Abstract
To explore the association of incident hip fractures with metabolic syndrome (MetS) and its single components, we designed a prospective cohort study of hip fracture incidence among 117,053 participants of a population-based health surveillance program in Vorarlberg, the westernmost Austrian province. Incident hip fractures were recorded between 5 and 10 years after inclusion at baseline from 2003 to 2009. Applying Cox proportional hazard models for each MetS component and for a composite z-score for MetS, hazards for fracture were estimated in quintiles, as continuous z-score variables, and as pathological cut off values. Mean age was 50.1 ± 15.6 years at baseline, 5–10 years after which 947 incident hip fractures occurred. An association of a higher composite MetS score with decreased hip fracture risk was observed in women (HR 0.80, 95%-CI 0.88–0.96, p < 0.01) which disappeared upon adjustment for BMI. BMI was inversely associated with hip fracture risk in women and men (HR for the highest compared with the lowest quintile: 0.83 (95%-CI: 0.63–1.10, ptrend < 0.05) and 0.55 (95%-CI: 0.38–0.79, ptrend < 0.001), respectively). Only in women, hip fracture risk was reduced at high cholesterol levels (HR for the highest relative to the lowest quintile: 0.64, 95%-CI: 0.48–0.84, ptrend < 0.05) and in hypercholesterolemic patients (HR 0.82, 95%-CI: 0.67–0.99, p < 0.05), but elevated in hyperglycemic patients (HR 1.33, 95%-CI: 1.05–1.70, p < 0.05). Hypertriglyceridemia was associated with increased male hip fracture risk (HR 1.33, 95%-CI: 1.03–1.72, p < 0.05). The inverse association between the MetS and hip fracture risk is mainly driven by one single component, namely BMI.
Keywords: Metabolic syndrome, Hip fractures, Body mass index, Serum cholesterol, Triglycerides, Vorarlberg Health Monitoring and Prevention Program
Highlights
-
•
Increasing BMI was associated with reduced female and male hip fracture risk.
-
•
Higher MetS score reduced female fractures, adjustment for BMI abolished the effect.
-
•
Increasing cholesterol and hypercholesterolemia reduced female hip fracture risk.
-
•
Hyperglycemia was associated with increased female hip fracture risk.
-
•
Hypertriglyceridemia was accompanied by increased male hip fracture risk.
1. Introduction
As one consequence of osteoporosis, hip fracture is among the most prevalent and devastating injuries among the elderly (Morris and Zuckerman, 2002; Keen, 2003). It is associated with increased mortality, morbidity, disability and economic costs to both the patient and society (Johnell and Kanis, 2004, Johnell and Kanis, 2005; Dhanwal et al., 2011). Because of the growing proportion of the aging population after all in industrialized countries, in recent years hip fractures have emerged as a tremendous global public health concern (WHO Scientific Group on the Prevention and Management of Osteoporosis, 2003). 1.6 million annual cases worldwide were estimated for the year 2000 with a predicted threefold increase of this number by the year 2050 (Gullberg et al., 1997). In the European Union among the population aged ≥50 years, hip fracture incidence in 2010 ranged between 231 and 640/100,000 in Romania and Denmark, respectively (Hernlund et al., 2013). In Austria, the number of hip fractures was estimated to amount to well above 15,000 in 2008, yielding a fairly high incidence of 456/100,000 among those aged ≥50, however, a decreasing secular trend since 2006 was observed (Dimai et al., 2011).
Likewise, the metabolic syndrome (MetS) must be regarded as an eminent public health issue, given its considerable prevalence that was estimated to affect 20–30% of the adult population in most countries worldwide, rising with advanced age and economic prosperity (Grundy, 2008). MetS is a compound condition consisting of various pathologic factors including obesity, hypertension, hyperglycemia and dyslipidemia, and is associated with high risk for the development of cardiovascular diseases (CVD) and type II diabetes mellitus (Levesque and Lamarche, 2008). In view of numerous reports of an association of osteoporotic fractures, particularly hip fractures, with ischemic heart disease, heart failure, hypertension, and diabetes, a relation between MetS and bone health can be expected (Janghorbani et al., 2007; Melton et al., 2008; Sennerby et al., 2009; Vestergaard et al., 2009a; Gerber et al., 2013). Findings describing risk of bone fractures in MetS patients have, however, been inconsistent. A recent meta-analysis including prospective cohort studies reported that MetS was associated with overall reduced fracture risk, which was, upon stratification for gender, only observed in men, and this gender-related discrepancy remained when only non-vertebral fractures were considered (Yang et al., 2016).
Moreover, the relation of each of the MetS components with fracture risk is not clear. In hyperglycemia, accumulation of advanced glycation endproducts (AGEs) in bone and enhanced oxidative stress are deemed to effectuate deterioration of bone material properties (Kume et al., 2005; Napoli et al., 2017). Whereas patients with type I and type II diabetes mellitus are indeed at elevated fracture risk (Janghorbani et al., 2007; Vestergaard et al., 2009a), impaired fasting blood glucose was not associated with increased fracture incidence (Strotmeyer et al., 2005), and in non-diabetics, hyperglycemia was even inversely correlated with fracture incidence (Gagnon et al., 2010). Also, hypertension has been associated with increased fracture risk, which was explained by increased urinary calcium excretion (Vestergaard et al., 2009b; Cappuccio et al., 2000), but also no association in women and even an inverse correlation in men have been observed (Ahmed et al., 2006). The role of lipids such as triglycerides and cholesterol in fracture risk is controversial, as reflected by conflicting reports on bone-related effects of lipid-lowering statins (Esposito et al., 2013a). High levels of triglycerides have been associated with decreased fracture risk (Ahmed et al., 2006; Szulc et al., 2010) which was attributed to their function in mediating the interaction between bone matrix and mineral, thus contributing towards improved bone quality (Xu and Yu, 2006; Szulc et al., 2010). High levels of cholesterol were reported to convey long-term risk of osteoporotic fractures (Trimpou et al., 2011) but also to protect against osteoporotic vertebral fractures (Sivas et al., 2009). An important role of cholesterol in the differentiation of bone marrow stem cells has been proposed (Parhami et al., 2002), otherwise the mechanism by which cholesterol affects bone is still largely elusive. Finally, BMI is inversely associated with bone fracture risk, mainly via action of mechanical load, and adipocyte-derived estrogen and adipokines (De Laet et al., 2005; Tang et al., 2013; Shapses and Sukumar, 2012).
There is no single investigation that has examined risk of hip fractures specifically in both MetS and its components. In view of inconclusive reports on bone fractures by other studies, we therefore conducted a population-based prospective cohort study to explore the relation between incident hip fractures with respect to both 1) MetS and 2) each MetS component.
