Abstract
Background
Multiple studies have identified COPD subtypes by using visual or quantitative evaluation of CT images. However, there has been no systematic assessment of a combined visual and quantitative CT imaging classification. We integrated visually defined patterns of emphysema with quantitative imaging features and spirometry data to produce a set of 10 nonoverlapping CT imaging subtypes, and we assessed differences between subtypes in demographic features, physiological characteristics, longitudinal disease progression, and mortality.
Methods
We evaluated 9,080 current and former smokers in the COPDGene study who had available volumetric inspiratory and expiratory CT images obtained using a standardized imaging protocol. We defined 10 discrete, nonoverlapping CT imaging subtypes: no CT imaging abnormality, paraseptal emphysema (PSE), bronchial disease, small airway disease, mild emphysema, upper lobe predominant centrilobular emphysema (CLE), lower lobe predominant CLE, diffuse CLE, visual without quantitative emphysema, and quantitative without visual emphysema. Baseline and 5-year longitudinal characteristics and mortality were compared across these CT imaging subtypes.
Results
The overall mortality differed significantly between groups (P < .01) and was highest in the 3 moderate to severe CLE groups. Subjects having quantitative but not visual emphysema and subjects with visual but not quantitative emphysema were unique groups with mild COPD, at risk for progression, and with likely different underlying mechanisms. Subjects with PSE and/or moderate to severe CLE had substantial progression of emphysema over 5 years compared with findings in subjects with no CT imaging abnormality (P < .01).
Conclusions
The combination of visual and quantitative CT imaging features reflects different underlying pathological processes in the heterogeneous COPD syndrome and provides a useful approach to reclassify types of COPD.
TRIAL REGISTRY
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov;
KEY WORDS: COPD, CT imaging, epidemiology, heterogeneity, subtype
Abbreviations: 6MWD, 6-minute walking distance; CLE, centrilobular emphysema; PFT, pulmonary function test; PRISm, preserved ratio impaired spirometry; PRM, parametric response mapping; PSE, paraseptal emphysema; SGRQ, St. George’s Respiratory Questionnaire
Substantial heterogeneity exists among patients with COPD with respect to clinical presentation, physiological characteristics, imaging characteristics, response to therapy, disease progression, and, ultimately, survival.1 COPD typically is diagnosed by demonstration of airflow limitation by using pulmonary function tests (PFTs). PFTs are also useful tools to assess COPD severity.2 However, PFTs have several limitations in the diagnosis of COPD and assessment of COPD progression. First, the rate of lung function decline varies with COPD severity, with subjects with more advanced COPD often having slower rates of absolute change in spirometric measures.3, 4 Second, PFTs do not distinguish the different pathophysiological contributors to COPD, including emphysema, airway inflammation, and small airway destruction.5
Visual CT imaging evaluation shows that subjects with COPD have a heterogeneous group of abnormalities, composed of a variety of patterns of emphysema, large airway inflammation, and nonemphysematous obstruction due to small airway disease.6 Although visual CT imaging evaluation can provide information about emphysema distribution, it does not provide a quantitative assessment of emphysema severity. Quantitative CT imaging evaluation can help assess the severity and lobar distribution of emphysema. However, quantitative CT imaging also has limitations, including overestimation of emphysema in patients with severe airflow obstruction.7 Visual categorization of emphysema was proposed by the Fleischner Society,8 and these visual emphysema categories were associated independently with differences in mortality.9
In this study, we sought to integrate visual and quantitative CT imaging phenotypes with spirometry to develop a comprehensive, nonoverlapping set of subtypes based on CT imaging and to assess demographic features, longitudinal progression, and mortality differences between these subtypes. We hypothesized that visual and quantitative CT imaging features along with spirometry would provide complementary information to define subtypes of smokers with significantly different cross-sectional features and longitudinal outcomes, including mortality. In addition, we hypothesized that there would be differences in cross-sectional features and longitudinal outcomes in several specific subtype comparisons: (1) subjects with visual but no quantitative emphysema vs subjects with quantitative but no visual emphysema, (2) large airway disease vs small airway disease, (3) paraseptal emphysema (PSE) vs diffuse centrilobular emphysema (CLE), and (4) upper vs lower vs diffuse CLE.
Materials and Methods
Subjects
COPDGene10 is a longitudinal multicenter prospective cohort study focused on the genetic and epidemiological characteristics of COPD. Between 2007 and 2011, 10,263 current and former smokers (with at least 10 pack-years of smoking history) with and without COPD were enrolled in COPDGene. Institutional review board approval for the study was obtained at all clinical centers, and written informed consent was obtained from all participants. All subjects were self-identified as non-Hispanic black or non-Hispanic white; additional details regarding the subjects analyzed in this study are provided in e-Appendix 1.
Quantitative CT Imaging Analysis
All subjects underwent volumetric inspiratory and expiratory CT imaging with use of a standardized imaging protocol.10, 11 Details regarding quantitative CT imaging analysis are provided in e-Appendix 1.
Visual Analysis and CT Imaging Subtypes
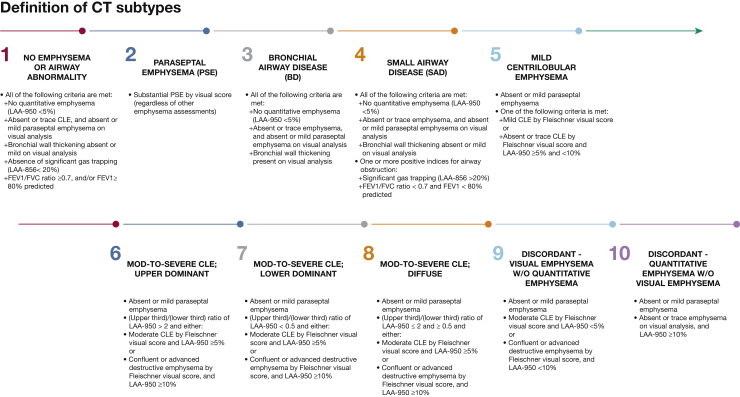
Visual CT imaging analysis was based on the Fleischner Society classification system.8 Two analysts performed the visual scoring, as previously described.9 Subjects were categorized into 10 specific subtypes based on previously defined visual and quantitative CT imaging parameters and spirometry8, 9, 12 (Fig 1, e-Fig 1). Subjects with significant PSE, as defined by the Fleischner criteria, were placed in the PSE category, regardless of other imaging characteristics. Quantitative thresholds for significant emphysema13 (mild ≥ 5%, moderate to severe ≥ 10%) and for emphysema distribution (ratio of upper lung to lower lung emphysema of > 2 for upper lobe predominant, < 0.5 for lower lobe predominant, or 0.5 to 2 for diffuse emphysema) were selected based on previous COPDGene publications.12
Figure 1.
Definition of CT imaging subtypes. Ten consensus CT imaging subtypes based on a combination of visual and quantitative CT imaging assessments. CLE = centrilobular emphysema; LAA = low attenuation area; PSE = paraseptal emphysema.
