Abstract
Great attention has been focused on the discovery of anti-angiogenic natural and synthetic compounds to be finally used as or at least a part of the treatment of tumors. The marine ecosystems provide diversity in natural chemicals with the potential of being exploited as medicines in the treatment of diseases. Several studies have investigated Ophiuroids as a source of anti-tumor and anti-metastatic organisms. Here, we described the inhibitory effects of an ethanolic crude extract of brittle star (Ophiocoma erinaceus) on angiogenesis and the expression level of TGF-β, VEGF, and bFGF in chicken chorioallantoic membrane (CAM) as an experimental model. To do this 45 embryonated eggs were randomly divided into six groups including the control group, sham, three experimental groups and positive. The number and the length of vessels were calculated using ImageJ® software. The relative mRNA levels of the genes in different groups were evaluated by qRT-PCR method. Our study was suggestive of an anti-angiogenesis effect of brittle star ethanolic crude extract in a CAM model. The extract also showed a pharmacological effect of down-regulation of mRNA related to VEGF, TGF-β, and bFGF genes on chicken vascular endothelial cells. It was also showed that the observed inhibitory effect is with a dose-dependent manner in which the highest inhibitory effect belonged to the highest used dose. We indicated the anti-angiogenesis properties of the Persian Gulf brittle star. Further studies are needed in other aspects of the brittle star extract in the treatment of angiogenesis, hyperplasia, and cancers.
Keywords: Cell biology, Pharmaceutical science, Molecular biology, Cancer research, Ophiocoma erinaceus, Vascular endothelial growth factor, Chorioallantoic membrane, Angiogenesis inhibitors, Hyperplasia
Cell biology; Pharmaceutical science; Molecular biology; Cancer research; Ophiocoma erinaceus; Vascular endothelial growth factor; Chorioallantoic membrane; Angiogenesis inhibitors; Hyperplasia
1. Introduction
Angiogenesis is defined as the function of formation of new blood vessels from pre-existing vessels. In physiological conditions, angiogenesis occurs in wound healing, embryonic development, and the menstrual cycle, which is tightly regulated by several stimulatory and inhibitory mechanisms [1, 2, 3, 4]. During angiogenesis, endothelial cells of blood vessels are stimulated by angiogenic factors such as vascular endothelial growth factors (VEGFs), and fibroblast growth factors (FGFs) to secrete proteases and plasminogen activators. Consequently the activity of proteases and plasminogen activators, results in the releasing of the endothelial cells by degradation of the blood vessel basement membrane, gives the cells ability to migrate, proliferate, differentiate to form a new, lumen-containing vessel in the surrounding matrix [5, 6]. TGF-β1 contributed in angiogenesis through VEGF-mediated apoptosis [7, 8]. In pathological conditions, excessive angiogenesis occurs in tumor progression, and rheumatoid arthritis, where blood vessels develop in an uncontrolled or disorganized manner. In many types of cancers it is generally believed that angiogenesis is highly required for growth of the tumors [9, 10]. Excessive angiogenesis seen in most of solid tumors can also be exploited by transformed cells as an entrance to the host blood stream and a path for metastasis [11]. Several other studies have given enough evidences for deep relations between tumors and angiogenesis to mark the angiogenesis as a target for anti-cancer drug development [12]. There are numbers of different cytokines and growth factors modulate angiogenesis. Among different angiogenesis factors, fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF) and transforming growth factor-beta 1 (TGF-β1) are considered as important factors which play key roles [6, 8, 13]. It appears endothelial cells of vessels in tumors produce more than one type of angiogenesis factor such VEGF, FGF in high amounts [7]. High levels of TGF-β1 expression are reported in many types of human carcinomas [14]. Currently, wide range of anti-angiogenic agents have been developed many of them with the inhibitory effects on VEGF, bFGF, and TGF-β1 factors [7, 8]. Several methods have been used for the study of angiogenesis and investigations on the effects of different compounds on the process of angiogenesis. Most frequently used of the methods, human amnion, air sacs, mouse cornea, and Chick Chorioallantoic Membrane (CAM) can be mentioned. Ease of use, low costs, high repeatability, has made the CAM method of choice for many angiogenesis studies [15, 16, 17, 18].