2. Patients and methods
2.1. Data source and selection of subjects
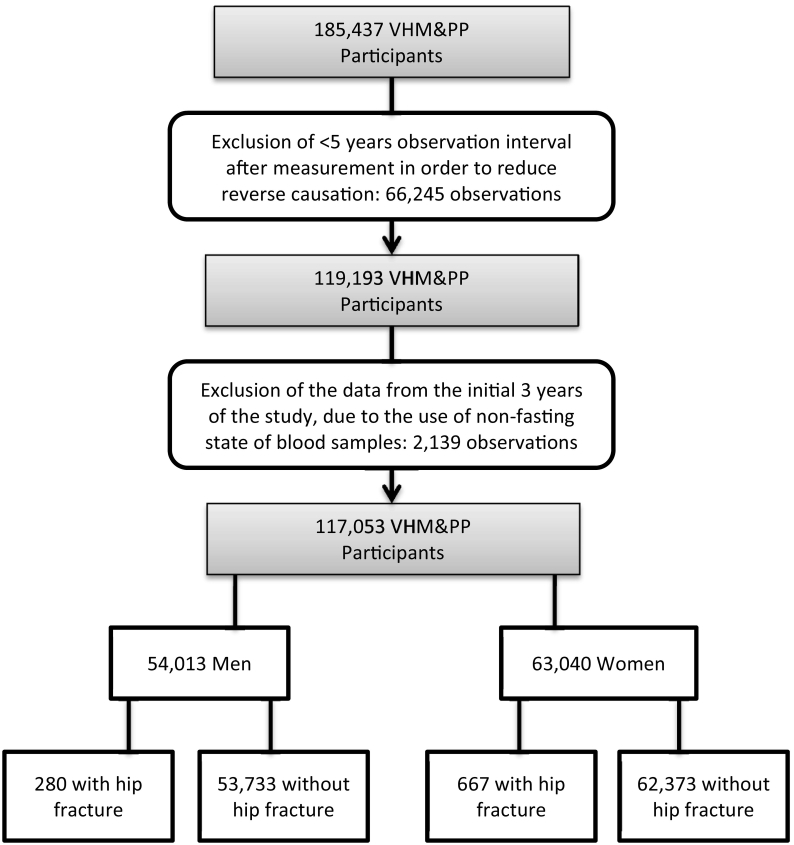
All study subjects participated in the Vorarlberg Health Monitoring and Prevention Program (VHM&PP), a population-based risk factor surveillance program in Vorarlberg, the westernmost province of Austria. Detailed descriptions of the subject selection and data collection procedures have been published previously (Stocks et al., 2010). In brief, the electronical documentation of the MetS cohort was started in 1985 and carried out until 2005, conducted by the Agency of Social and Preventive Medicine (aks). Within the framework of this program, all adult residents of Vorarlberg were invited to undergo a health examination free of charge up to once a year, and >185,000 participants, approximately two thirds of the inhabitants, followed the invitation (Fig. 1). Data were prospectively acquired to examine the association between MetS and hip fracture incidence. Information on hip fractures on the individual level was available from discharge diagnoses of all hospitals in Vorarlberg between January 1st, 2003 and December 31st, 2009, amounting to a total of 2955 hospitalizations due to hip fractures according to the ICD-10 code classes of S72. Since an effect of altered metabolic factors on bone and susceptibility to hip fractures is expected to be subject to considerable time lag and in order to minimize effects of immortal time bias and reverse causation, we required each individual to be alive at least five years before fracture, or censoring for death or end of follow-up on December 31st, 2009. Moreover, examinations from 1985 to 1987 were excluded because blood glucose was not measured in the fasting state during this period. The final data set consisted of a total of 117,053 subjects who sustained 947 first hip fractures. A flow diagram provides the selection criteria for participants in this study (Fig. 1). All participants gave written informed consent to use their data for scientific purposes. The Ethics Committee of the State of Vorarlberg approved this study which was performed according to the Declaration of Helsinki.
Fig. 1.
Flow diagram of study participants. VHM&PP, Vorarlberg Health Monitoring & Prevention Program.
2.2. Covariates
At recruitment to the study (January 1st 1988–December 31st 2004), participants underwent a physical examination according to a standard protocol, including measurement of blood pressure, body height, and body weight. Body mass index (BMI) was calculated, and information regarding smoking habits was recorded (classification as current, former, or never smoker). Concentrations of blood glucose, total cholesterol, and triglycerides were measured in blood samples taken from a cubital vein after at least 8 h of fasting. Within 1 to 4 h after blood sample collection, serum was obtained by centrifugation at 4000 rpm for 15 min. Subsequently, blood glucose, total cholesterol, and triglyceride concentrations were measured at 37 °C. To ensure comparability, three calibration samples were included in each run. The average value of the calibration samples of each run had to be within 3% of the true value, otherwise the run was repeated. Day-by-day variation had to be within 5%. Pathological cut-off levels were: BMI ≥30 kg/m2, blood glucose ≥126 mg/dl, cholesterol ≥200 mg/dl, triglyceride ≥150 mg/dl, systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg. Since no information was available on medications, pathological conditions were defined solely based upon blood concentrations of these parameters.
2.3. Outcome
From January 1st, 2003 to December 31st, 2009, all discharge diagnoses of incident hip fracture were identified in all hospitals in Vorarlberg (Landeskrankenhaus Bregenz, Landeskrankenhaus Bludenz, Krankenhaus Dornbirn, Landeskrankenhaus Feldkirch, Landeskrankenhaus Hohenems, and Sanatorium Schruns) according to the code classes of S72 of the International Classification of Diseases, 10th Revision (ICD-10). Only a patient's first hip fracture was included in the analysis. The available information did not permit us to distinguish between osteoporotic fragility fractures and non-osteoporotic fractures.
2.4. Statistical analysis
Continuous variables, i.e. age, BMI, fasting blood glucose, triglycerides, cholesterol, and systolic and diastolic blood pressure, are expressed as mean ± standard deviation (SD) or median with interquartile range (IQR), categorical variables, i.e. smoking habit and fracture incidence, are expressed as percentages. Since the incidence of osteoporotic fractures is highest in subjects >50 years, we performed a stratified analysis according to age at baseline <50 and ≥50 years. Using Cox proportional hazard models, risk of hip fracture was examined for each MetS component (i.e. BMI, blood glucose, total cholesterol, triglycerides, and mean arterial pressure defined as average of systolic and diastolic blood pressure) split into quintiles, expressed as standardized continuous z-scores, and divided by pathological cut off values. Quintile cut-off values were calculated for genders separately, and z-scores, obtained by subtraction of each value from the mean and division by the standard deviation, were calculated for age group at baseline and genders separately. Moreover, blood glucose and triglyceride values were log transformed before standardization because of the right-skewed data distribution. The composite MetS score was constructed by summing up z-scores of all five individual components. Hazard ratios were adjusted for age, BMI and smoking status (all of which are known risk factors for hip fracture) at baseline and controlled for categories of birth year (before 1937, 1937–1947, 1948–1957, 1958–1966, and after 1966). p values <0.05 (two-sided) were considered statistically significant.