Statistical Evaluation
Both univariate and multivariate analyses were conducted to compare the 10 specific CT imaging subtypes. Statistical approaches are described in e-Appendix 1.
Results
Overall Comparison of 10 CT Imaging Subtypes
Among the 9,080 smokers, approximately 25.8% (n = 2,339) had no visual or quantitative CT imaging emphysema, no visual airway disease at CT imaging, and normal spirometry results. These control subjects were compared with the other nine CT imaging subtypes. Of these remaining nine CT imaging subtypes, PSE (22.2%) and mild emphysema (19.3%) were the most common. The cumulative smoking intensity (measured in pack-years) was highest in the diffuse moderate to severe CLE group (Table 1). Functional small airway disease (on the basis of parametric response mapping [PRM]) and quantitative emphysema were highest in subjects within the three moderate to severe CLE groups. The airway wall area of a hypothetical 10-mm internal perimeter airway (Pi10) was greatest in the bronchial disease group. FEV1 values were the lowest in the 3 moderate to severe CLE subtypes (Table 2), especially among subjects with lower lobe predominance. Resting oxygen saturation and physical function measured by means of the 6-minute walking distance (6MWD) were the lowest in the 3 groups with moderate to severe CLE (Table 3). Comparisons of the airway-predominant groups (bronchial disease and small airway disease) with the 3 moderate to severe CLE-predominant groups showed multiple differences, including more current smoking and higher BMI in the airway-predominant groups (e-Table 1). Box plots of key clinical features in the 10 CT subtypes are shown in e-Figure 2.
Table 1.
Demographic and Imaging Characteristics of COPDGene Subjects in 10 CT Imaging Subtypes
| Characteristic | No. of Subjects | Moderate to Severe Centrilobular Emphysema |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No CT Imaging Abnormality | Paraseptal Emphysema | Bronchial Disease | Small Airway Disease | Mild Emphysema | Upper Lung Predominant | Lower Lung Predominant | Diffuse | Discordant Visual | Discordant Quantitative | P Valuea | ||
| No. of subjects | 2,339 | 2,018 | 861 | 381 | 1,755 | 309 | 53 | 706 | 527 | 131 | … | |
| Age at enrollment, y | 9,080 | 56.3 ± 8.2 | 60.4 ± 8.7 | 56.7 ± 8.8 | 61.0 ± 9.3 | 60.6 ± 9.0 | 64.1 ± 7.4 | 67.5 ± 8.1 | 66.6 ± 7.6 | 61.7 ± 8.8 | 63.1 ± 8.0 | < .001 |
| Female, No. (%) | 9,080 | 1,303 (55.7) | 571 (28.3) | 377 (43.8) | 191 (50.1) | 838 (47.7) | 180 (58.3) | 28 (52.8) | 350 (49.6) | 344 (65.3) | 29 (22.1) | < .001 |
| Race, NHW, No. (%) | 9,080 | 1,503 (64.3) | 1,267 (62.8) | 529 (61.4) | 280 (73.5) | 1,278 (72.8) | 241 (78.0) | 48 (90.6) | 597 (84.6) | 355 (67.4) | 122 (93.1) | < .001 |
| BMI, kg/m2 | 9,080 | 30.6 ± 6.3 | 26.8 ± 5.5 | 30.8 ± 6.3 | 30.0 ± 6.5 | 28.7 ± 6.0 | 27.6 ± 5.3 | 24.7 ± 4.3 | 25.8 ± 5.1 | 28.8 ± 5.9 | 28.0 ± 5.3 | < .001 |
| Current smoker, No. (%) | 9,080 | 1,301 (55.6) | 1,174 (58.2) | 583 (67.7) | 171 (44.9) | 917 (52.3) | 84 (27.2) | 11 (20.8) | 149 (21.1) | 308 (58.4) | 23 (17.6) | < .001 |
| Smoking intensity, pack-years | 9,078 | 35.4 ± 19.8 | 50.5 ± 26.8 | 41.2 ± 23.7 | 39.3 ± 21.1 | 45.0 ± 24.1 | 55.6 ± 27.6 | 50.8 ± 25.3 | 55.8 ± 27.1 | 50.0 ± 24.3 | 40.7 ± 25.4 | < .001 |
| Quantitative CT imaging | ||||||||||||
| PRMfSADb | 8,117 | 5.7 ± 4.0 | 20.2 ± 14.0 | 11.4 ± 9.6 | 17.3 ± 8.9 | 15.9 ± 12.3 | 28.6 ± 9.4 | 39.1 ± 9.1 | 33.6 ± 9.5 | 15.8 ± 11.4 | 26.0 ± 13.3 | < .001 |
| Emphysema, Perc15 | 9,078 | −899.3 ± 23.1 | −927.7 ± 32.5 | −893.8 ± 28.5 | −913.0 ± 17.7 | −916.5 ± 24.7 | −955.7 ± 17.4 | −953.3 ± 12.7 | −957.9 ± 16.4 | −908.8 ± 19.2 | −947.6 ± 5.1 | < .001 |
| Percentage of emphysema at −950 HU | 9,078 | 1.1 ± 1.1 | 10.5 ± 12.0 | 1.1 ± 1.2 | 2.0 ± 1.3 | 4.1 ± 4.2 | 20.1 ± 10.7 | 19.1 ± 10.0 | 22.6 ± 12.3 | 2.8 ± 2.2 | 13.7 ± 3.4 | < .001 |
| Upper lobe percentage of emphysema at −950 HU | 9,078 | 1.2 ± 1.3 | 12.1 ± 13.6 | 1.3 ± 1.4 | 2.3 ± 1.6 | 4.4 ± 4.4 | 27.2 ± 14.1 | 10.6 ± 6.1 | 23.5 ± 13.5 | 3.5 ± 2.9 | 14.6 ± 3.6 | < .001 |
| Lower lobe percentage of emphysema at −950 HU | 9,075 | 0.9 ± 1.1 | 7.8 ± 11.1 | 0.9 ± 1.2 | 1.7 ± 1.3 | 3.7 ± 4.3 | 9.4 ± 6.2 | 26.7 ± 13.6 | 21.4 ± 12.1 | 1.9 ± 1.9 | 12.5 ± 5.0 | < .001 |
| Ratio of upper lobe to lower lobe emphysema | 3,995 | 1.3 ± 0.7 | 4.5 ± 17.7 | 1.4 ± 0.8 | 1.6 ± 0.9 | 1.4 ± 0.9 | 3.7 ± 2.8 | 0.4 ± 0.1 | 1.2 ± 0.4 | 3.1 ± 3.4 | 1.3 ± 0.5 | < .001 |
| Adjusted lung density, g/L | 9,077 | 101.1 ± 21.2 | 76.7 ± 30.5 | 100.9± 22.3 | 90.0 ± 17.6 | 88.5 ± 22.2 | 51.7 ± 19.8 | 56.8 ±15.2 | 50.9 ± 19.3 | 96.1 ± 19.6 | 61.3 ± 8.8 | < .001 |
| Pi10 | 9,078 | 2.0 ± 0.4 | 2.4 ± 0.6 | 2.9 ± 0.6 | 2.2 ± 0.5 | 2.3 ± 0.6 | 2.6 ± 0.5 | 2.8 ± 0.5 | 2.6 ± 0.5 | 2.5 ± 0.6 | 2.0 ± 0.5 | < .001 |
Data are mean ± SD unless indicated otherwise. HU = Hounsfield units; NHW = non-Hispanic white; Perc15 = the 15th percentile of the lung density histogram; Pi10 = hypothetical 10-mm internal perimeter airway; PRMfSAD = parametric response mapping functional small airway disease.