Great attention has been focused on discovery of anti-angiogenic natural and synthetic compounds to be finally used as or at least as a part of treatment of tumors. Marine ecosystems enjoying high diversity of living creatures contain wide range of different materials with different chemical structures. The marine ecosystems provides diversity in natural chemicals with the potential of being exploited as medicines in treatments of diseases specially cancers [19, 20]. Several studies have investigated Ophiuroids as a source of anti-tumor and anti-metastatic organisms. Considerable number of studies are indicative of the noticeable anti-tumor properties of the Ophiuroids [21, 22, 23]. The Persian Gulf as a rich marine ecosystem is a territory of different species of Ophiuroids containing components characteristics of this part of the world. Brittle star (Ophiocoma erinaceous), inhabitant of the Persian Gulf isles noticeably Qeshm [24, 25]. Recent studies have shown the anti-angiogenic and anti-tumor properties of extracts prepare from brittle star. In this study, we investigated the inhibitory effects of an ethanolic crude extract of brittle star (Ophiocoma erinaceus) on angiogenesis and the expression level of TGF-β, VEGF, and bFGF in chicken chorioallantoic membrane (CAM) as an experimental model.
2. Material and methods
2.1. Preparation of ethanolic extract of brittle star
The brittle star O. erinaceus were collected from Qeshm Island, Northern Persian Gulf, Iran and supplied kindly by Baharara's group. Extraction was done based on the method of Baharara et al. [26], with certain modifications by the different solvents. Specimens of brittle stars washed with water and dried in a dark room and then were ground. For extraction, the powder (10 g) of specimens was dissolved in 100 ml of ethanol 100% and was shaken for 72 h. The solvent was filtered through Whatman filter paper with a pore size of 0.4 μm. The solvent was removed using rotary evaporator. The resulting extract was placed in incubator (FanAzma Gostar, Iran) until all the ethanol evaporates then the extract stored at 4 °C in a refrigerator for future use. The extract was dissolved in DMSO, dimethyl sulfoxide, (Merck, Germany) to prepare the stock solutions for use.
2.2. Preparation of the filter disks
Small filter paper discs with 6 mm in diameter were generated using a standard puncher and sterilized by autoclave. Different concentrations of extract were applied on CAM, by placing a filter paper disk and charging the test solution on it.
2.3. Chick chorioallantoic membrane (CAM) assay
Fertilized SPF eggs of Gallus gallus were obtained (from Razi Vaccine and Serum Research Institute, Iran), cleaned with 70% v/v ethanol, and placed in a 37.8 °C incubator (FanAzma Gostar, Iran) at in a relative humidity (60–70%) for the duration of their development.
At 6th day of incubation, the eggs were candled using a hand-held egg Candler at the blunt end of the egg to identify the air sac and prominent blood vessels. Using a sterile needle, the CAM were separated from the shell by making a shallow burr hole at the blunt end of the egg and another burr hole made perpendicular to the previously identified blood vessels in the center of the egg. A mild suction was applied to the blunt end burr hole to displace the air sac and drop the CAM away from the shell. Fine forceps were then used to pick away the shell over the false air sac [18, 27], so a square window of one cm2 was opened in the egg shell in aseptic conditions and under laminar flow (Bioflux, Japan) and then, sealed with parafilm to prevent dehydration and the eggs were returned for incubation. On the 8th day of incubation (Day 8), the eggs were removed from the incubator [28]. The different treatments used for each of the groups are shown in Table 1. The test specimens were divided into the negative control, positive control (treat with 20 μl of 100 μg/ml of Sutent® (Pfizer, USA)), Sham-exposed group (treated with 20 μl of DMSO) and 3 treatment group (treatment with 20 μl of 25, 50 and 100 μg/ml of brittle star extract) and then the places of the windows were again covered and the eggs were returned to the incubator.
Table 1.