3. Results
Mean age at the initial examination (baseline) was 49.9 ± 15.0 years in 54,013 men and 50.2 ± 16.1 years in 63,040 women (Table 1). During median follow-up of 7.5 years (IQR: 6.7–8.2 years), 947 incident hip fractures occurred, 70% of which in women. Mean age at hip fracture was 72.4 ± 14.3 years and 78.9 ± 9.7 years in men and women, respectively. In general, individuals sustaining a hip fracture were older, had higher mean blood pressure, higher median levels of blood glucose and cholesterol, and smoked less (Table 2).
Table 1.
Baseline characteristics of the study population.
| Total (n = 117,053) | Men (n = 54,013) | Women (n = 63,040) | |
|---|---|---|---|
| Age at baseline (years), mean ± SD | 50.1 ± 15.6 | 49.9 ± 15.0 | 50.2 ± 16.1 |
| Age at fracture (years), mean ± SD | 77.0 ± 11.6 | 72.4 ± 14.3 | 78.9 ± 9.7 |
| BMI (kg/m2), median (P20, P40, P60, P80) | 25.2 (21.9, 24.2, 26.2, 29.0) | 25.8 (23, 25, 26.7, 29.1) | 24.3 (21, 23.2, 25.6, 29.0) |
| MAP (mm Hg), median (P20, P40, P60, P80) | 96.7 (86.7, 93.3, 100, 106.7) | 98 (90, 96.6, 100, 100.3) | 95 (85, 93.3, 98.3, 106.7) |
| Blood glucose (mg/dl), median (P20, P40, P60, P80) | 91 (81, 88, 94, 102) | 93 (83, 90, 96, 105) | 89 (80, 86, 92, 100) |
| Triglycerides (mg/dl), median (P20, P40, P60, P80) | 102 (68, 89, 112, 166) | 116 (74, 101, 134, 193) | 92 (62, 82, 105, 145) |
| Cholesterol (mg/dl), median (P20, P40, P60, P80) | 213 (179, 203, 224, 251) | 213 (180, 203, 224, 250) | 213 (178, 202, 224, 252) |
| BMI ≥ 30 (kg/m2), n (%) | 18,023 (15.4) | 7985 (14.8) | 10,038 (15.9) |
| Blood glucose ≥ 126 mg/dl, n (%) | 5691 (4.9) | 3067 (5.7) | 2624 (4.2) |
| Cholesterol ≥ 200 mg/dl, n (%) | 73,554 (62.8) | 34,270 (63.4) | 39,284 (62.3) |
| Triglycerides ≥ 150 mg/dl, n (%) | 29,496 (25.2) | 17,875 (33.1) | 11,621 (18.4) |
| Hypertension, n (%) | 47,810 (40.8) | 24,269 (44.9) | 23,541 (37.3) |
| Smoking status, n (%) | |||
| Non-smoker | 76,711 (65.54) | 30,847 (57.11) | 45,864 (72.75) |
| Ex-smoker | 15,463 (13.21) | 10,030 (18.57) | 5433 (8.62) |
| Smoker | 24,879 (21.25) | 13,136 (24.32) | 11,743 (18.63) |
| Hip fractures, n (%) | 947 (0.81) | 280 (0.52) | 667 (1.06) |
BMI, body mass index; MAP, mean arterial pressure. P percentile. Hypertension: systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg.
Table 2.
Comparison of baseline characteristics between individuals with and without hip fracture according to gender.
| Men n = 54,013 |
Women n = 63,040 |
|||
|---|---|---|---|---|
| With |
Without |
With |
Without |
|
| Hip fracture |
Hip fracture |
|||
| n = 280 | n = 53,733 | n = 667 | n = 62,373 | |
| Age at baseline (years), mean ± SD | 64.9 ± 14.2 | 49.8 ± 15.0 | 71.4 ± 9.7 | 50.0 ± 16.0 |
| BMI (kg/m2), mean ± SD | 25.6 ± 4.0 | 26.2 ± 3.8 | 25.8 ± 4.2 | 25.2 ± 4.9 |
| MAP, mean ± SD | 102.3 ± 12.0 | 99.5 ± 12.1 | 104.2 ± 12.7 | 96.4 ± 13.6 |
| Blood glucose (mg/dl), median (Q1;Q3) | 94 (84;106.5) | 93 (85;102) | 93 (83;105) | 89 (82;98) |
| Triglycerides (mg/dl), median (Q1;Q3) | 114 (81.5;175) | 116 (81;174) | 111 (80;154) | 92 (67;132) |
| Cholesterol (mg/dl), median (Q1;Q3) | 219 (187;250) | 213 (186;242) | 234 (205;263) | 213 (185;244) |
| Smoking status, n (%) | ||||
| Non-smoker | 144 (51.43) | 30,703 (57.14) | 561 (84.11) | 45,303 (72.63) |
| Ex-smoker | 77 (27.50) | 9953 (18.52) | 36 (5.40) | 5397 (8.65) |
| Smoker | 59 (21.07) | 13,077 (24.34) | 70 (10.49) | 11,673 (18.71) |
BMI, body mass index; MAP, mean arterial pressure; Q1, 1st quartile; Q3, 3rd quartile.
As shown in Table 3, hip fracture risk decreased with increasing BMI in both women and men (HRs for the highest compared to the lowest quintile: 0.83 (95%-CI: 0.63–1.10, ptrend < 0.05) and 0.55 (95%-CI: 0.38–0.79, ptrend < 0.001), respectively). A statistically significant inverse association was also found for cholesterol in women (HR for the highest compared to the lowest quintile: 0.64, 95%-CI: 0.48–0.84, ptrend < 0.05). These BMI- and cholesterol-related effects remained when data expressed as continuous z-scores were analyzed (Table 4). An increase in BMI z-score by 1 was accompanied by a 21% and 14% risk reduction for hip fractures in men (p < 0.001) and women (p < 0.001), respectively, and cholesterol was inversely associated with hip fracture risk in women only (HR 0.90, 95%-CI: 0.83–0.97, p < 0.01 in the adjusted model). Likewise, in women only, an increment in the composite MetS score was associated with significantly decreased hip fracture risk in the crude analysis (HR 0.88, 95%-CI: 0.80–0.96, p < 0.01). Risk reduction was also observed in men (HR 0.91, 95%-CI: 0.80–1.04) but failed to reach statistical significance. Both in women and men, this association was abolished upon adjustment for smoking and BMI (Table 4).
Table 3.