P value tests for differences across all 10 groups.
The PRMfSAD of quantitative CT imaging integrates inspiratory and expiratory images to produce relative percentages of functional small airway disease defined by lung area < −856 HU at normal expiration.
Table 2.
Baseline Pulmonary Function Characteristics of Subjects in 10 CT Imaging Subtypes
| Characteristic | No. of Subjects | Moderate to Severe Centrilobular Emphysema |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No CT Imaging Abnormality | Paraseptal Emphysema | Bronchial Disease | Small Airway Disease | Mild Emphysema | Upper Lung Predominant | Lower Lung Predominant | Diffuse | Discordant Visual | Discordant Quantitative | P Valuea | ||
| No. of subjects | 2,339 | 2,018 | 861 | 381 | 1,755 | 309 | 53 | 706 | 527 | 131 | … | |
| Baseline lung function | ||||||||||||
| FEV1 % predicted | 9,080 | 92.61 ± 14.71 | 68.69 ± 26.70 | 76.03 ± 19.57 | 79.24 ± 20.36 | 80.33 ± 22.11 | 50.04 ± 22.97 | 40.36 ± 16.86 | 44.98 ± 22.10 | 73.11 ± 21.24 | 82.44 ± 27.59 | < .001 |
| Ratio of FEV1 to FVC | 9,080 | 0.79 ± 0.06 | 0.60 ± 0.17 | 0.71 ± 0.10 | 0.69 ± 0.09 | 0.68 ± 0.12 | 0.47 ± 0.12 | 0.39 ± 0.09 | 0.43 ± 0.13 | 0.64 ± 0.12 | 0.65 ± 0.13 | < .001 |
| FVC % predicted | 9,080 | 91.36 ± 14.72 | 86.44 ± 19.28 | 83.35 ± 17.50 | 87.74 ± 16.44 | 89.50 ± 17.20 | 78.77 ± 21.03 | 75.90 ± 21.81 | 77.10 ± 21.29 | 87.04 ± 17.59 | 93.56 ± 20.13 | < .001 |
| BDRb % of baseline FEV1, No. (%) | 8,972 | 252 (10.9) | 450 (22.6) | 246 (28.9) | 97 (25.9) | 370 (21.4) | 101 (32.8) | 17 (32.1) | 220 (31.2) | 129 (24.6) | 20 (15.4) | < .001 |
| Spirometry classification, No. (%) | < .001 | |||||||||||
| PRISmc | 1,072 | 437 (18.7) | 167 (8.3) | 204 (23.7) | 18 (4.7) | 178 (10.1) | 3 (1.0) | 0 (0.0) | 7 (1.0) | 55 (10.4) | 3 (2.3) | … |
| Normal spirometry results,d | 3,832 | 1,795 (76.7) | 571 (28.3%) | 311 (36.1) | 128 (33.6) | 803 (45.8) | 10 (3.2) | 0 (0.0) | 18 (2.5) | 145 (27.5) | 52 (39.7) | … |
| GOLD I | 744 | 107 (4.6) | 223 (11.1) | 59 (6.9) | 25 (6.6) | 173 (9.9) | 29 (9.4) | 1 (1.9) | 42 (5.9) | 62 (11.8) | 23 (17.6) | … |
| GOLD II | 1,817 | 0 (0.0) | 498 (24.7) | 207 (24.0) | 189 (49.6) | 416 (23.7) | 91 (29.4) | 13 (24.5) | 181 (25.6) | 192 (36.4) | 30 (22.9) | … |
| GOLD III | 1,078 | 0 (0.0) | 368 (18.2) | 67 (7.8) | 21 (5.5) | 159 (9.1) | 118 (38.2) | 22 (41.5) | 246 (34.8) | 57 (10.8) | 20 (15.3) | … |
| GOLD IV | 537 | 0 (0.0) | 191 (9.5) | 13 (1.5) | 0 (0.0) | 27 (1.5) | 58 (18.8) | 17 (32.1) | 212 (30.0) | 16 (3.0) | 3 (2.3) | … |
Data are mean ± SD unless indicated otherwise. BDR = bronchodilator response; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
P value tests for differences across all 10 groups.
The number of subjects who met American Thoracic Society criteria for BDR.
PRISm is ratio of FEV1 to FVC ≥ 0.7 and FEV1 < 80% predicted.
GOLD 0 indicates smokers with normal spirometry results, not necessarily without symptoms.
Table 3.
Baseline Physical Function and Comorbidities in 10 CT Imaging Subtypes
| Characteristic | No. of Subjects | Moderate to Severe Centrilobular Emphysema |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No CT Imaging Abnormality | Paraseptal Emphysema | Bronchial Disease | Small Airway Disease | Mild Emphysema | Upper Lung Predominant | Lower Lung Predominant | Diffuse | Discordant Visual | Discordant Quantitative | P Valuea | ||
| No. of subjects | 2,339 | 2,018 | 861 | 381 | 1,755 | 309 | 53 | 706 | 527 | 131 | … | |
| Oxygen saturation at rest, % | 9,095 | 96.9 ± 2.1 | 95.8 ± 3.2 | 96.4 ± 2.6 | 96.5 ± 2.4 | 96.3 ± 2.5 | 94.0 ± 4.2 | 93.9 ± 4.2 | 94.0 ± 4.2 | 95.8 ± 3.0 | 96.5 ± 2.1 | < .001 |
| 6-Minute walking distance, feet | 8,974 | 1,487.2 ± 359.3 | 1,288.3 ± 395.3 | 1,331.3 ± 392.5 | 1,407.5 ± 359.3 | 1,423.5 ± 377.3 | 1,111.7 ± 395.6 | 1,063.0 ± 341.7 | 1,151.0 ± 407.2 | 1,251.9 ± 378.8 | 1,550.8 ± 394.6 | < .001 |
| SGRQ total score | 9,097 | 17.8 ± 18.5 | 33.1 ± 23.6 | 30.5 ± 23.3 | 21.9 ± 20.3 | 23.1 ± 21.5 | 43.2 ± 20.9 | 46.8 ± 18.1 | 41.6 ± 20.4 | 31.2 ± 23.0 | 17.8 ± 19.6 | < .001 |
| Frequency of acute exacerbations of COPD per year | 9,098 | 0.2 ± 0.6 | 0.5 ± 1.0 | 0.4 ± 1.0 | 0.3 ± 0.8 | 0.3 ± 0.9 | 0.8 ± 1.2 | 1.2 ± 1.6 | 0.7 ± 1.2 | 0.5 ± 1.1 | 0.3 ± 0.8 | < .001 |
Data are mean ± SD. SGRQ = St. George’s Respiratory Questionnaire.