Experimental groups and treatments.
| Group | Treatment |
|---|---|
| Negative control | No treated |
| Sham-exposed group | 20 μl of DMSO |
| Group 1 | 20 μl of 25 μg/ml of brittle star extract |
| Group 2 | 20 μl 50 μg/ml of brittle star extract |
| Group 3 | 20 μl of 100 μg/ml of brittle star extract |
| Positive control | 20 μl of 100 μg/ml of Sutent® |
In 12th day of incubation, all cases were photographed by research photo stereomicroscope (Jenus, Japan); ×5.5 magnification images were taken. The tissues of the treated area were isolated, washed with PBS, and stored in liquid nitrogen for the next steps.
2.4. Quantification of angiogenesis
Secondary and tertiary capillaries were evaluated for anti-angiogenic response, in order to, images of the treated area were prepared, the number and length of vessels were measured four sides of the filter disc were using ImageJ® software (v1.45e, Massachusetts, USA).
2.5. Gene expression analysis using qRT-PCR
The effect of brittle star extract on TGFβ4, VEGF, and bFGF gene expression was examined by qRT-PCR analysis using gene specific primers. TGFβ4 roles in avian species are similar to TGFβ1 in mammalian species. For this reason, the expression of TGFβ4 was investigated instead of TGFβ1 in this study [29]. Total RNA from each sample was isolated using RNA extraction kit (GenAll®, Korea). The purified total RNA was quantified with a NanoDrop spectrophotometer (Thermo Scientific, Netherlands). Then, the extracted RNA was reverse transcribed into cDNA using oligo dT18 primer and cDNA Synthesis Kit (Vivantis, Malaysia) as described in the manufacturer's handbook.
Quantification of cDNA was performed by real-time PCR using SYBR® Green I (SG) detection and the Rotor-Gene® Q System (Qiagen, USA). For each sample, 200 ng of a cDNA template was added to 10 μL of Maxima SYBR Green qPCR Master Mix 2X (Thermo Scientific™, France), 0.4 μL of both forward and reverse primers and RNA-free water to a final volume of 20 μL. An initial denaturing step at 95 °C for 10 min was followed by 40 cycles with a denaturing step at 94 °C (20 s), an annealing step at 62 °C (30 s), and an elongation step at 68 °C (30 s). All reactions were performed in triplicate. For all analyses, quantification was made relative to expression of chicken beta actin as a reference gene. Primer sequences (Bioneer, Korea) are shown in Table 2.
Table 2.
Primer sequences of target genes.
| Target gene | Primers sequences (5′-3′); F: Forward, R: Reverse | Amplicon size | Reference |
|---|---|---|---|
| VEGF |
F: TGAGGGCCTAGAATGTGTCC R: TCTTTTGACCCTTCCCCTTT |
194 | [30] |
| TGf-β4 |
F: CACCGACTACTGCTTCGGC R: GTCGGCGCTCCAGATGTAC |
136 | [31] |
| bFGF |
F: GGCACTGAAATGTGCAACAG R: TCCAGGTCCAGTTTTTGGTC |
151 | [32] |
| β-actin |
F: CTGTGTTCCCATCTATCGTG R: GATCTTCTCCATATCATCCCAG |
170 | [33] |
2.6. Statistical analysis
Data was analyzed using SPSS software, version 16 (New York, USA). The statistical significance between groups was determined using one-way ANOVA at a level of p < 0.05. Graphs were plotted using Excel software and changes in gene expression were analyzed using the REST-2009® software.