Risk of incident hip fracture with respect to metabolic factors in quintiles; HR, hazard ratio.
| Exposures in quintiles | Quintile level (1, lowest; 5, highest) | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI)a | ptrend | HR (95% CI)b | ptrend | HR (95% CI)a | ptrend | HR (95% CI)b | ptrend | ||
| BMI | 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 0.61 (0.42,0.87) | 0.0006 | 0.61 (0.43,0.88) | 0.0006 | 1.08 (0.81,1.44) | 0.0159 | 1.09 (0.81,1.45) | 0.0111 | |
| 3 | 0.62 (0.44,0.88) | 0.62 (0.44,0.88) | 0.95 (0.72,1.26) | 0.96 (0.73,1.27) | |||||
| 4 | 0.51 (0.35,0.73) | 0.51 (0.35,0.73) | 0.81 (0.61,1.07) | 0.82 (0.62,1.08) | |||||
| 5 | 0.55 (0.38,0.79) | 0.55 (0.38,0.79) | 0.82 (0.62,1.08) | 0.83 (0.63,1.10) | |||||
| Blood glucose | 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 0.87 (0.59,1.29) | 0.8811 | 0.90 (0.61,1.34) | 0.5612 | 0.94 (0.72,1.22) | 0.1702 | 0.94 (0.72,1.23) | 0.3426 | |
| 3 | 0.99 (0.67,1.45) | 1.05 (0.72,1.54) | 0.93 (0.72,1.20) | 0.94 (0.73,1.22) | |||||
| 4 | 0.73 (0.50,1.08) | 0.79 (0.54,1.18) | 0.73 (0.57,0.95) | 0.76 (0.58,0.98) | |||||
| 5 | 1.01 (0.72,1.43) | 1.15 (0.81,1.64) | 0.90 (0.72,1.12) | 0.94 (0.75,1.17) | |||||
| Cholesterol | 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 0.79 (0.54,1.16) | 0.9667 | 0.82 (0.56,1.21) | 0.7472 | 0.72 (0.52,0.99) | 0.0498 | 0.72 (0.52,0.99) | 0.0459 | |
| 3 | 0.59 (0.39,0.89) | 0.62 (0.41,0.94) | 0.57 (0.42,0.78) | 0.57 (0.42,0.78) | |||||
| 4 | 0.84 (0.58,1.22) | 0.89 (0.61,1.29) | 0.74 (0.56,0.98) | 0.74 (0.56,0.98) | |||||
| 5 | 0.91 (0.63,1.30) | 0.97 (0.68,1.40) | 0.64 (0.49,0.85) | 0.64 (0.48,0.84) | |||||
| Triglycerides | 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 0.91 (0.63,1.32) | 0.6235 | 0.96 (0.66,1.39) | 0.1258 | 1.01 (0.74,1.39) | 0.1243 | 1.02 (0.74,1.40) | 0.2540 | |
| 3 | 0.73 (0.50,1.07) | 0.83 (0.56,1.22) | 0.82 (0.60,1.12) | 0.83 (0.61,1.14) | |||||
| 4 | 1.01 (0.70,1.45) | 1.18 (0.81,1.71) | 0.83 (0.61,1.12) | 0.85 (0.63,1.16) | |||||
| 5 | 1.06 (0.73,1.54) | 1.28 (0.87,1.90) | 0.85 (0.63,1.14) | 0.88 (0.65,1.19) | |||||
| MAP | 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 0.81 (0.52,1.26) | 0.4956 | 0.88 (0.56,1.38) | 0.7837 | 0.90 (0.60,1.36) | 0.1431 | 0.92 (0.61,1.40) | 0.3600 | |
| 3 | 0.83 (0.56,1.24) | 0.93 (0.62,1.39) | 0.79 (0.54,1.16) | 0.82 (0.56,1.20) | |||||
| 4 | 0.81 (0.55,1.20) | 0.95 (0.63,1.41) | 0.72 (0.50,1.04) | 0.77 (0.53,1.10) | |||||
| 5 | 0.84 (0.57,1.23) | 1.01 (0.68,1.49) | 0.78 (0.54,1.11) | 0.84 (0.58,1.20) | |||||
BMI, body mass index; MAP, mean arterial pressure.
Cox regression models are stratified by categories of birth year.
Cox regression models are stratified by categories of birth year, additionally adjusted for smoking and BMI (except for BMI).
Table 4.
Risk of incident hip fracture for continuous z-scores; HR, hazard ratio.
| Exposure | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| z-Scores, crudea |
z-Score, adjustedb |
z-Scores, crudea |
z-Score, adjustedb |
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| BMI | 0.79 (0.69,0.90) | 0.0004 | 0.79 (0.69,0.90) | 0.0004 | 0.86 (0.78,0.93) | 0.0005 | 0.86 (0.79,0.94) | 0.0009 |
| Log blood glucose | 0.98 (0.88,1.09) | 0.7199 | 1.02 (0.92,1.14) | 0.6771 | 1.02 (0.95,1.08) | 0.6424 | 1.04 (0.98,1.10) | 0.2507 |
| Cholesterol | 0.99 (0.88,1.12) | 0.9270 | 1.01 (0.89,1.14) | 0.8704 | 0.90 (0.83,0.98) | 0.0105 | 0.90 (0.83,0.97) | 0.0091 |
| Log triglycerides | 1.06 (0.93,1.20) | 0.3892 | 1.13 (1.00,1.29) | 0.0583 | 0.97 (0.89,1.05) | 0.4366 | 1.00 (0.91,1.09) | 0.9371 |
| MAP | 0.93 (0.82,1.05) | 0.2342 | 0.99 (0.87,1.12) | 0.8187 | 0.92 (0.85,1.00) | 0.0546 | 0.95 (0.87,1.04) | 0.2511 |
| MetS score | 0.91 (0.80,1.04) | 0.1538 | 1.08 (0.92,1.27) | 0.3469 | 0.88 (0.80,0.96) | 0.0064 | 0.95 (0.85,1.06) | 0.3497 |
BMI, body mass index; MAP, mean arterial pressure; MetS, metabolic syndrome.
Cox regression models are stratified by categories of birth year.
Cox regression models are stratified by categories of birth year, additionally adjusted for smoking and BMI (except for BMI).
Also when separate analyses were conducted for patients aged ≤50 and >50 years at baseline, BMI in both genders and cholesterol in women were linked with decreased hip fracture risk (Table 5). HRs were generally lower in men and women ≤50 years at baseline compared with those aged >50 years, which was, however, not reflected by p-values due to the considerably smaller number of patients ≤50 years. Blood pressure, triglycerides, and blood glucose levels did not show any statistically significant association with hip fracture. An increment in the continuous z-score for triglyceride levels, however, was associated with increased male hip fracture risk at borderline significance (HR 1.13, 95%-CI: 1.00–1.29, p = 0.06 in the adjusted model, Table 4), in particular in men aged >50 years (HR 1.15, 95%-CI: 1.00–1.32, p = 0.05, Table 5).