P value tests for differences across all 10 groups.
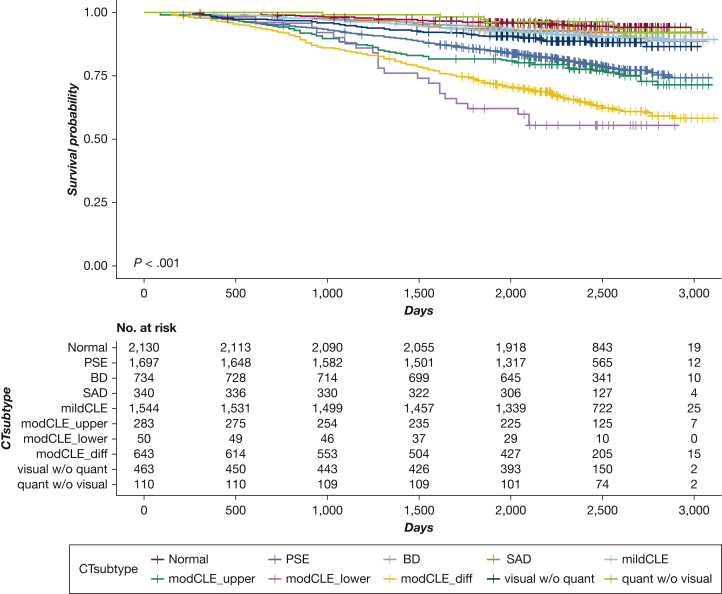
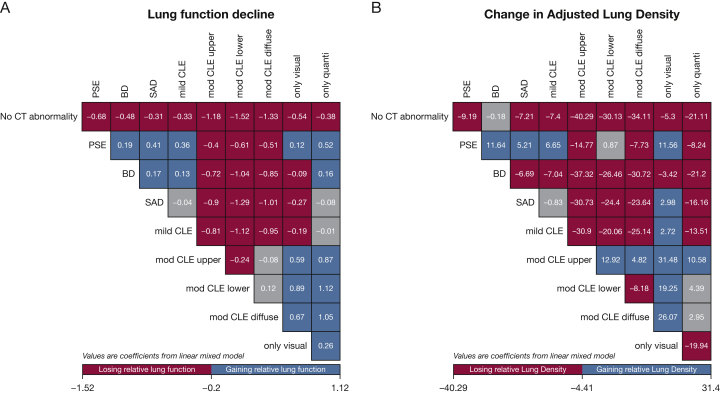
During follow-up, FEV1 declined the most in subjects with quantitative emphysema but without visual emphysema. Health-related quality of life (St. George’s Respiratory Questionnaire [SGRQ]) and physical function (6MWD) deteriorated the most in subjects with the diffuse moderate to severe CLE subtype. Quantitative emphysema worsened the most in subjects with lower lobe predominant moderate to severe CLE. Functional small airway disease (on the basis of PRM) increased the most in the CT imaging subtype with visual emphysema but without quantitative emphysema (3.6 % ± 8.5) (Table 4). The overall mortality was the highest in the 3 moderate to severe CLE subtypes and the PSE subtype (Fig 2). In multivariable linear mixed models, change in lung function during follow-up was significantly greater in all subjects with abnormal CT imaging findings than in control subjects (Fig 3A). The most substantial deterioration of health-related quality of life (SGRQ scores) occurred in the diffuse CLE and PSE groups. Subjects in the 3 moderate to severe CLE categories showed the highest risk for mortality.
Table 4.
Change in Lung Function, Chest CT Imaging Phenotypes, and Other Longitudinal Characteristics Over 5 Years in 10 CT Imaging Subtypes
| Characteristic | No. of Subjects | Moderate to Severe Centrilobular Emphysema |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No CT Imaging Abnormality | Paraseptal Emphysema | Bronchial Disease | Small Airway Disease | Mild Emphysema | Upper Lung Predominant | Lower Lung Predominant | Diffuse | Discordant Visual | Discordant Quantitative | P Valuea | ||
| No. of subjects | 1,593 | 1,060 | 525 | 276 | 1,159 | 189 | 22 | 345 | 328 | 112 | … | |
| Change in FEV1, mL | 5,013 | −203.63 ± 267.65 | −246.45 ± 315.32 | −186.51 ± 336.40 | −150.12 ± 305.45 | −223.15 ± 277.94 | −246.41 ± 263.71 | −175.67 ± 267.17 | −234.26 ± 245.17 | −221.69 ± 254.25 | −287.17 ± 320.44 | < .001 |
| Change in FEV1, mL/y | 5,013 | −36.13 ± 48.06 | −43.64 ± 56.54 | −32.27 ± 58.68 | −26.45 ± 53.14 | −39.23 ± 49.51 | −45.51 ± 49.40 | −35.20 ± 53.76 | −41.48 ± 41.06 | −38.84 ± 44.88 | −50.31 ± 55.59 | < .001 |
| Change in GOLD spirometry grade, No. (%) | 4,148 | < .001 | ||||||||||
| −1 | 293 | 32 (2.9) | 50 (6.1) | 30 (9.0) | 36 (18.1) | 90 (10.0) | 13 (7.9) | 1 (6.7) | 15 (5.4) | 14 (6.1) | 12 (12.2) | … |
| No change | 3,140 | 1,001 (89.8) | 562 (69.0) | 254 (76.0) | 143 (71.9) | 649 (71.9) | 107 (65.2) | 11 (73.3) | 191 (69.2) | 156 (68.1) | 66 (67.3) | … |
| +1 | 715 | 82 (7.4) | 203 (24.9) | 50 (15.0) | 20 (10.1) | 164 (18.2) | 44 (26.8) | 3 (20.0) | 70 (25.4) | 59 (25.8) | 20 (20.4) | … |
| Change in SGRQ total score | 5,194 | −0.69 ± 15.08 | 2.12 ± 16.79 | −1.46 ± 19.13 | 0.60 ± 13.83 | 0.19 ± 14.29 | 1.52 ± 12.52 | 0.13 ± 11.60 | 4.14 ± 12.75 | 0.71 ± 16.01 | 0.24 ± 11.81 | < .001 |
| Change in 6-minute walking distance | 4,945 | −121.00 ± 351.94 | −150.05 ± 390.70 | −138.73 ± 374.55 | −135.34 ± 323.90 | −131.36 ± 334.22 | −208.92 ± 379.85 | −94.87 ± 349.95 | −219.47 ± 351.80 | −169.96 ± 350.88 | −112.24 ± 237.06 | < .001 |
| Change in adjusted lung density, g/L | 4,744 | 1.41 ± 13.86 | −3.35 ± 13.51 | −0.99 ± 15.13 | 2.65 ± 14.15 | 2.35 ± 14.24 | −4.18 ± 11.93 | −10.10 ± 8.66 | −1.16 ± 11.28 | −5.12 ± 13.51 | 10.15 ± 12.53 | < .001 |
| Change in Perc15 | 4,744 | 1.70 ± 17.44 | −3.06 ± 15.37 | −7.44 ± 24.20 | 2.62 ± 17.27 | 1.75 ± 16.15 | −4.01 ± 11.84 | −8.27 ± 6.77 | −1.24 ± 10.04 | −7.63 ± 13.92 | 10.40 ± 13.03 | < .001 |
| Change in PRMfSADb | 4,020 | 0.83 ± 4.65 | 2.16 ± 8.11 | 2.02 ± 7.99 | −1.64 ± 8.64 | 1.31 ± 9.46 | 1.66 ± 6.45 | 0.68 ± 10.25 | 1.20 ± 7.77 | 3.60 ± 8.53 | −0.64 ± 8.52 | < .001 |
| Survival rates, y, No. (%) | 7,994 | 2,130 | 1,697 | 734 | 340 | 1,544 | 283 | 50 | 643 | 463 | 110 | … |
| 1 | 7,580 | 2,018 (94.7) | 1,636 (96.4) | 699 (95.2) | 313 (92.1) | 1,456 (94.3) | 260 (91.9) | 50 (100.0) | 593 (92.2) | 455 (98.3) | 100 (90.9) | … |
| 3 | 7,341 | 1,990 (93.4) | 1,554 (91.6) | 684 (93.2) | 306 (90.0) | 1,430 (92.6) | 244 (86.2) | 48 (96.0) | 545 (84.8) | 440 (95.0) | 100 (90.9) | … |
| 5 | 6,971 | 1,948 (91.5) | 1,451 (85.5) | 666 (90.7) | 296 (87.1) | 1,379 (89.3) | 212 (74.9) | 38 (76.0) | 460 (71.5) | 422 (91.1) | 99 (90.0) | … |