3. Results
3.1. The effects of brittle star ethanolic extract on number and length of CAM blood vessels
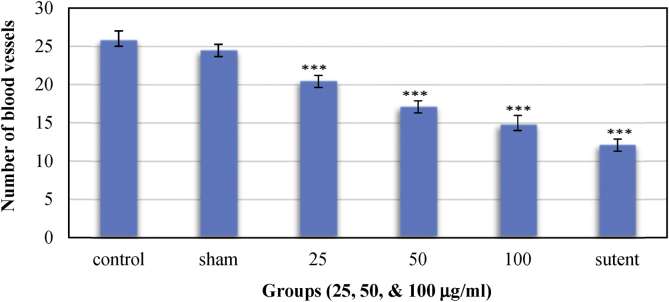
Three different concentrations of brittle star ethanolic extract were exposed to blood vessels network on CAM using a sterile filter disc. Embryonated chicken eggs at the age of 8th day were used for different treatments. Four days later CAM was assessed for changes in number and length of blood vessels. Figure 1 shows six photomicrographs of egg CAMs after different treatments (A, C, D, E, and F) and normal blood vessels network (B). The number and the length of capillaries in photo of each treated CAM was measured using ImageJ® software. Figures 2 and 3 show the average number of capillaries and length of them calculated in the experiment. Each number represents the average value for three tested eggs. In the negative control, normal chicken embryonated eggs, the average number of capillaries on selected area was 26 ± 0.81. The average number of capillaries in CAM of sham exposure group was 24.67 ± 0.47. The differences between the average number of blood vessels in negative control group (Figure 1B) and sham exposure (Figure 1C) was not statistically significant (p > 0.05). In experimental group treated with 100 μg/ml of ethanolic extract, the average number of vessels branches was 15.33 ± 0.47 (Figures 2 and 3). The average number in the experimental groups treated with 50 μg/ml and 25 μg/ml of brittle star ethanolic extract were 17.33 ± 0.47 and 20.66 ± 0.48 respectively. The average number of blood vessels in these two experimental groups was significantly decreased compare with control group (p < 0.001). The average number of capillaries in positive control group (Figure 1A) treated with 100 μg/ml Sutent® was 12.67 ± 0.47 which is significantly reduced in comparison with negative control group (p < 0.001).
Figure 1.
Photomicrographs of CAM in the control and treatment groups. The effect of the extract on angiogenesis is showed in these figures. (A) positive control (100 μg/ml sutent®), (B) negative control (C) sham exposure, (D) first treatment (25 μg/ml), (E) second treatment (50 μg/ml), (F) third treatment (100 μg/ml). As indicated, a dose dependent manner was seen in treatment groups by extracts. The picture magnification is 5.5.
Figure 2.
Mean of the number of blood vessels in experimented (treated) groups (n = 3, Mean ± SD, ***p < 0.001). The decreasing effect of the extract on the number of blood vessels was shown quantitatively. As seen, the extract affected the number of blood vessels in a dose dependent manner.
Figure 3.
Mean of the length of blood vessels in experimented (treated) groups (n = 3, Mean ± SD, ***p < 0.001). The effect of the extract on the length of blood vessels was shown quantitatively. As seen, the extract affected the length of blood vessels in a dose dependent manner.
Figures 1, 2, and 3 show the anti-angiogenic effects of ethanolic extract of brittle star on CAM. As indicated, the examined extract, has affected the number and length of blood vessels. The differences between the mean length of blood vessels branches in negative control group (B) (34.5 ± 1.08) and sham exposure group was not statistically significant (p). The differences between the mean of length of blood branches in negative control group (B) (34.5 ± 1.08) and positive control (A) (17.63 ± 0.45) and first treatment (D) (29.55 ± 0.82) and second treatment (E) (26 ± 0.7) and third treatment (F) (19.78 ± 0.61) groups was statistically significant (p). The differences between the mean of number of blood vessels in negative control group (B) (26 ± 0.81) and sham exposure (C) (24.67 ± 0.47) was not statistically significant (p). While the differences between the mean of number of blood vessels in negative control group (B) (26 ± 0.81) and positive control (A) (12.67 ± 0.47) and first treatment (D) (20.66 ± 0.47), second treatment (E) (17.33 ± 0.47) and third treatment (F) (15.33 ± 0.47) groups were statistically significant (p).