Table 5.
Risk of incident hip fracture for continuous z-scores stratified by age at baseline. HR, hazard ratio.
| Exposure | Men (n = 54,013) |
Women (n = 63,040) |
||||||
|---|---|---|---|---|---|---|---|---|
| Age ≤ 50 (n = 19,494) 28 hip fractures |
Age > 50 (n = 34,519) 251 hip fractures |
Age ≤ 50 (n = 23,209) 12 hip fractures |
Age > 50 (n = 39,831) 655 hip fractures |
|||||
| HR (95% CI)a | p | HR (95% CI)a | p | HR (95% CI)a | p | HR (95% CI)a | p | |
| BMI | 0.64 (0.41,1.01) | 0.0527 | 0.81 (0.70,0.93) | 0.0024 | 0.53 (0.21,1.31) | 0.1682 | 0.87 (0.80,0.95) | 0.0018 |
| Log blood glucose | 0.92 (0.57,1.49) | 0.7352 | 1.03 (0.93,1.15) | 0.5694 | 1.12 (0.58,2.18) | 0.7376 | 1.04 (0.97,1.10) | 0.2589 |
| Cholesterol | 1.13 (0.76,1.67) | 0.5551 | 1.00 (0.88,1.14) | 0.9791 | 0.56 (0.26,1.22) | 0.1446 | 0.90 (0.83,0.98) | 0.0136 |
| Log triglycerides | 1.04 (0.71,1.53) | 0.8511 | 1.15 (1.00,1.32) | 0.0530 | 1.12 (0.62,2.03) | 0.7055 | 1.00 (0.91,1.09) | 0.9036 |
| MAP | 0.75 (0.46,1.22) | 0.2398 | 1.00 (0.88,1.14) | 0.9912 | 0.57 (0.24,1.39) | 0.2153 | 0.96 (0.88,1.04) | 0.3223 |
| MetS score | 0.93 (0.52,1.67) | 0.8114 | 1.10 (0.93,1.29) | 0.2860 | 0.66 (0.22,1.93) | 0.4440 | 0.95 (0.85,1.07) | 0.4031 |
BMI, body mass index; MAP, mean arterial pressure; MetS, metabolic syndrome.
Cox regression models are stratified by categories of birth year and adjusted for age at baseline, smoking and BMI (except for BMI).
We finally compared hip fracture risk between levels above and below pathologic cut-off values of each MetS component (Table 6). In most pathologic conditions, hip fracture risk was either significantly increased (triglycerides ≥ 150 mg/dl in men: HR 1.33, 95%-CI: 1.03–1.72, p < 0.05; and blood glucose ≥ 126 mg/dl in women: HR 1.33, 95%-CI: 1.05–1.70, p < 0.05) or (near) significantly decreased (BMI ≥ 30 kg/m2 in women: HR 0.83, 95%-CI: 0.68–1.02, p = 0.08; and cholesterol ≥ 200 mg/dl in women: HR 0.82, 95%-CI: 0.67–0.99, p < 0.05).
Table 6.
Risk of incident hip fracture for pathologic values of metabolic factors; HR, hazard ratio.
| Exposure | Men (n = 54,013) |
Women (n = 63,040) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Hip fractures (n) | HR (95% CI)a | p | n | Hip fractures (n) | HR (95% CI)a | p | |
| BMI ≥ 30 (kg/m2) | 7985 | 36 | 0.81 (0.57,1.16) | 0.2493 | 10,038 | 111 | 0.83 (0.68,1.02) | 0.0777 |
| Blood glucose ≥ 126 mg/dl | 3067 | 29 | 1.38 (0.93,2.04) | 0.1098 | 2624 | 77 | 1.33 (1.05,1.70) | 0.0201 |
| Cholesterol ≥ 200 mg/dl | 34,270 | 186 | 0.89 (0.69,1.14) | 0.3552 | 39,284 | 532 | 0.82 (0.67,0.99) | 0.0366 |
| Triglycerides ≥ 150 mg/dl | 17,875 | 97 | 1.33 (1.03,1.72) | 0.0316 | 11,621 | 185 | 1.03 (0.87,1.23) | 0.7476 |
| Hypertension | 24,269 | 176 | 1.11 (0.86,1.44) | 0.4323 | 23,541 | 458 | 0.94 (0.79,1.11) | 0.4466 |
BMI, body mass index; Hypertension: systolic blood pressure ≥140 mm Hg; diastolic blood pressure ≥90 mm Hg.
Cox regression models are stratified by categories of birth year and adjusted for age at baseline, smoking and BMI (except for BMI).
4. Discussion
In this prospective cohort study, high BMI and increased levels of cholesterol and the composite MetS score were associated with reduced hip fracture risk among women, whereas elevated hip fracture risk was found for pathologically increased blood glucose. In men, hip fracture risk was negatively associated with increasing BMI, and increased at pathologically elevated levels of triglycerides. To interpret our findings, how each of the MetS components separately and MetS as a whole might exert influence on bone and modulate (hip) fracture risk is discussed in the following.
4.1. Blood glucose
We herein found increased hip fracture risk in hyperglycemic women and men, which was statistically significant only in women presumably because of the low number of fracture events in men. Indeed, both type 1 and type 2 diabetes were associated with increased risk of bone fractures (Janghorbani et al., 2007; Vestergaard et al., 2009a). It has been suggested that in hyperglycemia advanced glycation end-products (AGEs) that bind to bone collagen and oxidative stress compromise bone quality (Kume et al., 2005; Napoli et al., 2017), which could underly increased hip fracture risk as observed in the present study.
4.2. Total cholesterol
Our result of an inverse correlation of high cholesterol levels and hip fracture risk in women is at odds with a longitudinal study among 1396 men and women over a period of 20 years demonstrating an independent positive association between cholesterol and osteoporotic fracture (Trimpou et al., 2011). Compared with our study, genders were not analyzed separately, participants were several years younger at baseline, and all osteoporotic bone fractures were included, not only hip fractures. In addition, in Chinese women but not men, a positive association with risk of osteoporotic fractures has recently been reported for total cholesterol (on the verge of statistical significance) and, interestingly, for HDL cholesterol (Wang et al., 2018). Contrary to our investigation, this was a cross-sectional study with assessment of fracture history using a questionnaire at the time when blood was taken, and sub-analyses by fracture site yielded no associations with hip fracture. Moreover, a recent meta-analysis confirmed the positive association of total cholesterol with risk of osteoporotic fractures which was also lower at decreased levels of HDL (<40 mg/dl) (Ghorabi et al., 2019). Accordingly, high and low levels of total and HDL cholesterol, respectively, both risk factors for cardiovascular diseases, would be expected to exert differential effects on bone. Since only total cholesterol was available for the present study, hip fracture risk associated with low HDL thus remains speculative.
