & Olsen, 1980; Nicoll, Eccles, Oshima, & Rubia, 1975), the definitive study was based on the N265M mutation in the second transmembrane helix of the β3 subunit. This mutation both attenuated the action of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5463 on heterologously expressed GABAA receptors in vitro (Belelli, Lambert, Peters, Wafford, & Whiting, 1997) and attenuated etomidate's anaesthetic potency in the β3 N265M knock‐in mouse (Jurd et al., 2003). However, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5461 and the mephobarbital derivative, R‐mTFD‐MPAB, had the same general anaesthetic potency in wild‐type and the N265M knock‐in mouse (Amlong, Perkins, Houle, Miller, & Pearce, 2016), but the N265M mutation did not ablate their ability to enhance GABA currents at anaesthetic concentrations in vitro (Siegwart, Krahenbuhl, Lambert, & Rudolph, 2003; Szabo, Nourmahnad, Halpin, & Forman, 2019), suggesting, but not proving, that their in vivo actions are mediated by GABAA receptors.
GABAA receptors belong to the superfamily of pentameric Cys‐loop ligand‐gated ion channels. Synaptic GABAA receptors have the stoichiometry (α)2(β)2γ arranged as shown in Figure 1a. There is a high degree of homology between subunits, each being composed of a ~200 residue extracellular domain (ECD) containing the GABA sites, a transmembrane domain (TMD) consisting of a bundle of four transmembrane helices, and an unstructured intracellular domain between the third and fourth transmembrane helices (Figure 1b; Laverty et al., 2019). Photolabelling, site‐directed cysteine accessibility mutagenesis experiments (reviewed in Forman & Miller, 2016) and more recently structural studies (Chen et al., 2018; Laverty et al., 2017; Miller et al., 2017) have identified three distinct general anaesthetic binding sites located at subunit interfaces in the TMD (Figure 1c). Each distinct site consists of two homologous binding pockets. In the extracellular third of the TMD, etomidate binds at the two β+/α− interfaces (Site 1), whereas R‐mTFD‐MPAB binds at the γ+/β− and the α+/β− interfaces (Site 2; Chiara et al., 2013). Neurosteroids also bind at the two β+/α− interfaces but at a separate sites in the intracellular third of the TMD (Site 3; Figure 1a,b; Hosie, Wilkins, da Silva, & Smart, 2006; Ziemba et al., 2018).
Figure 1.
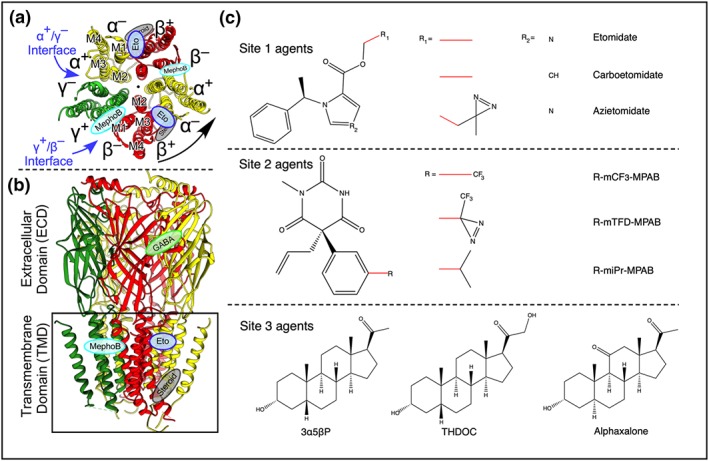
The structure, binding sites, and agonists of the α1β3γ2 GABAA receptor. (a) Cross section through the TMD viewed from the extracellular side showing previously established drug binding sites and the arrangement of the five subunits around a central pore. (b) Side view; the black box indicates the plasma membrane. Lozenges indicate the binding sites for GABA (green), etomidate (blue), mephobarbital derivatives (cyan), and steroids (grey). The protein backbone is in ribbon notation (Forman & Miller, 2016; Laverty et al., 2019). (c) The chemical structures of agents used in this study
Site‐selective agents all stabilize the open state of the GABAA receptor because their binding sites have higher affinity for that state than for the resting state (reviewed in Forman & Miller, 2011). Consequently, binding to one site will allosterically enhance the affinity of the other sites, leading to the hypothesis that combinations of general anaesthetics should act synergistically if they bind selectively to distinct sites. Pioneering studies have shown that steroids interact allosterically with etomidate and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5464 both in vivo and in vitro (Cao et al., 2018; Chen et al., 2014; Drexler, Balk, & Antkowiak, 2016). Here, using three sets of general anaesthetics that bind with high selectivity to one of the three sites shown in Figure 1c, we find that the potencies of combinations of site‐selective anaesthetics that bind to the same site are additive, whereas those that bind to two or three distinct sites are increasingly synergistic.
2. METHODS
2.1. Tadpole behavioural assay
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, Altman, & Group, 2010) and with the recommendations made by the British Journal of Pharmacology. All animal studies were conducted according to animal protocols preapproved by the Massachusetts General Hospital (MGH) Subcommittee on Research Animal Care (Protocols #2006N000124 and 2015N000012) with the approval of the MGH Institutional Animal Care and Use Committee.
Early pre‐limb bud stage Xenopus laevis tadpoles (XEP Cat#: Xla100, RRID:XEP_Xla100, approximately 2–2.5 cm in length) were purchased from Xenopus One (Ann Arbor, MI) and were housed in the MGH Center for Comparative Medicine Facilities. Fifty tadpoles were housed in 4‐L tanks with circulating dechlorinated water under a 12‐hr light/dark cycle. They were allowed 24 hr after arrival before experiments were conducted. Loss of righting reflexes (LoRR) assays were conducted in 100 ml of Tris buffered water (2.5 mM, pH = 7.4) in vessels containing five tadpoles. LoRR was assessed by inverting a tadpole with a bent Pasteur pipette. Tadpoles that then righted themselves within 5 s were scored 0, whereas those that failed to do so were scored 1. LoRR was assessed after 10, 30, and 60 min. Responses stabilized after 30 min. Values recorded after 1 hr of exposure were used in the analysis. After 1 hr, tadpoles were transferred to recovery beakers filled with 200 ml of dechlorinated tap water and were assessed 24 hr later to ensure recovery from anaesthesia. Once recovered, tadpoles were killed with 5% tricaine or a lethal concentration of pentobarbital. Tadpoles that did not recover (<0.01% of animals tested) were excluded from the analysis. All data were obtained by the first author and were not blinded.
Because the tadpole extracellular fluid readily equilibrates with the anaesthetic concentration in which they swim, the data obtained represent concentration–response rather than dose–response relationships. Data were fitted to a logistic curve to yield the EC50 (Waud, 1972). The average slope of site‐selective anaesthetics (3.3) was used in fitting all experimental concentration–response curves.
2.2. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Statistical analysis was performed using independent values. For control experiments with one drug alone, a varying number of tadpoles was required to accurately determine each compound's EC50. This was necessary because the EC50s were often unknown, so choosing concentrations that gave sufficient points on the steep part of the curve was by trial and error. In no case were less than five animals used at a given concentration. For combination experiments, one drug's concentration was held constant in solution while the other's concentration was varied to determine its new EC50. Forty tadpoles were normally sufficient to define each concentration–response curve, except when propofol was fixed at 0.158 and 0.315 μΜ and etomidate was varied (n = 50 and 55, respectively) because additional concentrations were needed for the reasons stated above. These results were analysed using isobolographic analysis.