Data are mean ± SD unless indicated otherwise. See Tables 1, 2 and 3 legends for expansion of abbreviations.
P value tests for differences across all 10 groups.
The PRMfSAD of quantitative CT imaging integrates inspiratory and expiratory images to produce relative percentages of functional small airway disease defined by lung area < −856 HU at normal expiration.
Figure 2.
Kaplan-Meier curves of risk for all-cause death in smokers according to CT imaging subtypes. P values were calculated with the use of a log-rank test. BD = bronchial airway disease; diff = diffuse; mod = moderate; Normal = no emphysema or airway abnormality; quant = quantitative; quant w/o visual = discordant: quantitative emphysema without visual emphysema; SAD = small airway disease; visual w/o quant = discordant: visual emphysema without quantitative emphysema. See Figure 1 legend for expansion of other abbreviations.
Figure 3.
Change in FEV1 (A) and adjusted lung density (B) in each pair of CT imaging subtypes. Values in each figure panel are coefficients from linear mixed regression for each comparison pair, adjusting for age, race, sex, current smoking status, cumulative smoking intensity, BMI, and FEV1. If the corrected P value is less than .05, the box is displayed in red or blue; a gray box indicates no statistical significance. Red (losing lung function or lung density faster) and blue (losing lung function or lung density slower) in each group of columns and in each group of rows indicates levels of statistical significance. For example, those with PSE had a coefficient of −0.68 for FEV1 decline, and −9.19 for change in adjusted lung density, indicating significantly faster decline for these parameters than for those with no CT imaging abnormality. See Figures 1 and 2 legends for expansion of abbreviations.
Comparisons of Specific CT Imaging Subtypes
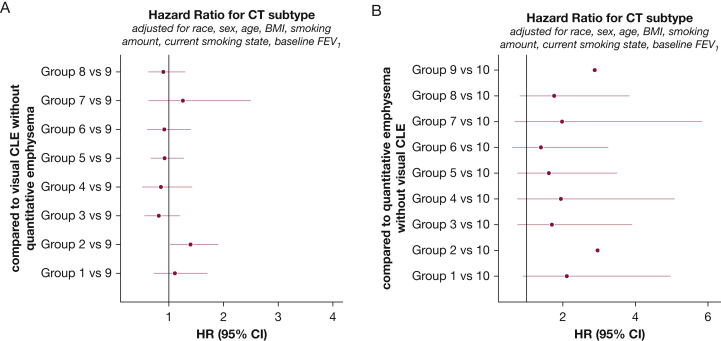
The Discordant Visual vs Quantitative Emphysema Subtypes: Compared with subjects with quantitative but not visual emphysema, subjects having visual but not quantitative emphysema were more likely to be female and current smokers and less likely to be black (P < .01, respectively) (Table 1). Subjects with visual but not quantitative emphysema had higher adjusted lung density and lower lung function (P < .01). There was a higher percentage of subjects with preserved ratio impaired spirometry (PRISm) in the visual but no quantitative emphysema group. During follow-up, subjects with the visual-only emphysema subtype had slower lung function decline but greater deterioration in SGRQ scores and exercise capacity than did subjects with the quantitative-only subtype. The subjects with visual-only emphysema lost substantial lung density over 5 years, whereas the subjects with quantitative-only emphysema gained lung density (P < .01). The visual-only emphysema group had a significantly higher risk for mortality (hazard ratio, 2.88; 95% CI, 1.22- 6.82) (Fig 4).
Figure 4.
Cox regression proportional hazard model analysis of all-cause mortality: comparison of discordant groups with other subtypes. Adjusted hazard ratios (solid circles) and 95% CIs (horizontal lines) for death at 5-year follow-ups comparing each of the other CT imaging subtypes with the visual only (A) and quantitative only (B) subtypes. Hazard ratios are adjusted for race, sex, age, BMI, pack-years, current smoking status, and baseline FEV1. See Figures 1 and 2 legends for expansion of abbreviations.
Bronchial Disease vs Small Airway Disease: Compared with individuals with physiologically defined airway disease without visual evidence (small airway disease), subjects with visually defined airway disease (bronchial disease) were younger (P < .01) and had a higher proportion of current smokers (P < .01). Furthermore, individuals in the bronchial disease subtype had less emphysema and higher adjusted lung density (P < .01). There were substantially more subjects in the bronchial disease group who were classified as being in the PRISm category. Subjects with bronchial disease had greater reductions in SGRQ scores (P < .01) than did subjects with small airway disease. During follow-up, subjects with bronchial disease had increased lung function decline, greater deterioration in SGRQ scores and exercise capacity, greater loss of adjusted lung density, and increased functional small airway disease. No significant differences in survival were observed between these groups.