3.2. The effects of ethanolic extract of brittle star on expression levels of mRNA VEGF, bFGF, and TGF- genes
The levels of expression of mRNA derived from VEGF, bFGF, and TGF- genes in tissues isolated from chorioallantoic membrane in controls and treatment groups were analyzed using real time PCR assay. The real time PCR assay showed that the levels of expression of mRNA derived from VEGF, bFGF, and TGF- genes in sham exposure as compared to negative control were not reduced significantly (p). This indicates that the used solvent (DMSO) does not affect the angiogenesis. As well as the levels of expression of VEGF gene in first and second treatments (B, C) with a significance level of p < 0.05 and third treatment and positive control (D, E) with a significance level of p were reduced as compared to negative control. Down-expression of TGF- gene in first and second treatments (B, C) as compared to negative control were not statistically significant (p) and only in the third treatment and positive control groups, significant decrease (p) were observed in expression of TGF- gene as compared to negative control. The expression of bFGF gene were reduced in first treatment (B) as compared to negative control with a significance level of and in second and third treatment groups (C, D) and positive group (E) with a significance level of . This reduction in expression of VEGF, bFGF, and TGF- genes in experimental groups was in a dose dependent manner so that the highest reduction in expression was related to third treatment group (D) and the lowest reduction was related to first treatment group (B). The results of real time PCR were shown in Figure 4.
Figure 4.
The relative expression of VEGF, bFGF, and TGF- genes in exprimented (treated) groups (n = 3, Mean ± SD, ***P, **P). The figure shows the results of real time PCR assay using bar chart. As it can be seen, the extract of brittle star reduces the expression of VEGF, bFGF, and TGF- genes in treatment and positive control groups so that the highest reduction in mRNA was related to positive control group and the treatment groups also showed a dose dependent decrease in expression of these genes. This reduction in mRNA levels related to TGF- gene in the first and second treatment groups were not significant (p) and was significant for third treatment and positive control groups with a level significance of p The reduction of the mRNA levels related to bFGF gene was significant for first treated group with a level significance of p and for second and third groups with a level significance of p. The reduction level related to VEGF gene was significant for first and second treated groups with a level significance of p and for third group and positive control with a level significance of p1.
4. Discussion
Our study was indicative of an anti-angiogenesis effect of brittle star ethanolic crude extract, in a CAM model. The extract also showed a pharmacological effect of down-regulation of mRNA related with VEGF, TGF-β, and bFGF genes on chicken vascular endothelial cells. It was also showed that, the observed inhibitory effect is with a dose dependent manner in which the highest inhibitory effect belonged to the highest used dose. The down-regulation of VEGF and bFGF was stronger than the down-regulation of TGF-β. This finding, at gene expression levels, confirms the Baharar's study showing the anti-angiogenesis effects of a brittle star extract in a CAM model [22].
There are several kinds of brittle stars living in different marine ecosystems [34]. Many drug discovery studies have focused on different kinds of brittle stars, living in different parts of the world, to reach to substances with anti-bacterial, anti-fungal, anti-inflammatory, anti-diabetic, and anti-tumor activities [21, 35, 36]. It has been shown that the Persian Gulf brittle star (Ophiocoma erinaceus), inhabitant of the Persian Gulf, is potentially a source of anti-angiogenic components [26, 37]. Different mechanisms are involved in angiogenesis that can be targeted for suppression of the process. The inhibition of formation of secondary and tertiary vessels branches is a consequence of reduced expression of angiogenic factors such as VEGF, bFGF, and TGF-β in endothelial vascular cells [7, 8, 38], which is in agreement with the our study. Our finding is an indication of the mechanism of inhibition caused by extract on angiogenesis of chicken vascular epithelial cells.
Down-regulatory effects of methanolic extract of brittle star on VEGF, bFGF, and TGF-β has also been shown on human ovary cancer cells, an in vitro confirmation to our findings [26, 35]. Anti-apoptotic and cytotoxic effects of a dichloromethanic extract of brittle star was also shown by another study on a melanoma cell line [39]. Our study along with these data collectively indicates the potential for the use of the Persian Gulf brittle star in treatment of disorders related to angiogenesis and also treatment of hyperplasia and cancers.
Anti-angiogenesis effect of brittle star extract is shown to be by mean of inhibition of VEGF, bFGF, and TGF-β expression on an ovary cancer cell line model [26, 35], a fact that was confirmed in the CAM model by present study.