An isobologram plots the mixtures of drugs eliciting the desired response (EC50, in our case). For simplicity, we normalized the concentrations of each drug relative to the EC50, creating fractional values rather than concentrations. The sum of the coordinates for each point gives the total effective fractional sum of drug, which has been characterized by Tallarida as the interaction index, or simply α, according to Equation (1). The line for additivity spans across the graph (α = 1). Points below this line (α < 1) indicate synergy, and points above this line (α > 1) indicate antagonism (Tallarida, 2002; Tallarida, 2016).
| (1) |
The determined α values for groups of data were averaged together, and the SDs were calculated based on Equation (2) (σ total is the combined SD, σi = the SD from an α value).
| (2) |
The GraphPad one sample t test and unpaired t test were used to compare average α values of experiments to the expected value of one or to one another respectively (RRID:SCR_000306). The one‐way ANOVA was used to compare groups of three sets of experiments with one another (RRID:SCR_016762). For all analyses, significance was set at 1% (P < .01).
2.3. Materials
R‐miPr‐MPAB and R‐mCF3‐MPAB were synthesized by methods similar to R‐mTFD‐MPAB as previously described (Savechenkov et al., 2012). See Supporting Information for details on the full synthesis. The chemicals 3α5βP, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4321, propofol, methanol, DMSO, and Tris were bought from Sigma‐Aldrich (St. Louis, MO). Alphaxalone was bought from Tocris Bioscience (Bristol, UK). Etomidate was purchased from Bachem (Bubendorf, Switzerland).
Sites 1 and 2 are subtly different homologous sites, causing some overlap in binding selectivity. The anaesthetics used in this study had at least a 20‐fold binding selectivity for their respective site and are considered site‐selective. The etomidate derivatives are site‐selective for Site 1 while the mephobarbital derivatives are site‐selective for Site 2 (Chiara et al., 2013; Cohen, J. 2018, unpublished data). Neurosteroids have only been shown to act on Site 3, due to their unique structure, and are considered site‐selective.
2.5‐mM Tris buffer was used for all assays and was made from a 1‐M Tris stock. Stocks of 16 L of dechlorinated tap water (AmQuel Plus; from Kordon, Hayward, CA) were used to fill the recovery beakers for the tadpoles post anaesthesia. Stock solutions of anaesthetics were dissolved in either DMSO or methanol. The final DMSO concentration did not exceed 0.04%. Control experiments showed that 0.2% DMSO caused no LoRR (0/10 animals). For drug stocks in methanol, the methanol was evaporated in glass containers using argon gas before buffer was added. All stocks of photo‐sensitive drugs were stored in amber glassware and covered in aluminium foil while in use.
2.4. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Fabbro et al., 2017; Alexander, Peters et al., 2017).
3. RESULTS
3.1. Potencies of individual anaesthetics
The concentration dependence of the LoRR was determined for the 10 anaesthetics in Table 1 as described in Section 2. For example, in the case of alphaxalone, groups of tadpoles were equilibrated in one of seven concentrations of alphaxalone and the fraction of tadpoles experiencing LoRR is shown in Figure 2a. The EC50s of individual anaesthetics are reported in Table 1 and are consistent with the previously published literature (alphaxalone: 1.12 ± 0.14, Stastna et al., 2011; etomidate: 2.3 μΜ [95% confidence limits, 1.9–2.7 μΜ], Belelli et al., 2003; azietomidate: 2.2 ± 0.14 μΜ, Husain et al., 2003; R‐mTFD‐MPAB: 3.7 ± 0.25 μΜ, Savechenkov et al., 2012; propofol: 0.52 ± 0.08 μΜ, Germann et al., 2016; carboetomidate: 5.4 ± 0.5 μM, Cotten et al., 2010; and 3α5βP: 63 ± 3 nM, Chen et al., 2014). Additionally, the primary binding site of these anaesthetics are listed in Table 1.
Table 1.
Tadpole LoRR EC50s of individual anaesthetics
| Drug | Site | EC50 (μΜ) | n a |
|---|---|---|---|
| Etomidate | 1 | 2.22 ± 0.18 | 95 |
| Azietomidate | 1.22 ± 0.11 | 50 | |
| Carboetomidate | 5.43 ± 0.59 | 50 | |
| R‐mTFD‐MPAB | 2 | 2.34 ± 0.14 | 130 |
| R‐mCF3‐MPAB | 7.33 ± 0.81 | 55 | |
| R‐miPr‐MPAB | 1.14 ± 0.20 | 40 | |
| Propofol | 1 and 2 | 0.63 ± 0.09 | 85 |
| Alphaxalone | 3 | 1.42 ± 0.13 | 55 |
| 3α5βP | 0.31 ± 0.03 | 50 | |
| THDOC | 2.50 ± 0.16 | 85 |
n is the number of tadpoles used to determine the concentration–response curve.
Figure 2.
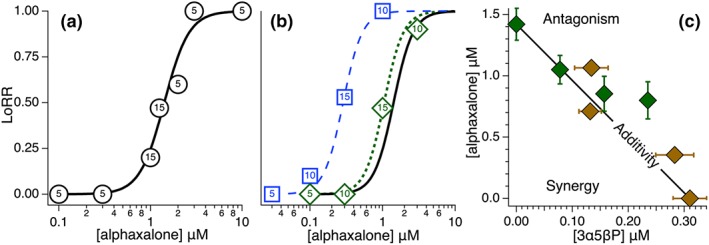
The EC50 of alphaxalone (Site 3) is shifted differently by the presence of 25% of the EC50 of either 3α5βP (Site 3) or etomidate (Site 1). In (a), the loss of righting reflexes (LoRR) is plotted against the free concentration of alphaxalone in the aqueous phase surrounding the tadpoles. The fraction of animals experiencing LoRR is represented by the vertical placement of the circles, with the number of animals tested displayed within them (total n = 55). The quantal data was fit as described in Methods and the EC50 is displayed in Table 1. (b): Alphaxalone concentration response curves (C.R.C.) are shown in the presence of 25% of either 3α5βP's or etomidate's EC50. (c): The concentrations of mixtures of alphaxalone and 3α5βP that together produce an EC50 response are plotted. The concentration of either alphaxalone or 3α5βP was varied while the concentration of the other anaesthetic was fixed. In these and succeeding figures, the thick black line represents the theoretical line for additivity where α = 1 (Equation 1; Tallarida, 2016). The error bars shown are the SDs of the EC50s.
25% of either 3α5βP's (Site 3) or etomidate's (Site 1) EC50 was held fixed in solution and alphaxalone's new concentration–response curve was determined and is shown in Figure 2b. The concentration–response curves both shifted left, but the alphaxalone + etomidate mixture shifted much more so, indicating synergy. The α values for alphaxalone + 3α5βP and alphaxalone + etomidate are reported in Tables 2 and 3, respectively. The principles established here will guide our comparison of synergy and additivity among different anaesthetic mixtures.
Table 2.