PSE Compared With Diffuse Moderate to Severe CLE: Compared with subjects with diffuse moderate to severe CLE, subjects with significant PSE were younger, were more often male, and included a higher proportion of current smokers (P < .01, respectively). Subjects with PSE had less functional small airway disease and higher adjusted lung density at baseline (P < .01, respectively). They also responded less to inhaled bronchodilators (P < .01). Subjects with PSE had better lung function, SGRQ scores, and exercise capacity (P < .01, respectively) than diffuse CLE. During follow-up, adjusted lung density decreased faster in subjects with PSE (Fig 3B) (P < .01).
Comparison of Upper, Lower, and Diffuse Moderate to Severe CLE Subtypes: Compared with upper lobe predominant or diffuse moderate to severe CLE subtypes, the lower lobe predominant CLE subtype had greater PRM functional small airway disease, and Pi10 values (P < .01, respectively), and subjects with lower lobe predominant CLE had the lowest baseline lung function. During follow-up, individuals with lower lobe predominant CLE had a slower rate of lung function decline. The adjusted lung density and the 15th percentile of the lung density histogram decreased most rapidly and functional small airway disease increased most in the lower lobe predominant CLE subtype. In addition, of the subjects with the 3 moderate to severe CLE subtypes, individuals with lower lobe predominant CLE had the highest 5-year mortality in the univariate analysis. However, after adjusting for age, sex, race, current smoking status, pack-years of smoking, BMI, and baseline FEV1, mortality was not significantly different across the 3 moderate to severe CLE subtypes.
Discussion
In this study, we investigated the clinical and physiological characteristics of smokers from the COPDGene study who were categorized into 10 nonoverlapping subtypes based on chest CT imaging and spirometric characteristics. These 10 subtypes had substantial differences in their rates of radiological, physiological, and symptom progression, as well as in mortality. These results suggest that the combination of visual and quantitative CT imaging features, which may reflect different underlying pathobiological processes in COPD, may provide a superior approach to classify COPD compared with the use of visual or quantitative CT imaging features alone.
By using a combination of visual and quantitative CT imaging assessments, we identified discordant subtypes for both emphysema and airway disease. We suspect that the finding of visual but no quantitative emphysema occurs when the degree of emphysema is insufficient to result in significantly reduced lung density, or the resultant density decrease may be masked by an accompanying increase in density from smoking-related lung inflammation.14, 15 The converse discordance, quantitative emphysema without visual emphysema, suggests that there is loss of lung density while the lung parenchyma retains enough definition that “holes” are not seen at visual inspection. High inflation volumes or overextension of regions of the lung (because of loss of elastic recoil) could produce this effect. It also would be an expected event in mild panlobular emphysema in which a diffuse loss of lung parenchymal density occurs.
Compared with subjects having quantitative but no visual emphysema, current smokers, women, and whites were substantially more common among subjects with visually defined emphysema without quantitative evidence. The subjects with visual-only emphysema had higher lung density, lower lung function, and worse SGRQ and 6MWD scores, suggesting that many of the subjects in the visual-only emphysema subtype have areas of low lung density due to emphysema masked by smoking-induced lung inflammation. In these discordant emphysema groups, the visual-only emphysema group had greater progression of quantitative emphysema, greater reduction in 6MWD, and increased mortality, even after adjustment for current smoking status and smoking intensity. This finding may indicate an effect of inflammation of the lung parenchyma or airways in subjects with otherwise relatively mild disease. In contrast, the quantitative-only emphysema group had the most rapid decline in lung function (FEV1), which suggests that this is another group of subjects with an early marker of disease who are at high risk for disease progression.
Through our subtyping groups, we also were able to investigate the differences between visually defined disease of the larger bronchi (bronchial disease) and physiologically defined small airway disease. The proportion of PRISm (approximately 24%) was higher in bronchial disease, and the proportion of Global Initiative for Chronic Obstructive Lung Disease spirometry grade II in bronchial disease was lower than in small airway disease. Although subjects with bronchial disease had less emphysema at baseline, they had more rapid progression of emphysema and worsening of SGRQ scores than did subjects with small airway disease. Again, some of this difference could be due to a higher proportion of current smokers in the bronchial disease group. Subjects with visually defined bronchial disease also showed evidence of substantial small airway disease. The presence of visually identified bronchial wall thickening could be a marker for extensive airway inflammation. This finding could occur before the development of significant emphysema and provide an important marker of subjects at high risk for disease progression.
Our subtyping system also allowed comparison of PSE and CLE. Although subjects with PSE had better physiological and functional characteristics than did subjects with diffuse moderate to severe CLE at baseline, they had substantial progression of emphysema and loss of lung function over time. On the basis of our approach of including subjects in the PSE group regardless of other characteristics, there were subjects with moderate to severe CLE in the PSE group. However, PSE is likely an important pattern of emphysema, and its presence should be recognized as an important marker of subjects at high risk of disease progression.
Finally, we compared the subtypes with varying anatomical distributions of moderate to severe CLE. Compared with subjects with upper lobe predominant CLE, subjects with lower lobe predominant CLE had more difficulties in physical function and health-related quality of life. Survival across the three moderate to severe CLE subtypes was similar; however, statistical power to detect differences in survival was limited by the small sample size of the lower lobe predominant CLE subtype. Lower lobe predominant and diffuse emphysema should be recognized as markers of subjects with high disease effect.
There have been many efforts to unravel COPD heterogeneity to understand better its biological mechanisms, predict outcomes, and determine specific therapeutic options.16, 17 Previous efforts to create COPD subtypes based on CT imaging information have been performed in various ways.8, 13 The Fleischner Society used CT imaging to divide COPD into five subtypes based on a visual assessment of emphysema severity and pattern; however, they relied on concordant quantitative CT imaging findings when describing the cross-sectional characteristics and progression of these five subtypes.8 Machine learning approaches also have been used to create discrete groups of subjects with COPD. For example, Castaldi et al13 performed k-means cluster analysis and identified four subtypes by using a set of four features: FEV1, emphysema quantified by means of CT imaging, segmental airway wall area, and emphysema distribution. They noted that the cluster of subjects with severe emphysema was associated more strongly with known COPD genetic risk factors (HHIP and the cluster of genes including CHRNA3, CHRNA5, and IREB2). In our study, the three subtypes of moderate to severe CLE seemed to share the distribution of Global Initiative for Chronic Obstructive Lung Disease stages and clinical characteristics with the cluster of severe emphysema of Castaldi et al.13 Although CT imaging characteristics were not included in their cluster analysis approach, the International COPD Genetics Consortium comparison of clustering solutions across COPD study populations suggested that continuous disease axes may be more relevant than discrete clusters for COPD.18 However, designating specific groups has advantages in certain situations, such as the phosphodiesterase 4 inhibitor treatments in patients with the chronic bronchitis phenotype.19 Investigators in future studies should evaluate whether patients with imaging-based subtypes also benefit from specific treatment; for example, it is possible that patients with emphysema-predominant subtypes may benefit less from inhaled corticosteroid treatment.