As a strong anti-angiogenic drug, Sutent® was also incorporated in our experiments as positive control. Results of our study were also indicative of down-regulation of VEGF, bFGF, and TGF-β gene expression, caused by Sutent®, the generic name of Sunitinib malate. It is cleared that anti-angiogenic effects of Sutent® is due to inhibition of tyrosine kinase receptors by blocking the ATP-binding side [40]. In fact, the drug inhibits signaling in different cells by targeting receptor tyrosine kinases (RTKs) [41]. In our experiments Sutent® showed stronger inhibitory effect comparing with the brittle star extract at the same concentrations used for both of the substances at 100 μg/ml. In both of these treatments the highest inhibitory effect was seen on bFGF and the lowest one was seen on TGF-β genes expressions. Recent studies has shown that the mechanism of TGF-β1 and VEGF are related together conclusively [7, 8], the inhibitory effects on these two genes are expected to be similar, a fact that was also observed in our study. Obviously, the mechanism of action of the brittle star extract, on inhibition of angiogenesis can be followed in future studies.
The ethanolic extract used in the present study is a crude preparation which according to studies on closely related brittle stars contains a wide range of different components such as saponins, polysaccharides, trepens, sulphated sterols, sulphated carotenoids, phenil propanoids, and naphtoquinons [21, 35]. Anti-tumor effects on human and mouse lymphoma cells were demonstrated to be caused by saponin and saponin-like fractions prepared from some other brittle star extracts [42].
Saponins of brittle star showed anti-metastatic properties on HeLa cell, a human cervical cancer cell line [37]. Anti-proliferative effects of the methanolic extract of brittle star was also observed on these cells [26]. The active component(s) of the Persian Gulf brittle star crude ethanolic extract remains to be investigated by fractionating and testing the anti-angiogenic and down-regulatory properties of the extract in future studies.
5. Conclusion
We indicated the anti-angiogenesis properties of the Persian Gulf brittle star. Further studies are needed in other aspects of the brittle star extract in the treatment of angiogenesis, hyperplasia, and cancers.
Declarations
Author contribution statement
Saeed Ataei, Roya Rahmani, Nasrin Zareh, Fatemeh Donyadideh, Saba Ataei Kachooei, Mohammad Nabiuni, Sajjad Yazdansetad: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported partially by the Kharazmi University as three MSc theses, and the Razi Vaccine and Serum Research Institute in the form of supplying the CAM tests.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge the Research Center for Animal Development Applied Biology of Mashhad Branch, Islamic Azad University, IR Iran for supplying the brittle stars. The author would like to thank department of bacterial poultry diseases at Razi Vaccine and Serum Research Institute for technical assistance in CAM assay. The helps of Ms. Latifeh Karimzadeh and the rest of the Cell and Developmental Biology Lab staff at Kharazmi University, Karaj, IR Iran in experiments are highly acknowledged.
References
- 1.Adair T., Montani J. San Rafael (CA): Morgan & Claypool Life Sciences. 2010. Angiogenesis.www.ncbi.nlm.nih.gov/books/NBK53242/ Williston, USA. [PubMed] [Google Scholar]
- 2.Tahergorabi Z., Khazaei M. A review on angiogenesis and its assays. Iran. J. Basic Med. Sci. 2012;15:1110–1126. http://www.ncbi.nlm.nih.gov/pubmed/23653839 [PMC free article] [PubMed] [Google Scholar]
- 3.Héroult M., Reiss Y., Augustin H.G. Encycl. Mol. Pharmacol. Springer Berlin Heidelberg; Berlin, Heidelberg: 2008. Angiogenesis and vascular morphogenesis; pp. 80–88. [Google Scholar]
- 4.Johnson K.E., Wilgus T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care. 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross M.J., Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. http://www.ncbi.nlm.nih.gov/pubmed/11282421 [DOI] [PubMed] [Google Scholar]