Fixed concentrations, EC50s, and αs of all same‐site anaesthetic mixtures
| Drug A | [Drug A] μM | Drug B | [Drug B] μM | Drug C | [Drug C] μM | α | Average α | |
|---|---|---|---|---|---|---|---|---|
| Site 1 | Etomidate | 0.60 | Azietomidate | 0.77 ± 0.12 | — | — | 0.90 ± 0.10 | |
| 1.00 | 0.86 ± 0.11 | — | — | 1.15 ± 0.09 | ||||
| 1.78 | 0.34 ± 0.07 | — | — | 1.08 ± 0.06 | ||||
| 1.46 ± 0.16 | 0.305 | — | — | 0.91 ± 0.07 | ||||
| 1.05 ± 0.12 | 0.53 | — | — | 0.90 ± 0.05 | ||||
| 0.42 ± 0.09 | 1.20 | — | — | 1.12 ± 0.04 | 1.01 ± 0.14 | |||
| 0.111 | 1.04 ± 0.15 | Carboetomidate | 0.27 | 0.95 ± 0.12 | ||||
| 0.222 | 0.96 ± 0.54 | 0.54 | 0.98 ± 0.12 | |||||
| 0.555 | 0.34 ± 0.06 | 1.36 | 0.78 ± 0.05 | |||||
| 0.111 | 0.06 | 4.22 ± 0.55 | 0.88 ± 0.10 | |||||
| 0.222 | 0.12 | 3.51 ± 0.49 | 0.85 ± 0.09 | |||||
| 0.555 | 0.305 | 1.44 ± 0.39 | 0.77 ± 0.07 | |||||
| 1.93 ± 0.26 | 0.06 | 0.27 | 0.97 ± 0.12 | |||||
| 1.67 ± 0.22 | 0.12 | 0.54 | 0.95 ± 0.10 | |||||
| 1.00 ± 0.13 | 0.305 | 1.36 | 0.95 ± 0.06 | 0.90 ± 0.12 | ||||
| Site 2 | R‐mTFD‐MPAB | 0.585 | R‐mCF3‐MPAB | 6.93 ± 0.85 | — | — | 1.20 ± 0.12 | |
| 1.17 | 2.75 ± 0.39 | — | — | 0.88 ± 0.05 | ||||
| 1.755 | 1.12 ± 0.21 | — | — | 0.90 ± 0.03 | ||||
| 1.78 ± 0.20 | 1.82 | — | — | 1.01 ± 0.09 | ||||
| 1.23 ± 0.12 | 3.64 | — | — | 1.03 ± 0.05 | ||||
| 1.20 ± 0.12 | 5.46 | — | — | 1.26 ± 0.05 | 1.05 ± 0.17 | |||
| 0.12 | 4.42 ± 0.67 | R‐miPr‐MPAB | 0.06 | 0.70 ± 0.09 | ||||
| 0.234 | 3.39 ± 0.54 | 0.11 | 0.66 ± 0.07 | |||||
| 0.585 | 3.07 ± 0.48 | 0.29 | 0.92 ± 0.07 | |||||
| 0.12 | 0.37 | 0.97 ± 0.15 | 0.95 ± 0.13 | |||||
| 0.23 | 0.73 | 0.83 ± 0.15 | 0.93 ± 0.13 | |||||
| 0.585 | 1.83 | 0.30 ± 0.04 | 0.77 ± 0.04 | |||||
| 1.09 ± 0.17 | 0.37 | 0.06 | 0.56 ± 0.07 | |||||
| 1.06 ± 0.13 | 0.73 | 0.11 | 0.65 ± 0.07 | |||||
| 0.82 ± 0.17 | 1.83 | 0.285 | 0.85 ± 0.07 | 0.78 ± 0.17 a | ||||
| Site 3 | Alphaxalone | 0.355 | 3α5βP | 0.28 ± 0.03 | — | — | 1.16 ± 0.11 | |
| 0.71 | 0.13 ± 0.02 | — | — | 0.93 ± 0.06 | ||||
| 1.065 | 0.13 ± 0.03 | — | — | 1.18 ± 0.10 | ||||
| 1.05 ± 0.12 | 0.078 | — | — | 0.99 ± 0.08 | ||||
| 0.85 ± 0.14 | 0.157 | — | — | 1.10 ± 0.10 | ||||
| 0.80 ± 0.15 | 0.235 | — | — | 1.31 ± 0.11 | 1.11 ± 0.17 | |||
| 0.07 | 0.26 ± 0.04 | THDOC | 0.125 | 0.94 ± 0.13 | ||||
| 0.14 | 0.20 ± 0.03 | 0.25 | 0.85 ± 0.10 | |||||
| 0.355 | 0.13 ± 0.02 | 0.625 | 0.92 ± 0.06 | |||||
| 0.07 | 0.016 | 1.84 ± 0.35 | 0.83 ± 0.14 | |||||
| 0.14 | 0.031 | 1.34 ± 0.20 | 0.74 ± 0.08 | |||||
| 0.355 | 0.078 | 0.93 ± 0.13 | 0.87 ± 0.05 | |||||
| 0.86 ± 0.11 | 0.016 | 0.125 | 0.71 ± 0.08 | |||||
| 0.95 ± 0.11 | 0.031 | 0.25 | 0.87 ± 0.08 | |||||
| 0.45 ± 0.08 | 0.078 | 0.625 | 0.82 ± 0.05 | 0.84 ± 0.12 a |
Note. α values are reported as ±SD. SD of average αs are calculated according to Equation (2). Each concentration–response curve had 40 animals.
Differs significantly (P < .01) from the value of one expected for additivity.
Table 3.
Fixed concentrations, EC50s, and αs for all combinations of Site 1, 2, and 3 anaesthetics
| Site 1 | Site 2 | Site 3 | α | Average α |
|---|---|---|---|---|
| [Etomidate] μM | [R‐mTFD‐MPAB] μΜ | [Alphaxalone] μΜ | ||
| 0.555 | — | 0.29 ± 0.03 | 0.45 ± 0.02 | |
| 1.11 | — | 0.10 ± 0.02 | 0.57 ± 0.01 | |
| 1.665 | — | 0.03 ± 0.01 | 0.77 ± 0.00 | |
| 0.74 ± .08 | — | 0.355 | 0.58 ± 0.04 | |
| 0.26 ± 0.03 | — | 0.71 | 0.62 ± 0.01 | |
| 0.31 ± .04 | — | 1.065 | 0.89 ± 0.02 | 0.65 ± 0.16 a |
| 0.22 | 1.33 ± 0.20 | — | 0.67 ± 0.08 | |
| 0.55 | 1.16 ± 0.22 | — | 0.75 ± 0.09 | |
| 1.2 | 0.25 ± 0.05 | — | 0.65 ± 0.02 | |
| 1.32 ± 0.17 | 0.25 | — | 0.70 ± 0.08 | |
| 0.76 ± 0.10 | 0.625 | — | 0.61 ± 0.04 | |
| 0.23 ± 0.04 | 1 | — | 0.53 ± 0.02 | 0.69 ± 0.09 a |
| — | 0.585 | 0.79 ± 0.10 | 0.80 ± 0.07 | |
| — | 1.17 | 0.31 ± 0.05 | 0.72 ± 0.03 | |
| — | 1.755 | 0.21 ± 0.03 | 0.90 ± 0.02 | |
| — | 1.35 ± 0.15 | 0.355 | 0.83 ± 0.06 | |
| — | 0.31 ± 0.56 | 0.71 | 0.63 ± 0.04 | |
| — | 0.09 ± 0.01 | 1.065 | 0.79 ± 0.01 | 0.78 ± 0.10 a |
| 0.111 | 0.30 ± 0.03 | 0.07 | 0.23 ± 0.01 | |
| 0.222 | 0.12 ± 0.02 | 0.14 | 0.25 ± 0.10 | |
| 0.555 | 0.01 ± 0.01 | 0.355 | 0.51 ± 0.01 | |
| 0.111 | 0.12 | 0.26 ± 0.01 | 0.28 ± 0.02 | |
| 0.222 | 0.23 | 0.34 ± 0.01 | 0.44 ± 0.03 | |
| 0.555 | 0.585 | 0.03 ± 0.01 | 0.52 ± 0.01 | |
| 0.43 ± 0.06 | 0.12 | 0.07 | 0.29 ± 0.03 | |
| 0.96 ± 0.10 | 0.23 | 0.14 | 0.63 ± 0.04 | |
| 0.29 ± 0.04 | 0.585 | 0.355 | 0.63 ± 0.02 | 0.42 ± 0.16 a |
Note. α values are reported as ± SD. SD of average αs are calculated according to Equation (2). Each concentration–response curve had 40 animals.
Differs significantly (P < .01) from the value of one expected for additivity.