Our findings are, however, in line with a study in Turkish postmenopausal women reporting a decrease in risk of osteoporotic vertebral fractures by 2.2% for an increment of 1 mg/dl of total cholesterol (Sivas et al., 2009). Evidence for a protective role of cholesterol against hip fractures emanates from a study reporting increased hip fracture risk in the lowest vs. highest quartile of α-tocopherol serum levels (Holvik et al., 2014). Therein, fracture risk was not only mitigated upon adjustment for total cholesterol, but also when the α-tocopherol/total cholesterol ratio was the exposure compared with α-tocopherol alone, which demonstrates that effects of α-tocopherol and total cholesterol on hip fracture risk work in the same direction. Furthermore, participants of the Multi-Ethnic Study of Atherosclerosis with total cholesterol levels ≥240 mg/dl were less likely than those with lower levels to be affected by non-cardiovascular diseases like dementia and hip fracture, however, differences failed to reach statistical significance (Ogunmoroti et al., 2016). As for a potential mechanism, it was suggested that a baseline level of cholesterol biosynthesis plays an important role for the osteoblastic differentiation of marrow stromal cells, thus lower levels of cholesterol contribute towards impaired formation of a mineralized bone matrix and higher risk of fractures (Parhami et al., 2002).
4.3. Triglycerides
In the present investigation, men with hypertriglyceridemia were at statistically significantly increased risk of hip fracture, and a higher continuous z-score for triglycerides was associated with elevated hip fracture risk at borderline statistical significance. This result starkly contrasts with the report by Szulc et al. (2010) that the protective effect of MetS against bone fractures in men is mainly driven by elevated triglycerides. This study did not, however, examine hip fracture risk specifically, average age at baseline was considerably higher (65 years) than in our study, and education level, history of fractures, and self-reported comorbidities were included as co-variates. On the other hand, in the Tromsø Study no association of hypertriglyceridemia with non-vertebral fracture risk was found in men while in women a protective effect was observed (Ahmed et al., 2006). Hip fracture was not an end-point either in this large study comprising >27,000 participants, and age at baseline (around 47 years) was slightly younger than herein. Our results could tentatively be explained by elevated risk of falls because of lowered muscular strength that has recently been associated with increasing serum triglyceride concentrations in elderly hip fracture patients (Amador Licona et al., 2018). The gender-related difference might be traced to an inverse relationship between male central obesity and BMD (Eckstein et al., 2016).
4.4. Blood pressure
Our study is in line with other investigations that found no significant association between blood pressure and fracture risk (von Muhlen et al., 2007; Szulc et al., 2010; Lee et al., 2014). However, different blood pressure related effects have been associated with bone health and fracture risk. On the one hand, high blood pressure was associated with general fracture and hip fracture risk in a large case-control study comprising register-based information about a large number of comorbidities and medications that were adjusted for (Vestergaard et al., 2009b), as well as with increased bone loss of the femoral neck (Capuccio et al., 1999). Excess excretion of calcium through the urine due to hypertension resulting in bone mineral loss could be the underlying explanation (Cappuccio et al., 2000). By contrast, a positive association between hypertension and BMD at the lumbar spine and femoral neck in women, and at the lumbar spine in men aged 50 years and older was found in the Canadian Multicenter Osteoporosis Study (Hanley et al., 2003). The pathophysiological mechanisms were difficult to explain, since potential confounding factors such as medication with thiazide diuretics and BMI were accounted for (Hanley et al., 2003). In other studies, increased wrist bone density in women (Lidfeldt et al., 2002) and decreased non-vertebral fracture risk in men (Ahmed et al., 2006) were associated with high blood pressure. In addition, risk of falls may be exacerbated due to dizziness from cerebrovascular diseases. Indeed, high blood pressure was found to decrease risk of falls in women, and in men, risk went up when blood pressure was low (Klein et al., 2013). It might be hypothesized that in studies that found no association like in ours, a possible negative effect of high blood pressure on bone mineral density is counter-balanced by a reduced propensity for falls. Alternatively, since use of most anti-hypertensive drugs has been associated with decreased hip fracture risk (Ruths et al., 2015; Barzilay et al., 2017), initiation of anti-hypertensive treatment after baseline by at least a proportion of participants with high baseline blood pressure might explain our results.
4.5. BMI
Our finding that BMI is inversely associated with hip fracture risk in both genders agrees with previous notions that low BMI is correlated with high fracture risk and that overweight and obesity are protective against bone loss (De Laet et al., 2005; Tang et al., 2013). The protective effect of obesity against fractures was attributed to mechanical loading on bone and higher serum levels of estrogen and adipokines, leading to increased BMD and bone mineral content (Shapses and Sukumar, 2012). Moreover, a BMD independent mechanism has also been suggested based on the effect of soft tissue padding, reducing the impact of trauma (Shapses and Sukumar, 2012).
4.6. Metabolic syndrome
Decreased risk of MetS-related bone fractures has been reported in a meta-analysis including 1350 incident and 1628 prevalent fractures, with most of the inverse association identified in prospective studies, independent of sex, fracture site and the definition of MetS (Esposito et al., 2013b). We herein likewise observed an inverse association of MetS z-scores with hip fracture risk, which was, however, statistically significant in women only and disappeared upon adjustment for BMI, which indicates that this effect mainly relies upon BMI. In a previous investigation in Korean men, lower risk of incident fractures in subjects with MetS than in subjects without MetS was eliminated upon adjustment for BMI (Lee et al., 2014). In a cross-sectional study, women with MetS displayed higher BMD than women without MetS, and this association disappeared upon adjustment for BMI (Hernández et al., 2010). This difference in BMD did not, however, translate into altered vertebral and non-vertebral fracture risk, and there were no MetS-related differences in men (Hernández et al., 2010). Likewise, in the Rotterdam Study, Muka et al. (2015) reported no statistically significant association of MetS with total and non-vertebral fracture risk in either sex upon adjustment for various drugs, lifestyle and dietary factors in a cohort substantially older at baseline (72 years) than ours.
MetS is a complex entity consisting of various components each of which on its own could modify fracture risk. Moreover, several diagnostic criteria have been proposed for MetS, but none of them is widely accepted (Alberti et al., 2009). Most studies have applied the NCEP-ATPIII criteria to investigate MetS and bone metabolism, according to which MetS is diagnosed if at least three out of five criteria are present (Huang, 2009). This implies a particular amount of heterogeneity among MetS patients, since various combinations of components occur in these individuals. This could explain why single MetS components are more reliably associated with bone health and fracture risk than the diagnosis of MetS, as found also herein.