- 6.Ucuzian A.A., Gassman A.A., East A.T., Greisler H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010;31:158–175. doi: 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari G., Cook B.D., Terushkin V., Pintucci G., Mignatti P. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell. Physiol. 2009;219:449–458. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logue O.C., McGowan J.W.D., George E.M., Bidwell G.L. Therapeutic angiogenesis by vascular endothelial growth factor supplementation for treatment of renal disease. Curr. Opin. Nephrol. Hypertens. 2016;25:404–409. doi: 10.1097/MNH.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Adjei A.A. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. The Oncologist. 2015;20:660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta M.K., Qin R.-Y. Mechanism and its regulation of tumor-induced angiogenesis. World J. Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. http://www.ncbi.nlm.nih.gov/pubmed/12800214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 13.Presta M., Dell’Era P., Mitola S., Moroni E., Ronca R., Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis, Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Huang J.J., Blobe G.C. Dichotomous roles of TGF-β in human cancer. Biochem. Soc. Trans. 2016;44:1441–1454. doi: 10.1042/BST20160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribatti D. The chick embryo chorioallantoic membrane as an in vivo assay to study antiangiogenesis. Pharmaceuticals. 2010;3:482–513. doi: 10.3390/ph3030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBord L.C., Pathak R.R., Villaneuva M., Liu H.-C., Harrington D.A., Yu W., Lewis M.T., Sikora A.G. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am. J. Cancer Res. 2018;8:1642–1660. http://www.ncbi.nlm.nih.gov/pubmed/30210932 [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z., Wen Z., Bai X. In vivo chick chorioallantoic membrane (CAM) angiogenesis assays. BIO-PROTOCOL. 2013;3 [Google Scholar]
- 18.Gatne D.P., Mungekar S., Addepalli V., Mohanraj K., Ghone S.A., Rege N.N. Development of collateral vessels: a new paradigm in CAM angiogenesis model. Microvasc. Res. 2016;103:11–13. doi: 10.1016/j.mvr.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Song X., Xiong Y., Qi X., Tang W., Dai J., Gu Q., Li J. Molecular targets of active anticancer compounds derived from marine sources. Mar. Drugs. 2018;16:175. doi: 10.3390/md16050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W., Hong J., Lee C.-O., Cho H.Y., Shin S., Jung J.H. Bioactive metabolites from the brittle star Ophioplocus japonicus. Nat. Prod. Sci. 2004;10:253–261. http://agris.fao.org/agris-search/search.do?recordID=KR2005011947 [Google Scholar]
- 22.Baharara J., Amini E. Phytochemical screening, antioxidant effect and down regulation of TGF-β induced by Ophiocoma erinaceus brittle star crude extract. Zahedan J. Res. Med. Sci. 2015;17:29–33. [Google Scholar]
- 23.Baharara J., Amini E., Namvar F. Evaluation of the anti-proliferative effects of Ophiocoma erinaceus methanol extract against human cervical cancer cells. Avicenna J. Med. Biotechnol. (AJMB) 2016;8:29–35. http://www.ncbi.nlm.nih.gov/pubmed/26855733 [PMC free article] [PubMed] [Google Scholar]
- 24.Keshavarz M., Mohammadikia D., Dabbagh A.R. The echinoderms fauna in intertidal zone of southern oli village coast ( boushehr , Persian Gulf ) the echinoderms fauna in intertidal zone of southern oli village coast ( boushehr , Persian Gulf ) J. Anim. Sci. Adv. 2012;2:495–498. http://www.scopemed.org/?mno=20191 [Google Scholar]
- 25.Fatemi S.M.R., Jamili S., Valinassab T., Kuranlu N. Diversity of ophiuroidea from lengeh portand Qeshm Island in the Persian Gulf. J. Fish. Aquat. Sci. 2010;5:42–48. [Google Scholar]
- 26.Baharara J., Amini E. The potential of brittle star extracted polysaccharide in promoting apoptosis via intrinsic signaling pathway. Avicenna J. Med. Biotechnol. (AJMB) 2015;7:151–158. http://www.ncbi.nlm.nih.gov/pubmed/26605009 [PMC free article] [PubMed] [Google Scholar]