3.2. Two site‐selective anaesthetics acting on the same site interact additively
The Site 3 anaesthetics, alphaxalone and 3α5βP, were tested in six different combinations. In three cases, alphaxalone was fixed in solution at different concentrations while 3α5βP was varied, while in the remainder, the reverse procedure was followed. The fixed concentrations, EC50s determined, and α values of these experiments, as well as all same site mixture experiments in this study, are all reported in Table 2, and the isobologram analysis is given in Figure 2c. None differed significantly from the expected additive value of one.
For simplicity, we expressed the concentration of each drug as a fraction of its own EC50 and plotted the data according to Equation (1) in Figure 3c. In this method, the α values are simply the sum of the coordinates of each point. Of the six points tested, all are in close proximity to the line of additivity, with two below and four above it. The α values ranged from 0.93 to 1.31, as shown in Table 2.
Figure 3.
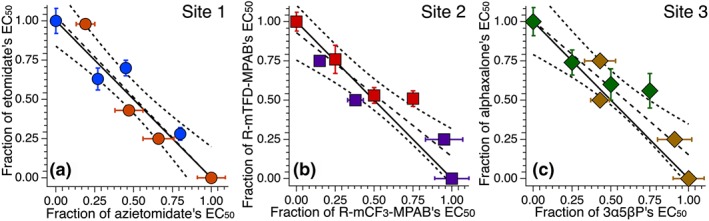
Isobolograms of mixtures of two anaesthetics that both act on the same site show additivity. The fractional concentrations of the drug mixtures required to produce 50% loss of righting reflexes (LoRR) are plotted with the theoretical line for additivity. Each point represents the concentrations of the two drugs, one of which was held constant and the other varied to determine the concentration‐response curve and EC50. L.R. is the linear least squares fit of the data and C.I. is its confidence interval. Shown are combinations of (a): etomidate (Eto) and azietomidate (Azi), both Site 1 anaesthetics; (b): R‐mTFD‐MPAB (MPAB) and R‐mCF3‐MPAB (CF3), both Site 2 anaesthetics, and (c): alphaxalone (Alphax) and 3α5βP, both Site 3 anaesthetics.
The Site 1 anaesthetics, etomidate and azi‐etomidate, were tested in the same manner as above, and the isobologram is displayed in Figure 3a. Of the six points tested, all are in close proximity to the line of additivity, with the α values ranging from 0.90 to 1.15.
The Site 2 anaesthetics, R‐mTFD‐MPAB and R‐mCF3‐MPAB, were tested in the same manner and the isobologram is displayed in Figure 3b. Of the six points tested, all are in close proximity to the line of additivity, with the α values ranging from 0.88 to 1.26. In addition, ANOVA was conducted between these three sets of same‐site mixtures, showing no significant differences.
3.3. Two site‐selective anaesthetics acting on different sites interact synergistically
Etomidate and alphaxalone, Sites 1 and 3 agents respectively, were tested in combination with one another in the same manner as described above. The fixed concentrations, EC50s, and α values of these experiments, as well as all experiments of combinations of anaesthetics acting on different sites, are displayed in Table 3, and the isobologram analysis is shown in Figure 4a. All α values for sets of two site‐selective anaesthetics were significantly lower than the value of one expected for additivity. Of the six points tested, all lay well below the line of additivity, with the α values ranging from 0.45 to 0.89.
Figure 4.
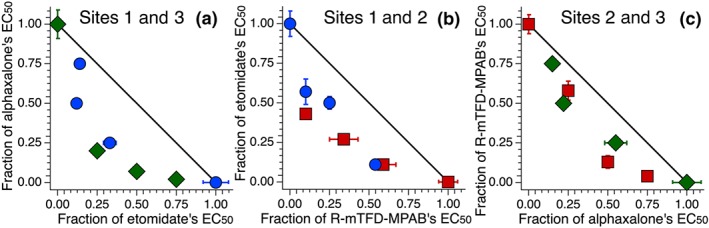
Isobolograms of mixtures of two anaesthetics acting on different sites show synergy. The fractional concentrations of the drugs required to produce 50% LoRR are plotted as in Figure 3. Shown are combinations of (a): etomidate (Eto) and alphaxalone (Alphax), Site 1 and Site 3 anaesthetics, respectively; (b): etomidate and R‐mTFD‐MPAB (MPAB), Site 1 and Site 2 anaesthetics, respectively, and (c): R‐mTFD‐MPAB and alphaxalone, Site 2 and Site 3 anaesthetics, respectively.
Similarly, etomidate was then tested with the Site 2 agent R‐mTFD‐MPAB, and the data are shown in Figure 4b. Of the six points tested, all lay well below the line of additivity, with the α values ranging from 0.53 to 0.75. Lastly, R‐mTFD‐MPAB was tested with alphaxalone, and the data are shown in Figure 4c. Of the six points tested, all lay well below the line of additivity, with the α values ranging from 0.63 to 0.90. In addition, ANOVA was conducted between these three sets of different‐site mixtures, showing no significant difference.
3.4. Three site‐selective anaesthetics acting on the same site interact nearly additively
Three Site 1 anaesthetics, etomidate, azietomidate, and carboetomidate, were tested in three‐way combination with one another by fixing the concentration of two drugs in solution and varying the third to determine the EC50 shift. The data from the nine mixtures tested, as well as all the other three‐way same‐site combinations, are shown in Table 2. Like the isobolograms of two‐way combinations, an isobologram was constructed with the fractional concentration of each drug on each axis, and the data are displayed as blue discs in the 3D isobologram in Figure 5a. Of the ninepoints tested, all lay below the surface of additivity with α values ranging from 0.77 to 0.98 but did not differ significantly from the expected value of one.
Figure 5.
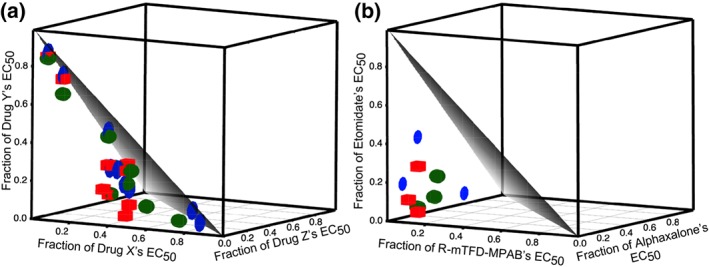
Three dimensional isobolograms of three–way mixtures of site–selective anaesthetics that either (a) act on a single site or (b) act on different sites show additivity and synergy, respectively. The triangular grey surface is the result expected for additivity (Surface of add.). (a) The drugs corresponding to the x, y, and z axes are: for the Site 1 agents, azietomidate, etomidate, and carboetomidate; for the Site 2 agents, R‐mTFD‐MPAB, R‐miPr‐MPAB, and R‐mCF3‐MPAB and for the Site 3 agents, THDOC, 3α5βP, and alphaxalone. (b) Data shown are from experiments where etomidate was varied and the other two (alphaxalone and R‐mTFD‐MPAB) were held constant, where R‐mTFD‐MPAB was varied while the other two anaesthetics were held constant and where alphaxalone was varied and R‐mTFD‐MPAB and etomidate were held constant.
The three Site 2 anaesthetics, R‐mTFD‐MPAB, R‐mCF3‐MPAB, and R‐miPr‐MPAB, were tested in the same manner as above, and the data are displayed in the same figure, but as red cubes. Of the nine points tested, all lay below the surface of additivity with the α values ranging from 0.56 to 0.95 and differed significantly from the expected value of one.
The three Site 3 anaesthetics, alphaxalone, 3α5βP, and THDOC, were tested, and the data are also displayed in the same figure, but as green spheres. Of the nine points tested, all lay below the surface of additivity with the α values ranging from 0.71 to 0.94, which differed significantly from the expected value of one.