4.7. Strengths and limitations
Strengths of this study include its prospective design, the large sample size, and the use of population-based data. Also, measurement of metabolic factors was carried out according to a standard protocol. Data on hip fractures were based on hospital discharge databases and collected on individual level. One limitation of our study is that a proportion of participants might have been lost to follow-up after their baseline examination because of relocation or admission to a hospital outside the study area, resulting in missed hip fracture cases. Whereas we could not account for subjects who moved away, exhaustive capture of hip fractures sustained by residents of Vorarlberg is very likely owing to the geographic situation and political factors (Concin et al., 2016). Extensive mountain ranges and borders with non-EU countries with a distinct social security system drastically limit treatment of local hip fractures in adjacent regions. Moreover, a hip fracture practically always entails admission to a hospital, thus ensuring completeness of cases. In addition, it was not possible to distinguish between hip fractures due to low and high trauma, nor could multiple, simultaneous fractures be identified. We attempted to mitigate this limitation by conducting separate analyses according to age >50 and ≤50 years, assuming prevalence of low traumatic osteoporotic fragility fractures above the age of 50 years. Another possible concern is with competing risk of mortality, which was moderate in our dataset (not shown). However, cause-specific Cox proportional hazards models as applied herein have been shown to appropriately capture relative hazards irrespective of competing risk of mortality in studies with explanatory and etiological rather than prognostic focus like ours (Wolbers et al., 2014). Further limitations include the availability of BMI only devoid of information on proportions of lean and fat mass, and the lack of detailed data on blood lipids, because of which total cholesterol was used as surrogate for HDL that is more specific than total cholesterol with respect to cardiovascular disease. Consequently, a continuous MetS score served as a proxy for the syndrome, as described previously (Franks and Olsson, 2007). Neither was information available on use of drugs that could have exerted influence on metabolic factors, modulated risk of falls and affected bone health, in particular in an elderly population characterized by polypharmacy. Whereas the effect of lipid-lowering statins on osteoporotic fracture risk is controversial (Esposito et al., 2013a), most anti-hypertensive drugs have been shown to confer reduced risk of hip fractures (Ruths et al., 2015; Barzilay et al., 2017). Likewise, information was unavailable on physical activity and alcohol consumption, behavioral factors which are known to affect fracture risk. Finally, when generalizing the results, it should be borne in mind that the analyses did not consider possible short-term effects on hip fractures.
5. Conclusions
In summary, we have found an inverse association of the MetS score with risk of hip fracture which was statistically significant in women only and abolished upon adjustment for BMI. The lowered incidence of hip fractures with higher MetS score can be attributed to high BMI and high cholesterol levels in women while this association in men is mainly driven by BMI only. Clinical significance of our results is reflected by increased hip fracture risk in hyperglycemic women and hypertriglyceridemic men, while hypercholesterolemia was associated with reduced female hip fracture risk. With the exception of the positive and inverse association of hypertriglyceridemia and hypercholesterolemia, respectively, with hip fracture risk, results of the present investigation are in line with at least a good part of previous findings for non-vertebral fractures. However, interpretation of results of the present study should account for the fact that our analyses focused on medium to long-term effects. Nevertheless, these results might facilitate early identification of individuals at increased risk for hip fracture.
Transparency document
Transparency document.
Declaration of competing interest
Kilian Rapp has taken part in an expert board and lecture for Amgen. Erlangga Dominic, Wolfgang Brozek, Raphael Simon Peter, Ella Fromm, Hanno Ulmer, Hans Concin, and Gabriele Nagel declare that they have no disclosures and no conflict of interest.
Acknowledgments
Acknowledgements
We thank Elmar Stimpfl for excellent technical support, and Markus Wallner, Christian Bernhard, and Gabriela Dür from the Vorarlberg State Government. This work received financial support by the state of Vorarlberg.
Author contributions
ED, RSP, GN, and HC conceived of the study; ED and EF conducted the data analysis; ED, WB, and GN drafted the manuscript; ED, WB, RSP, HU, KR, HC, and GN contributed to the interpretation of results. All authors approved the final version of the article.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Ahmed L.A., Schirmer H., Berntsen G.K., Fønnebø V., Joakimsen R.M. Features of the metabolic syndrome and the risk of non-vertebral fractures: the Tromsø study. Osteoporos. Int. 2006;17:426–432. doi: 10.1007/s00198-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.-C., James W.P.T., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Amador Licona N., Moreno Vargas E.V., Martinez-Cordero C. Protein intake, serum lipids and muscle strength in the elderly. Nutr. Hosp. 2018;35:65–70. doi: 10.20960/nh.1368. [DOI] [PubMed] [Google Scholar]
- Barzilay J.I., Davis B.R., Pressel S.L., Ghosh A., Puttnam R., Margolis K.L., Whelton P.K. The impact of antihypertensive medications on bone mineral density and fracture risk. Curr. Cardiol. Rep. 2017;19:76. doi: 10.1007/s11886-017-0888-0. [DOI] [PubMed] [Google Scholar]
- Cappuccio F.P., Kalaitzidis R., Duneclift S., Eastwood J.B. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J. Nephrol. 2000;13:169–177. [PubMed] [Google Scholar]
- Capuccio F.P., Meilahn E., Zmuda J.M., Cauley J.A. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- Concin H., Brozek W., Benedetto K.-P., Häfele H., Kopf J., Bärenzung T., Schnetzer R., Schenk C., Stimpfl E., Waheed-Hutter U., Ulmer H., Rapp K., Zwettler E., Nagel G. Hip fracture incidence 2003–2013 and projected cases until 2050 in Austria: a population-based study. Int. J. Public Health. 2016;61:1021–1030. doi: 10.1007/s00038-016-0878-9. [DOI] [PubMed] [Google Scholar]
- De Laet C., Kanis J.A., Odén A., Johanson H., Johnell O., Delmas P., Eisman J.A., Kroger H., Fujiwara S., Garnero P., McCloskey E.V., Mellstrom D., Melton L.J., 3rd, Meunier P.J., Pols H.A.P., Reeve J., Silman A., Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos. Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- Dhanwal D.K., Dennison E.M., Harvey N.C., Cooper C. Epidemiology of hip fracture: worldwide geographic variation. Indian J. Orthop. 2011;45:15–22. doi: 10.4103/0019-5413.73656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimai H.P., Svedbom A., Fahrleitner-Pammer A., Pieber T., Resch H., Zwettler E., Chandran M., Borgström F. Epidemiology of hip fractures in Austria: evidence for a change in the secular trend. Osteoporos. Int. 2011;22:685–692. doi: 10.1007/s00198-010-1271-9. [DOI] [PubMed] [Google Scholar]