- 27.Miller W.J., Kayton M.L., Patton A., O’Connor S., He M., Vu H., Baibakov G., Lorang D., Knezevic V., Kohn E., Alexander H.R., Stirling D., Payvandi F., Muller G.W., Libutti S.K. A novel technique for quantifying changes in vascular density, endothelial cell proliferation and protein expression in response to modulators of angiogenesis using the chick chorioallantoic membrane (CAM) assay. J. Transl. Med. 2004;2:4. doi: 10.1186/1479-5876-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribatti D., Nico B., Vacca A., Presta M. The gelatin sponge–chorioallantoic membrane assay. Nat. Protoc. 2006;1:85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- 29.Pan H., Halper J. Cloning, expression, and characterization of chicken transforming growth factor β4. Biochem. Biophys. Res. Commun. 2003;303:24–30. doi: 10.1016/s0006-291x(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 30.Mroczek-Sosnowska N., Sawosz E., Vadalasetty K.P., Łukasiewicz M., Niemiec J., Wierzbicki M., Kutwin M., Jaworski S., Chwalibog A. Nanoparticles of copper stimulate angiogenesis at systemic and molecular level. Int. J. Mol. Sci. 2015;16:4838–4849. doi: 10.3390/ijms16034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S.C., Isobe N., Nishibori M., Yoshimura Y. Expression of transforming growth factor-β isoforms and their receptors in utero-vaginal junction of hen oviduct in presence or absence of resident sperm with reference to sperm storage. Reproduction. 2006;132:781–790. doi: 10.1530/rep.1.01177. [DOI] [PubMed] [Google Scholar]
- 32.Chwalibog A., Wierzbicki M., Sawosz E., Grodzik M., Hotowy A., Prasek M., Jaworski S., Sawosz F. Carbon nanoparticles downregulate expression of basic fibroblast growth factor in the heart during embryogenesis. Int. J. Nanomed. 2013;8:3427. doi: 10.2147/IJN.S49745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury V.S., Nishibori M., Yoshimura Y. Changes in the expression of TGF$β$-isoforms in the anterior pituitary during withdrawal and resumption of feeding in hens. Gen. Comp. Endocrinol. 2003;133:1–7. doi: 10.1016/s0016-6480(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 34.Stöhr S., O’Hara T.D., Thuy B. Global diversity of brittle stars (echinodermata: ophiuroidea) PLoS One. 2012;7 doi: 10.1371/journal.pone.0031940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baharara J., Amini E., Mousavi M. The anti-proliferative and anti-angiogenic effect of the methanol extract from brittle star. Reports Biochem. Mol. Biol. 2015;3:68–75. http://www.ncbi.nlm.nih.gov/pubmed/26989740 [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu K. Biological properties of brittle star Ophiocnemis marmorata collected from Parangipettai, Southeast coast of India. J. Microbiol. Antimicrob. 2013;5:110–118. [Google Scholar]
- 37.Amini E., Nabiuni M., Baharara J., Parivar K., Asili J. Metastatic inhibitory and radical scavenging efficacies of saponins extracted from the brittle star (Ophiocoma erinaceus) Asian Pac. J. Cancer Prev. APJCP. 2015;16:4751–4758. doi: 10.7314/apjcp.2015.16.11.4751. http://www.ncbi.nlm.nih.gov/pubmed/26107236 [DOI] [PubMed] [Google Scholar]
- 38.Cross M.J., Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 39.Baharara J., Amini E., Nikdel N., Afzali M. The pro apoptotic effect of brittle Star dichloromethane extract on B16F10 melanoma cell line. J. Paramed. Sci. 2015;6:74–80. http://journals.sbmu.ac.ir/jps/article/view/9793 [Google Scholar]
- 40.Gotink K.J., Verheul H.M.W. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regad T. Targeting RTK signaling pathways in cancer. Cancers. 2015;7:1758–1784. doi: 10.3390/cancers7030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson L., Bohlin L., Iorizzi M., Riccio R., Minale L., Moreno-López W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon. 1989;27:179–188. doi: 10.1016/0041-0101(89)90131-1. http://www.ncbi.nlm.nih.gov/pubmed/2718189 [DOI] [PubMed] [Google Scholar]