Taken together, these data suggest in each of the three sets above that there is a small degree of synergy that reached the threshold of significance in two of the three sets. ANOVA showed that the α values of these three sets of agents did not differ from each other. The average α of the three sets of three‐way same‐site anaesthetics was 0.84 ± 0.15 (n = 27), which was significantly different from the value of one expected for additivity. However, this small degree of synergy was significantly less than that experienced when two agents acted on different sites (α = 0.69 ± 0.13; n = 18).
3.5. Three site‐selective anaesthetics acting at three different sites interact super synergistically
The anaesthetics etomidate, R‐mTFD‐MPAB, and alphaxalone, each site‐specific anaesthetics acting on Sites 1, 2, and 3 respectively, were tested in three‐way combinations in the same manner as described above, and the results are shown in Table 2 and Figure 5b. Of the nine points tested, all are well below the surface of additivity, with the α values ranging from 0.23 to 0.63. The mean and SD of α for this data set (n = 9) was significantly less than that for the three sets of three‐way same‐site anaesthetics above (n = 27).
3.6. Propofol is synergistic with anaesthetics acting on Site 1, 2, or 3
Although propofol and alphaxalone interact synergistically (Cao et al., 2018), propofol has only weak or no selectively between Sites 1 and 2. (Chiara et al., 2013). We first confirmed that alphaxalone and propofol interact synergistically (Table 4 and Figure 6b). Combinations of R‐mTFD‐MPAB and propofol interacted synergistically with α values ranging from 0.35 to 0.75 (Table 4 Figure 6a). Furthermore, combinations of etomidate and propofol also interacted synergistically with α values ranging from 0.38 to 0.78 (Table 4 and Figure 6c). In addition, ANOVA showed that the α values of these mixtures with propofol did not differ significantly from one another.
Table 4.
Fixed concentrations, EC50s, and αs of propofol with Site 1, 2, and 3 anaesthetics
| Drug A | [Drug A] μM | [Propofol] μΜ | α | Average α |
|---|---|---|---|---|
| Etomidate | 0.29 ± 0.03 | 0.158 | 0.38 ± 0.01 | |
| 0.25 ± 0.03 | 0.315 | 0.61 ± 0.01 | ||
| 0.07 ± 0.02 | 0.473 | 0.78 ± 0.01 | ||
| 0.555 | 0.09 ± 0.01 | 0.39 ± 0.02 | ||
| 1.11 | 0.03 ± 0.01 | 0.54 ± 0.01 | ||
| 1.655 | 0.01 ± 0.01 | 0.75 ± 0.01 | 0.58 ± 0.17 a | |
| R‐mTFD‐MPAB | 0.24 ± 0.04 | 0.158 | 0.35 ± 0.02 | |
| 0.14 ± 0.02 | 0.315 | 0.56 ± 0.01 | ||
| 0.03 ± 0.01 | 0.473 | 0.76 ± 0.01 | ||
| 0.585 | 0.09 ± 0.01 | 0.39 ± 0.02 | ||
| 1.17 | 0.03 ± 0.01 | 0.54 ± 0.01 | ||
| 1.755 | 0.01 ± 0.01 | 0.75 ± 0.01 | 0.56 ± 0.17 a | |
| Alphaxalone | 0.34 ± 0.04 | 0.158 | 0.59 ± 0.03 | |
| 0.29 ± 0.05 | 0.315 | 0.79 ± 0.04 | ||
| 0.09 ± 0.01 | 0.473 | 0.84 ± 0.01 | ||
| 0.355 | 0.08 ± 0.02 | 0.33 ± 0.03 | ||
| 1.710 | 0.03 ± 0.01 | 0.53 ± 0.01 | ||
| 1.065 | 0.03 ± 0.01 | 0.78 ± 0.01 | 0.64 ± 0.20 a |
Note. α values are reported as ±SD. SD of average αs are Calculated according to Equation (2). Each concentration–response curve had 40 animals except for the first two rows that had 50 and 55 animals respectively.
Differs significantly (P < .01) from the value of one expected for additivity.
Figure 6.
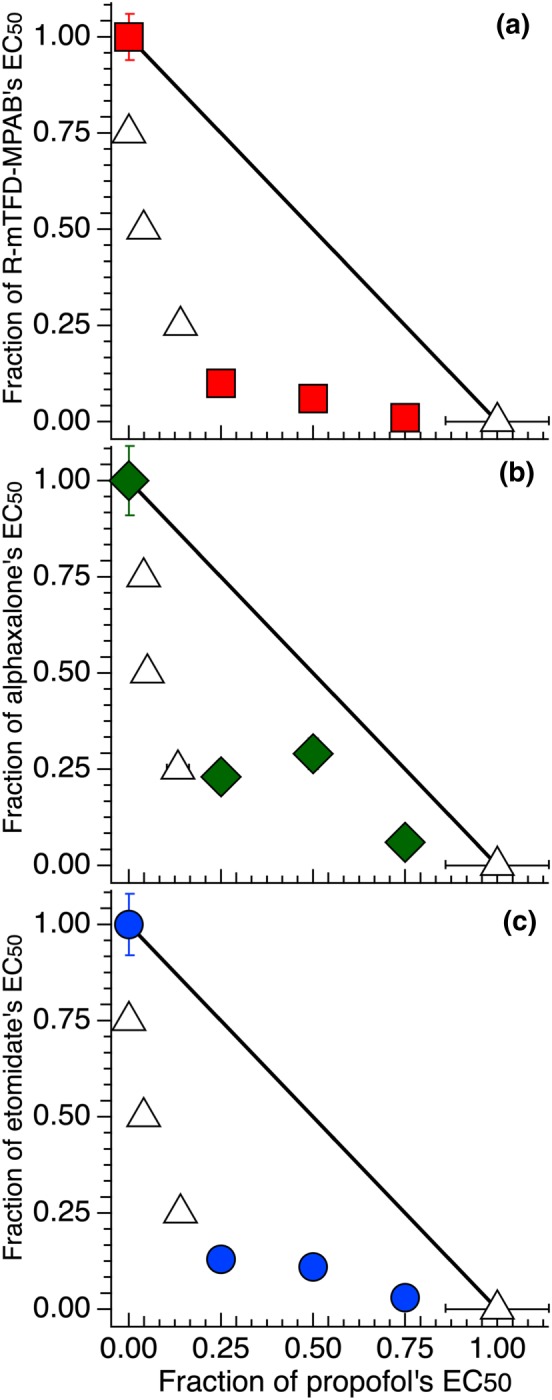
Isobolograms of propofol with either R‐mTFD‐MPAB, alphaxalone or etomidate all show synergy. Isobolograms are plotted as in Figure 3. Shown are combinations of propofol (both a Site 2 and 3 anaesthetic) with: (a) R‐mTFD‐MPAB (Site 2), (b) alphaxalone (Site 3) or (c) etomidate (Site 1).
4. DISCUSSION
Our work establishes, for the first time, some principles for predicting whether combinations of general anaesthetics that act on GABAA receptors will interact additively or synergistically. Early studies of anaesthetic mixtures were carried out with the relatively low affinity volatile and gaseous anaesthetics and all demonstrated additivity (Clarke et al., 1978; Eger et al., 2008; Hendrickx, Eger, Sonner, & Shafer, 2008). Richards and White (1981) were the first to recognize that higher potency intravenous agents with stricter structure activity relationships would be more likely to reveal interactions between binding sites. Monitoring sleep time in rats, they showed that mixtures of alphaxalone with either etomidate or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7233 were synergistic whereas mixtures with alphadalone, a steroid, were additive. They postulated that allosteric interactions between binding sites were responsible. These studies on the interaction of steroid anaesthetics with intravenous agents were later extended to alphaxalone + propofol and to 3α5βP + etomidate and parallels with actions on heterologously expressed GABAA receptors were established (Cao et al., 2018; Li et al., 2014; Shin, Germann, Covey, Steinbach, & Akk, 2019; Shin, Germann, Steinbach, & Akk, 2017). In this study, we have extended the pharmacology to include synergy between the three distinct TMD sites shown in Figure 1.