Some limitations need to be considered in the interpretation of our findings. First, even though COPDGene is a large COPD cohort, the number of subjects in some groups was relatively small. Second, the order of selection of the subtypes undoubtedly led to some of the observed differences; our findings for PSE well may have been different if we had focused on subjects with pure PSE (without CLE). We included subjects in the PSE group if substantial PSE was present, regardless of other CT imaging characteristics, which may explain in part why the prevalence of PSE (22.3%) was higher in our analysis than in other studies (3%-15%).13, 20, 21, 22 However, these results also may be due to substantial differences in study populations. There were differences in BMI between the COPD subtypes, which could have affected quantitative emphysema assessments. Multiple other clinical characteristics overlapped between the COPD subtypes, suggesting that they are not isolated clusters of subjects. Compared with previously reported studies, the COPDGene study had more smokers, a higher average intensity of smoking, and more severe COPD subjects. Despite these limitations, we were able systemically to investigate the prognostic significance of 10 discrete subtypes based on a combination of spirometry, airway features, emphysema patterns, and emphysema distribution, thus creating a set of CT imaging subtypes that eventually could be applied clinically. It is likely that further studies will be helpful in refining these subtypes.
Conclusions
In conclusion, our study introduces a potentially useful way to classify individuals with COPD at the time of diagnosis. This work confirms that COPD is highly heterogeneous. Specific COPD subtypes based on visual and quantitative CT imaging parameters and spirometry results provide a potentially useful tool to characterize this heterogeneity. Furthermore, the subtypes defined by means of CT imaging have different clinical characteristics, rates of progression, and risk of mortality. Further follow-up is needed to determine how these CT imaging subtypes change over longer periods. Moreover, assessment of genetic associations and other types of omics data across the subtypes may provide insight into whether these subtypes have distinct molecular causes.
Acknowledgments
Author contributions: J. P. and E. K. S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J. P., E. K. S., and B. D. H. performed the data analysis. J. P., E. K. S., B. D. H., and D. A. L. drafted the manuscript. All authors contributed substantially to the study design, interpretation of the results, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: B. J. M. reports grant funds from AstraZeneca, GlaxoSmithKline, Pearl Research, and Sunovion Pharmaceuticals; continuing medical education activities for Academy for Continued Healthcare Learning, Catamount Medical Education, Hybrid Communications, Medscape, Novartis, Projects in Knowledge, and Ultimate Medical Academy; medical advisory boards for AstraZeneca, Boehringer Ingelheim, Circassia, GlaxoSmithKline, Phillips, Science 24/7, Sunovion Pharmaceuticals, Theravance Biopharma, Third Pole Therapeutics, and Verona Pharma; and data safety and monitoring boards for Shire and Spiration. D. A. L. with colleagues has a pending patent for software to classify the severity of COPD. E. K. S. received grant and travel support from GlaxoSmithKline. None declared (J. P., B. D. H., J. D. C., E. A. R., S. H., V. J. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
* COPDGene Investigators—Core Units: Administrative Center: James D. Crapo, MD (principal investigator); Edwin K. Silverman, MD, PhD (principal investigator); Barry J. Make, MD; Elizabeth A. Regan, MD, PhD. Genetic Analysis Center: Terri Beaty, PhD; Ferdouse Begum, PhD; Adel R. Boueiz, MD; Peter J. Castaldi, MD, MSc; Michael Cho, MD; Dawn L. DeMeo, MD, MPH; Marilyn G. Foreman, MD, MS; Eitan Halper-Stromberg; Lystra P. Hayden, MD, MMSc; Craig P. Hersh, MD, MPH; Jacqueline Hetmanski, MS, MPH; Brian D. Hobbs, MD, MMSc; John E. Hokanson, MPH, PhD; Nan Laird, PhD; Christoph Lange, PhD; Sharon M. Lutz, PhD; Merry-Lynn McDonald, PhD; Margaret M. Parker, PhD; Dmitry Prokopenko, PhD; Dandi Qiao, PhD; Elizabeth A. Regan, MD, PhD; Phuwanat Sakornsakolpat, MD; Edwin K. Silverman, MD, PhD; Emily S. Wan, MD; Sungho Won, PhD. Imaging Center: Mustafa Al Qaisi, MD; Harvey O. Coxson, PhD; Teresa Gray; MeiLan K. Han, MD, MS; Eric A. Hoffman, PhD; Stephen Humphries, PhD; Francine L. Jacobson, MD, MPH; Philip F. Judy, PhD; Ella A. Kazerooni, MD; Alex Kluiber; David A. Lynch, MB; John D. Newell Jr, MD; Elizabeth A. Regan, MD, PhD; James C. Ross, PhD; Raul San Jose Estepar, PhD; Joyce Schroeder, MD; Jered Sieren; Douglas Stinson; Berend C. Stoel, PhD; Juerg Tschirren, PhD; Edwin Van Beek, MD, PhD; Bram van Ginneken, PhD; Eva van Rikxoort, PhD; George Washko, MD; Carla G. Wilson, MS. Pulmonary Function Test Quality Assurance Center, Salt Lake City, UT: Robert Jensen, PhD. Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Jim Crooks, PhD; Douglas Everett, PhD; Camille Moore, PhD; Matt Strand, PhD; Carla G. Wilson, MS. Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, MPH, PhD; John Hughes, PhD; Gregory Kinney, MPH, PhD; Sharon M. Lutz, PhD; Katherine Pratte, MSPH; Kendra A. Young, PhD. Mortality Adjudication Core: Surya Bhatt, MD; Jessica Bon, MD; MeiLan K. Han, MD, MS; Barry J. Make, MD; Carlos Martinez, MD, MS; Susan Murray, ScD; Elizabeth A. Regan, MD, PhD; Xavier Soler, MD; Carla G. Wilson, MS. Biomarker Core: Farnoush Banaei-Kashani, PhD; Russell P. Bowler, MD, PhD; Katerina Kechris, PhD. COPDGene Investigators – Clinical Centers: Ann Arbor VA: Jeffrey L. Curtis, MD; Perry G. Pernicano, MD; Baylor College of Medicine, Houston, TX: Nicola Hanania, MD, MS; Mustafa Atik, MD; Aladin Boriek, PhD; Kalpatha Guntupalli, MD; Elizabeth Guy, MD; Amit Parulekar, MD; Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, MD, MPH; Alejandro A. Diaz, MD, MPH; Lystra P. Hayden, MD; Brian D. Hobbs, MD; Craig Hersh, MD, MPH; Francine L. Jacobson, MD, MPH; George Washko, MD; Columbia University, New York, NY: R. Graham Barr, MD, DrPH; John Austin, MD; Belinda D’Souza, MD; Byron Thomashow, MD; Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr, MD; H. Page McAdams, MD; Lacey Washington, MD; HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, MD, MPH; Joseph Tashjian, MD; Johns Hopkins University, Baltimore, MD: Robert Wise, MD; Robert Brown, MD; Nadia N. Hansel, MD, MPH; Karen Horton, MD; Allison Lambert, MD, MHS; Nirupama Putcha, MD, MHS; Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, PhD, MD; Alessandra