To avoid pharmacokinetic uncertainties associated with studies in mammals, we used the traditional model of tadpoles because they equilibrate with the bath concentration of the agents investigated. We set out to test the hypothesis that the general anaesthetic potencies of mixtures of site‐selective agents binding to the same or to different sites would combine additively or synergistically respectively. The results proved to be remarkably consistent with this hypothesis, a success that may be attributed to the highly site‐selective agents employed and to the low occupancy of these sites at general anaesthetic concentrations (Rusch, Zhong, & Forman, 2004). First, we found that for each of the three sites, the potencies of two or three agents each acting on a single site were simply additive and consistent with Equation (1) with α = 1. Second, we found that pairs of selective agents acting on any two of the three possible pairwise combinations of sites always acted synergistically and that within the broad errors associated with in vivo studies, no pair interacted more strongly than any other based on their values of α, although it remains possible that studies in model systems such as those at the receptor level may reveal subtle differences (Cao et al., 2018). Third, combinations of three agents that acted selectively on different sites were even more synergistic.
Although our work firmly establishes the principles governing additivity and synergy of anaesthetic mixtures of site‐selective agents that act on GABAA receptors, clinically used non‐steroidal intravenous general anaesthetics often have low selectivity between the etomidate and R‐mTFD‐MPAB sites and do not bind the steroid sites at all. For example, the selectivity for the R‐mTFD‐MPAB sites over the etomidate sites is just 13‐fold for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2804, eightfold for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5480, 1.6‐fold for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2579, and 0.6‐fold to twofold for propofol (Chiara et al., 2013; Shin et al., 2018). These selectivities are much lower than those for the canonical agents etomidate and R‐mTFD‐MPAB, which have selectivity for their preferred sites of ~1000‐ and 60‐fold respectively (Chiara et al., 2013). We chose propofol to test the hypothesis that agents with weak selectivities would act synergistically with both etomidate‐ and R‐mTFD‐MPAB–selective agents because it is thoroughly characterized, it is a widely used intravenous anaesthetic in the clinical setting, and its interaction with the steroid site has been reported to be synergistic in mice and in vitro (Cao et al., 2018), a finding we have confirmed here in tadpoles (Table 2). Consistent with the hypothesis, we found that when propofol was mixed with either etomidate or R‐mTFD‐MPAB, the resultant combination produced LoRR synergistically (Table 3). That is, whichever site is occupied by a site‐selective anaesthetic, propofol can bind to the other site to produce a synergistic interaction. The corollary of this observation is that propofol must act synergistically with itself, which might account for the high potency of such a simple molecule.
Pharmacokinetics may compromise the use of mixtures of intravenous anaesthetics in mammals including humans (Hendrickx et al., 2008; McKay, 1991), but in the few cases where plasma levels have been measured, no major issues have emerged to date (Borowicz, Luszczki, & Czuczwar, 2004). Furthermore, the little data using site‐selective agents in mammals are consistent with our findings. For example, mixtures of alphaxalone and etomidate are synergistic in rats (Richards & White, 1981) and mixtures of propofol and thiopentone, which interact with little selectivity on both Site 1 and 2, are additive in humans (Jones et al., 1999; Vinik, Bradley, & Kissin, 1999).
A goal of using combinations of general anaesthetics in the clinical situation would be to reduce off‐target side effects. Compared to its use alone, the concentration of an agent used in a site‐selective triple mixture is reduced 6–7 fold. Whether this is sufficient to reduce a side effect that only one agent in the mixture exerts depends on the target. For example, in the case of etomidate's high affinity interaction with https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1359, this would be far from sufficient (Zolle et al., 2008). However, for reducing side effects that are only encountered at high anaesthetic concentrations, for example, respiratory and cardiac depression, the strategy might have some success. Furthermore, for other members of the pentameric ligand‐gated ion channel superfamily such as https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=76 in the autonomic nervous system, where the pharmacology does not parallel that of general anaesthesia, the strategy might be advantageous (Forman, 1998; Forman & Miller, 2011).
Side effects that are mediated by GABAA receptors are a special case. Although much remains to be learnt about GABAA receptor isoforms, most are expected to have two β+/α− interfaces because GABA binds in these interfaces in the ECD. Thus, mixtures of Site 1 and 3 agents might be expected to act equally on all GABAA receptor isoforms. But Site 2 agents and future agents that might target the undrugged α+/γ− interface might have their action confined to a subset of largely synaptic receptors. However, further characterization of Site 2 agents in non‐synaptic GABAA receptors is needed because they can also target the β+/β− interface in αβ and even some αβδ receptors (Chiara et al., 2016).
The principles discussed above may well be generalizable to other behaviours mediated by heteromeric pentameric ligand‐gated ion channel receptors. However, a prerequisite will be the development of agents that selectively target specific subunit interfaces, which in turn requires a knowledge of the stoichiometry and arrangement of the five subunits. In non‐synaptic GABAA receptors , the γ subunit is, with the exception of α5 subunit‐containing receptors, replaced by either an additional β subunit or a δ subunit so that more than two homologous sites are likely to exist in all GABAA receptors. An additional complexity is that some receptors may have no well‐defined stoichiometry. This is thought to be the case in δ subunit containing GABAA receptors and in α4β2 nAChRs (Nelson, Kuryatov, Choi, Zhou, & Lindstrom, 2003; Wongsamitkul, Baur, & Sigel, 2016).
In conclusion, the β3 N265M knock‐in mouse established that the general anaesthetic actions of etomidate were mediated by GABAA receptors (Jurd et al., 2003). The fact the steroids and the mephobarbital agents studied here allosterically enhance the action of etomidate strengthens the hypothesis that their general anaesthetic actions are also mediated by GABAA receptors, without providing definitive proof.
The concept that general anaesthetics interact allosterically with function in the pentameric ligand‐gated ion channel superfamily has long been recognized (Olsen, 2015; Changeux, 2013). Our work and that of Akk and colleagues adds to this concept by considering allosteric interactions between anaesthetic sites (Cao et al., 2018). We have shown that combinations of anaesthetics that act on just one of the three known sites interact additively, whereas those that act on one or two different sites interact synergistically leading to significant reductions in drug load. The principles demonstrated here may have wider implications for the development of drugs that act allosterically and for drug screening.
AUTHOR CONTRIBUTIONS
K.W.M. conceived the project. K.W.M. and D.E.K. designed the experiments, which were performed and analysed by D.E.K.. K.S.B. and P.Y.S. performed the synthesis of the barbiturates and azietomidate.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
This research was supported by a Grant from the National Institute for General Medical Science to K.W.M. (GM 58448) and by the Department of Anesthesia, Critical Care & Pain Medicine at Massachusetts General Hospital. We thank Dr D. E. Raines (MGH) for a gift of carboetomidate. The molecular graphic in Figure 1 was created with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41‐GM103311 (Pettersen et al., 2004).
Kent DE, Savechenkov PY, Bruzik KS, Miller KW. Binding site location on GABAA receptors determines whether mixtures of intravenous general anaesthetics interact synergistically or additively in vivo. Br J Pharmacol. 2019;176:4760–4772. 10.1111/bph.14843
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(Suppl 1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlong, C. A. , Perkins, M. G. , Houle, T. T. , Miller, K. W. , & Pearce, R. A. (2016). Contrasting efects of the γ‐aminobutyric acid type A receptor β3 subunit N265M mutation on loss of righting reflexes induced by etomidate and the novel anesthetic barbiturate R‐mTFD‐MPAB. Anesthesia and Analgesia, 123, 1241–1246. 10.1213/ANE.0000000000001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli, D. , Lambert, J. J. , Peters, J. A. , Wafford, K. , & Whiting, P. J. (1997). The interaction of the general anesthetic etomidate with the γ‐aminobutyric acid type A receptor is influenced by a single amino acid. Proceedings of the National Academy of Sciences of the United States of America, 94, 11031–11036. 10.1073/pnas.94.20.11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli, D. , Muntoni, A. L. , Merrywest, S. D. , Gentet, L. J. , Casula, A. , Callachan, H. , … Peters, J. A. (2003). The in vitro and in vivo enantioselectivity of etomidate implicates the GABAA receptor in general anaesthesia. Neuropharmacology, 45, 57–71. 10.1016/S0028-3908(03)00144-8 [DOI] [PubMed] [Google Scholar]
- Borowicz, K. K. , Luszczki, J. , & Czuczwar, S. J. (2004). Interactions between non‐barbiturate injectable anesthetics and conventional antiepileptic drugs in the maximal electroshock test in mice—An isobolographic analysis. European Neuropsychopharmacology, 14, 163–172. 10.1016/S0924-977X(03)00104-4 [DOI] [PubMed] [Google Scholar]
- Cao, L. Q. , Montana, M. C. , Germann, A. L. , Shin, D. J. , Chakrabarti, S. , Mennerick, S. , … Akk, G. (2018). Enhanced GABAergic actions resulting from the coapplication of the steroid 3α‐hydroxy‐5α‐pregnane‐11,20‐dione (alfaxalone) with propofol or diazepam. Scientific Reports, 8, 10341 10.1038/s41598-018-28754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux, J. P. (2013). The concept of allosteric interaction and its consequences for the chemistry of the brain. The Journal of Biological Chemistry, 288, 26969–26986. 10.1074/jbc.X113.503375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Wells, M. M. , Arjunan, P. , Tillman, T. S. , Cohen, A. E. , Xu, Y. , & Tang, P. (2018). Structural basis of neurosteroid anesthetic action on GABAA receptors. Nature Communications, 9, 3972 10.1038/s41467-018-06361-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. W. , Wang, C. , Krishnan, K. , Manion, B. D. , Hastings, R. , Bracamontes, J. , … Evers, A. S. (2014). 11‐trifluoromethyl‐phenyldiazirinyl neurosteroid analogues: Potent general anesthetics and photolabeling reagents for GABAA receptors. Psychopharmacology, 231, 3479–3491. 10.1007/s00213-014-3568-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara, D. C. , Jayakar, S. S. , Zhou, X. , Zhang, X. , Savechenkov, P. Y. , Bruzik, K. S. , … Cohen, J. B. (2013). Specificity of intersubunit general anesthetic‐binding sites in the transmembrane domain of the human α1β3γ2 γ‐aminobutyric acid type A (GABAA) receptor. The Journal of Biological Chemistry, 288, 19343–19357. 10.1074/jbc.M113.479725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara, D. C. , Jounaidi, Y. , Zhou, X. , Savechenkov, P. Y. , Bruzik, K. S. , Miller, K. W. , & Cohen, J. B. (2016). General anesthetic binding sites in human α4β3δ γ‐aminobutyric acid type A receptors (GABAARs). The Journal of Biological Chemistry, 291, 26529–26539. 10.1074/jbc.M116.753335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, R. F. , Daniels, S. , Harrison, C. B. , Jordan, M. J. , Paton, W. D. , Smith, E. B. , & Smith, R. A. (1978). Potency of mixtures of general anaesthetic agents. British Journal of Anaesthesia, 50, 979–983. 10.1093/bja/50.10.979 [DOI] [PubMed] [Google Scholar]
- Cotten, J. F. , Forman, S. A. , Laha, J. K. , Cuny, G. D. , Husain, S. S. , Miller, K. W. , … Raines, D. E. (2010). Carboetomidate: A pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology, 112, 637–644. 10.1097/ALN.0b013e3181cf40ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler, B. , Balk, M. , & Antkowiak, B. (2016). Synergistic modulation of γ‐aminobutyric acid type A receptor‐mediated synaptic inhibition in cortical networks by allopregnanolone and propofol. Anesthesia and Analgesia, 123, 877–883. 10.1213/ANE.0000000000001429 [DOI] [PubMed] [Google Scholar]
- Eger, E. I. 2nd , Tang, M. , Liao, M. , Laster, M. J. , Solt, K. , Flood, P. , … Sonner, J. M. (2008). Inhaled anesthetics do not combine to produce synergistic effects regarding minimum alveolar anesthetic concentration in rats. Anesthesia and Analgesia, 107, 479–485. 10.1213/01.ane.0000295805.70887.65 [DOI] [PubMed] [Google Scholar]
- Forman, S. A. (1998). Direct interactions of anesthetics and nonanesthetics with the nicotinic acetylcholine receptor pore. Toxicology Letters, 100‐101, 169–178. 10.1016/S0378-4274(98)00182-9 [DOI] [PubMed] [Google Scholar]
- Forman, S. A. , & Miller, K. W. (2011). Anesthetic sites and allosteric mechanisms of action on Cys‐loop ligand‐gated ion channels. Canadian Journal of Anaesthesia, 58, 191–205. 10.1007/s12630-010-9419-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman, S. A. , & Miller, K. W. (2016). Mapping general anesthetic sites in heteromeric γ‐aminobutyric acid type A receptors reveals a potential for targeting receptor subtypes. Anesthesia and Analgesia, 123, 1263–1273. 10.1213/ANE.0000000000001368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann, A. L. , Shin, D. J. , Manion, B. D. , Edge, C. J. , Smith, E. H. , Franks, N. P. , … Akk, G. (2016). Activation and modulation of recombinant glycine and GABAA receptors by 4‐halogenated analogues of propofol. British Journal of Pharmacology, 173, 3110–3120. 10.1111/bph.13566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickx, J. F. , Eger, E. I. 2nd , Sonner, J. M. , & Shafer, S. L. (2008). Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesthesia and Analgesia, 107, 494–506. 10.1213/ane.0b013e31817b859e [DOI] [PubMed] [Google Scholar]
- Hosie, A. M. , Wilkins, M. E. , da Silva, H. M. , & Smart, T. G. (2006). Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature, 444, 486–489. 10.1038/nature05324 [DOI] [PubMed] [Google Scholar]
- Husain, S. S. , Ziebell, M. R. , Ruesch, D. , Hong, F. , Arevalo, E. , Kosterlitz, J. A. , … Miller, K. W. (2003). 2‐(3‐Methyl‐3H‐diaziren‐3‐yl)ethyl 1‐(1‐phenylethyl)‐1H‐imidazole‐5‐carboxylate: A derivative of the stereoselective general anesthetic etomidate for photolabeling ligand‐gated ion channels. Journal of Medicinal Chemistry, 46, 1257–1265. 10.1021/jm020465v [DOI] [PubMed] [Google Scholar]
- Jones, D. , Prankerd, R. , Lang, C. , Chilvers, M. , Bignell, S. , & Short, T. (1999). Propofol‐thiopentone admixture‐hypnotic dose, pain on injection and effect on blood pressure. Anaesthesia and Intensive Care, 27, 346–356. 10.1177/0310057X9902700403 [DOI] [PubMed] [Google Scholar]