Adami, PhD; Matthew Budoff, MD; Hans Fischer, MD; Janos Porszasz, MD, PhD; Harry Rossiter, PhD; William Stringer, MD; Michael E. DeBakey; VAMC, Houston, TX: Amir Sharafkhaneh, MD, PhD; Charlie Lan, DO; Minneapolis VA: Christine Wendt, MD; Brian Bell, MD; Ken M. Kunisaki, MD, MS; Morehouse School of Medicine, Atlanta, GA: Marilyn G. Foreman, MD, MS; Eugene Berkowitz, MD, PhD; Gloria Westney, MD, MS; National Jewish Health, Denver, CO: Russell Bowler, MD, PhD; David A. Lynch, MB; Reliant Medical Group, Worcester, MA: Richard Rosiello, MD; David Pace, MD; Temple University, Philadelphia, PA: Gerard Criner, MD; David Ciccolella, MD; Francis Cordova, MD; Chandra Dass, MD; Gilbert D’Alonzo, DO; Parag Desai, MD; Michael Jacobs, PharmD; Steven Kelsen, MD, PhD; Victor Kim, MD; A. James Mamary, MD; Nathaniel Marchetti, DO; Aditi Satti, MD; Kartik Shenoy, MD; Robert M. Steiner, MD; Alex Swift, MD; Irene Swift, MD; Maria Elena Vega-Sanchez, MD; University of Alabama, Birmingham, AL: Mark Dransfield, MD; William Bailey, MD; Surya P. Bhatt, MD; Anand Iyer, MD; Hrudaya Nath, MD; J. Michael Wells, MD; University of California, San Diego, CA: Joe Ramsdell, MD; Paul Friedman, MD; Xavier Soler, MD, PhD; Andrew Yen, MD; University of Iowa, Iowa City, IA: Alejandro P. Comellas, MD; Karin F. Hoth, PhD; John Newell, Jr, MD; Brad Thompson, MD; University of Michigan, Ann Arbor, MI: MeiLan K. Han, MD, MS; Ella Kazerooni, MD; Carlos H. Martinez, MD, MPH; University of Minnesota, Minneapolis, MN: Joanne Billings, MD; Abbie Begnaud, MD; Tadashi Allen, MD; University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD; Jessica Bon, MD; Divay Chandra, MD, MSc; Carl Fuhrman, MD; Joel Weissfeld, MD, MPH; University of Texas Health Science Center at San Antonio, San Antonio, TX: Antonio Anzueto, MD; Sandra Adams, MD; Diego Maselli-Caceres, MD; Mario E. Ruiz, MD.
Additional information: The e-Appendix, e-Figures, and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: B. D. H. was funded by the National Institutes of Health [Grant K08HL136928] and the Parker B. Francis Research Opportunity Award. The COPDGene project (clinicaltrials.gov; Identifier: NCT00608764) was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Grants U01 HL089897, U01 HL089856] and the COPD Foundation through contributions made to an industry advisory board composed of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion Pharmaceuticals. This study also was funded by the National Institutes of Health [Grant P01 HL114501]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Supplementary Data
References
- 1.Han M.K., Agusti A., Calverley P.M. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusasco V., Martinez F. Chronic obstructive pulmonary disease. Compr Physiol. 2014;4(1):1–31. doi: 10.1002/cphy.c110037. [DOI] [PubMed] [Google Scholar]
- 3.Tantucci C., Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agusti A., Edwards L.D., Celli B. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42:636–646. doi: 10.1183/09031936.00195212. [DOI] [PubMed] [Google Scholar]
- 5.Andreeva E., Pokhaznikova M., Lebedev A., Moiseeva I., Kuznetsova O., Degryse J.M. Spirometry is not enough to diagnose COPD in epidemiological studies: a follow-up study. NPJ Prim Care Respir Med. 2017;27(1):62. doi: 10.1038/s41533-017-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta PP. High resolution computed tomography and chronic obstructive pulmonary disease. Chapter 9. In: MartAn-Loeches I, ed. Bronchitis. http://www.intechopen.com/books/bronchitis/high-resolution-computed-tomography-and-chronic-obstructive-pulmonary-disease. Accessed August 29, 2019.
- 7.Lynch D.A. Progress in imaging COPD, 2004-2014. Chronic Obstr Pulm Dis. 2014;1(1):73–82. doi: 10.15326/jcopdf.1.1.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch D.A., Austin J.H., Hogg J.C. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277(1):192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch D.A., Moore C.M., Wilson C. Genetic Epidemiology of COPD (COPDGene) Investigators. CT-based visual classification of emphysema: association with mortality in the COPDGene study. Radiology. 2018;288(3):859–866. doi: 10.1148/radiol.2018172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regan E.A., Hokanson J.E., Murphy J.R. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han M.K., Bartholmai B., Liu L.X. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 12.Boueiz A., Chang Y., Cho M.H. Lobar emphysema distribution is associated with 5-year radiological disease progression. Chest. 2018;153(1):65–76. doi: 10.1016/j.chest.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaldi P.J., San José Estépar R., Mendoza C.S. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188:1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashraf H., Lo P., Shaker S.B. Short-term effect of changes in smoking behaviour on emphysema quantification by CT. Thorax. 2011;66(1):55–60. doi: 10.1136/thx.2009.132688. [DOI] [PubMed] [Google Scholar]
- 15.Shaker S.B., Stavngaard T., Laursen L.C., Stoel B.C., Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD. 2011;8(1):2–7. doi: 10.3109/15412555.2010.541306. [DOI] [PubMed] [Google Scholar]
- 16.Hogg J.C., Chu F., Utokaparch S. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 17.Barker B.L., Brightling C.E. Phenotyping the heterogeneity of chronic obstructive pulmonary disease. Clin Sci (Lond) 2013;124(6):371–387. doi: 10.1042/CS20120340. [DOI] [PubMed] [Google Scholar]
- 18.Castaldi P.J., Dy J., Ross J. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airway disease and emphysema. Thorax. 2014;69(5):415–422. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennard S.I., Calverley P.M., Goehring U.M., Bredenbroker D., Martinez F.J. Reduction of exacerbations by the PDE4 inhibitor roflumilast: the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi: 10.1186/1465-9921-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith B.M., Austin J.H., Newell J.D., Jr. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1) doi: 10.1016/j.amjmed.2013.09.020. 94.e7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki T., Nishino M., Gao W. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax. 2015;70:1156–1162. doi: 10.1136/thoraxjnl-2015-207653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostridge K., Williams N., Kim V. Distinct emphysema subtypes defined by quantitative CT analysis are associated with specific pulmonary matrix metalloproteinases. Respir Res. 2016;17(1):92. doi: 10.1186/s12931-016-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.