- Jurd, R. , Arras, M. , Lambert, S. , Drexler, B. , Siegwart, R. , Crestani, F. , … Rudolph, U. (2003). General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB Journal, 17, 250–252. 10.1096/fj.02-0611fje [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , Altman, D. G. , & Group NCRRGW (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty, D. , Desai, R. , Uchanski, T. , Masiulis, S. , Stec, W. J. , Malinauskas, T. , … Aricescu, A. R. (2019). Cryo‐EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature, 565, 516–520. 10.1038/s41586-018-0833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty, D. , Thomas, P. , Field, M. , Andersen, O. J. , Gold, M. G. , Biggin, P. C. , … Smart, T. G. (2017). Crystal structures of a GABAA‐receptor chimera reveal new endogenous neurosteroid‐binding sites. Nature Structural & Molecular Biology, 24, 977–985. 10.1038/nsmb.3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb‐Lundberg, F. , Snowman, A. , & Olsen, R. W. (1980). Barbiturate receptor sites are coupled to benzodiazepine receptors. Proceedings of the National Academy of Sciences of the United States of America, 77, 7468–7472. 10.1073/pnas.77.12.7468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , Bracamontes, J. R. , Manion, B. D. , Mennerick, S. , Steinbach, J. H. , Evers, A. S. , & Akk, G. (2014). The neurosteroid 5β‐pregnan‐3α‐ol‐20‐one enhances actions of etomidate as a positive allosteric modulator of α1β2γ2L GABAA receptors. British Journal of Pharmacology, 171, 5446–5457. 10.1111/bph.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, A. C. (1991). Synergism among i.v. anaesthetics. British Journal of Anaesthesia, 67, 1–3. 10.1093/bja/67.1.1 [DOI] [PubMed] [Google Scholar]
- Miller, P. S. , Scott, S. , Masiulis, S. , De Colibus, L. , Pardon, E. , Steyaert, J. , & Aricescu, A. R. (2017). Structural basis for GABAA receptor potentiation by neurosteroids. Nature Structural & Molecular Biology, 24, 986–992. 10.1038/nsmb.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. E. , Kuryatov, A. , Choi, C. H. , Zhou, Y. , & Lindstrom, J. (2003). Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Molecular Pharmacology, 63, 332–341. 10.1124/mol.63.2.332 [DOI] [PubMed] [Google Scholar]
- Nicoll, R. A. , Eccles, J. C. , Oshima, T. , & Rubia, F. (1975). Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature, 258, 625–627. 10.1038/258625a0 [DOI] [PubMed] [Google Scholar]
- Olsen, R. W. (2015). Allosteric ligands and their binding sites define γ‐aminobutyric acid (GABA) type A receptor subtypes. Advances in Pharmacology, 73, 167–202. 10.1016/bs.apha.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Pettersen, E. F. , Goddard, T. D. , Huang, C. C. , Couch, G. S. , Greenblatt, D. M. , Meng, E. C. , & Ferrin, T. E. (2004). UCSF Chimera—A visualization system for exploratory research and analysis. Journal of Computational Chemistry, 25, 1605–1612. 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- Richards, C. D. , & White, A. E. (1981). Additive and non‐additive effects of mixtures of short‐acting intravenous anaesthetic agents and their significance for theories of anaesthesia. British Journal of Pharmacology, 74, 161–170. 10.1111/j.1476-5381.1981.tb09969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch, D. , Zhong, H. , & Forman, S. A. (2004). Gating allosterism at a single class of etomidate sites on α1β2γ2L GABAA receptors accounts for both direct activation and agonist modulation. The Journal of Biological Chemistry, 279, 20982–20992. 10.1074/jbc.M400472200 [DOI] [PubMed] [Google Scholar]
- Savechenkov, P. Y. , Zhang, X. , Chiara, D. C. , Stewart, D. S. , Ge, R. , Zhou, X. , … Bruzik, K. S. (2012). Allyl m‐trifluoromethyldiazirine mephobarbital: An unusually potent enantioselective and photoreactive barbiturate general anesthetic. Journal of Medicinal Chemistry, 55, 6554–6565. 10.1021/jm300631e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D. J. , Germann, A. L. , Covey, D. F. , Steinbach, J. H. , & Akk, G. (2019). Analysis of GABAA receptor activation by combinations of agonists acting at the same or distinct binding sites. Molecular Pharmacology, 95, 70–81. 10.1124/mol.118.113464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D. J. , Germann, A. L. , Johnson, A. D. , Forman, S. A. , Steinbach, J. H. , & Akk, G. (2018). Propofol is an allosteric agonist with multiple binding sites on concatemeric ternary GABAA receptors. Molecular Pharmacology, 93, 178–189. 10.1124/mol.117.110403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D. J. , Germann, A. L. , Steinbach, J. H. , & Akk, G. (2017). The actions of drug combinations on the GABAA receptor manifest as curvilinear isoboles of additivity. Molecular Pharmacology, 92, 556–563. 10.1124/mol.117.109595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart, R. , Krahenbuhl, K. , Lambert, S. , & Rudolph, U. (2003). Mutational analysis of molecular requirements for the actions of general anaesthetics at the γ‐aminobutyric acid A receptor subtype, α1β2γ2. BMC Pharmacology, 3, 13 10.1186/1471-2210-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastna, E. , Krishnan, K. , Manion, B. D. , Taylor, A. , Rath, N. P. , Chen, Z. W. , … Covey, D. F. (2011). Neurosteroid analogues. 16. A new explanation for the lack of anesthetic effects of Δ16‐alphaxalone and identification of a Δ17(20) analogue with potent anesthetic activity. Journal of Medicinal Chemistry, 54, 3926–3934. 10.1021/jm2002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, A. , Nourmahnad, A. , Halpin, E. , & Forman, S. A. (2019). Monod‐Wyman‐Changeux allosteric shift analysis in mutant α1β3γ2L GABAA receptors indicates selectivity and crosstalk among intersubunit transmembrane anesthetic sites. Molecular Pharmacology, 95, 408–417. 10.1124/mol.118.115048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida, R. J. (2002). The interaction index: A measure of drug synergism. Pain, 98, 163–168. 10.1016/S0304-3959(02)00041-6 [DOI] [PubMed] [Google Scholar]
- Tallarida, R. J. (2016). Drug combinations: Tests and analysis with isoboles. Current Protocols in Pharmacology, 72, 9 19 11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik, H. R. , Bradley, E. L. Jr. , & Kissin, I. (1999). Isobolographic analysis of propofol‐thiopental hypnotic interaction in surgical patients. Anesthesia and Analgesia, 88, 667–670. 10.1213/00000539-199903000-00037 [DOI] [PubMed] [Google Scholar]
- Waud, D. R. (1972). On biological assays involving quantal responses. The Journal of Pharmacology and Experimental Therapeutics, 183, 577–607. [PubMed] [Google Scholar]
- Wongsamitkul, N. , Baur, R. , & Sigel, E. (2016). Toward understanding functional properties and subunit arrangement of α4β2δ γ‐Aminobutyric Acid, Type A (GABAA) receptors. The Journal of Biological Chemistry, 291, 18474–18483. 10.1074/jbc.M116.738906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba, A. M. , Szabo, A. , Pierce, D. W. , Haburcak, M. , Stern, A. T. , Nourmahnad, A. , … Forman, S. A. (2018). Alphaxalone binds in inner transmembrane β+−α− interfaces of α1β3γ2 γ‐aminobutyric acid type A receptors. Anesthesiology, 128, 338–351. 10.1097/ALN.0000000000001978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolle, I. M. , Berger, M. L. , Hammerschmidt, F. , Hahner, S. , Schirbel, A. , & Peric‐Simov, B. (2008). New selective inhibitors of steroid 11β‐hydroxylation in the adrenal cortex. Synthesis and structure‐activity relationship of potent etomidate analogues. Journal of Medicinal Chemistry, 51, 2244–2253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