- Eckstein N., Buchmann N., Demuth I., Steinhagen-Thiessen E., Nikolov J., Spira D., Eckardt R., Norman K. Association between metabolic syndrome and bone mineral density – data from the Berlin Aging Study II (BASE-II) Gerontology. 2016;62:337–344. doi: 10.1159/000434678. [DOI] [PubMed] [Google Scholar]
- Esposito K., Capuano A., Sportiello L., Giustina A., Giugliano D. Should we abandon statins in the prevention of bone fractures? Endocrine. 2013;44:326–333. doi: 10.1007/s12020-013-9924-z. [DOI] [PubMed] [Google Scholar]
- Esposito K., Chiodini P., Capuano A., Colao A., Giugliano D. Fracture risk and bone mineral density in metabolic syndrome: a meta-analysis. J. Clin. Endocrinol. Metab. 2013;98:3306–3314. doi: 10.1210/jc.2013-1775. [DOI] [PubMed] [Google Scholar]
- Franks P.W., Olsson T. Metabolic syndrome and early death: getting to the heart of the problem. Hypertension. 2007;49:10–12. doi: 10.1161/01.HYP.0000251934.55488.ae. [DOI] [PubMed] [Google Scholar]
- Gagnon C., Magliano D.J., Ebeling P.R., Dunstan D.W., Zimmet P.Z., Shaw J.E., Daly R.M. Association between hyperglycaemia and fracture risk in non-diabetic middle-aged and older Australians: a national, population-based prospective study (AusDiab) Osteoporos. Int. 2010;21:2067–2074. doi: 10.1007/s00198-009-1164-y. [DOI] [PubMed] [Google Scholar]
- Gerber Y., Melton L.J., 3rd, McNallan S.M., Jiang R., Weston S.A., Roger V.L. Cardiovascular and noncardiovascular disease associations with hip fractures. Am. J. Med. 2013;126:169.e19–169.e26. doi: 10.1016/j.amjmed.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorabi S., Shab-Bidar S., Sadeghi O., Nasiri M., Khatibi S.R., Djafarian K. Lipid profile and risk of bone fracture: a systematic review and meta-analysis of observational studies. Endocr. Res. 2019;44:168–184. doi: 10.1080/07435800.2019.1625057. [DOI] [PubMed] [Google Scholar]
- Grundy S.M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- Gullberg B., Johnell O., Kanis J.A. World-wide projections for hip fracture. Osteoporos. Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- Hanley D.A., Brown J.P., Tenenhouse A., Olszynski W.P., Ioannidis G., Berger C., Prior J.C., Pickard L., Murray T.M., Anastassiades T., Kirkland S., Joyce C., Joseph L., Papaioannou A., Jackson S.A., Poliquin S., Adachi J.D. Associations among disease conditions, bone mineral density, and prevalent vertebral deformities in men and women 50 years of age and older: cross-sectional results from the Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2003;18:784–790. doi: 10.1359/jbmr.2003.18.4.784. [DOI] [PubMed] [Google Scholar]
- Hernández J.L., Olmos J.M., Pariente E., Martínez J., Valero C., García-Velasco P. Metabolic syndrome and bone metabolism: the Camargo Cohort study. Menopause. 2010;17:955–961. doi: 10.1097/gme.0b013e3181e39a15. [DOI] [PubMed] [Google Scholar]
- Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvik K., Gjesdal C.G., Tell G.S., Grimnes G., Schei B., Apalset E.M. Low serum concentrations of alpha-tocopherol are associated with increased risk of hip fracture. A NOREPOS study. Osteoporos. Int. 2014;25:2545–2554. doi: 10.1007/s00198-014-2802-6. [DOI] [PubMed] [Google Scholar]
- Huang P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janghorbani M., Van Dam R.M., Willett W.C., Hu F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007;166:495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- Johnell O., Kanis J.A. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos. Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- Johnell O., Kanis J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005;16(Suppl. 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- Keen R.W. Burden of osteoporosis and fractures. Curr. Osteoporos. Rep. 2003;1:66–70. doi: 10.1007/s11914-003-0011-x. [DOI] [PubMed] [Google Scholar]
- Klein D., Nagel G., Kleiner A., Ulmer H., Rehberger B., Concin H., Rapp K. Blood pressure and falls in community-dwelling people aged 60 years and older in the VHM&PP cohort. BMC Geriatr. 2013;13:50. doi: 10.1186/1471-2318-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S., Kato S., Yamagishi S., Inagaki Y., Ueda S., Arima N., Okawa T., Kojiro M., Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J. Bone Miner. Res. 2005;20:1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Baek S., Ahn S.H., Kim S.H., Jo M.-W., Bae S.J., Kim H.-K., Choe J., Park G.-M., Kim Y.-H., Koh J.-M., Kim B.-J., Kim G.S. Association between metabolic syndrome and incident fractures in Korean men: a 3-year follow-up observational study using national health insurance claims data. J. Clin. Endocrinol. Metab. 2014;99:1615–1622. doi: 10.1210/jc.2013-3608. [DOI] [PubMed] [Google Scholar]
- Levesque J., Lamarche B. The metabolic syndrome: definitions, prevalence and management. J. Nutrigenet. Nutrigenomics. 2008;1:100–108. doi: 10.1159/000112457. [DOI] [PubMed] [Google Scholar]
- Lidfeldt J., Holmdahl L., Samsioe G., Nerbrand C., Nyberg P., Scherstén B., Agardh C.-D. The influence of hormonal status and features of the metabolic syndrome on bone density: a population-based study of Swedish women aged 50 to 59 years. The women’s health in the Lund area study. Metabolism. 2002;51:267–270. doi: 10.1053/meta.2002.300001. [DOI] [PubMed] [Google Scholar]
- Melton L.J., 3rd, Leibson C.L., Achenbach S.J., Therneau T.M., Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J. Bone Miner. Res. 2008;23:1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.H., Zuckerman J.D. National Consensus Conference on Improving the Continuum of Care for Patients with Hip Fracture. J. Bone Joint Surg. Am. 2002;84:670–674. doi: 10.2106/00004623-200204000-00027. [DOI] [PubMed] [Google Scholar]
- von Muhlen D., Safii S., Jassal S.K., Svartberg J., Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos. Int. 2007;18:1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Muka T., Trajanoska K., Kiefte-de Jong J.C., Oei L., Uitterlinden A.G., Hofman A., Dehghan A., Zilikens M.A., Franco O.H., Rivadeneira F. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam Study. PLoS One. 2015;10:e0129116. doi: 10.1371/journal.pone.0129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- Ogunmoroti O., Allen N.B., Cushman M., Michos E.D., Rundek T., Rana J.S., Blankstein R., Blumenthal R.S., Blaha M.J., Veledar E., Nasir K. Association between life’s simple 7 and noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhami F., Mody N., Gharavi N., Ballard A.J., Tintut Y., Demer L.L. Role of the cholesterol biosynthetic pathway in osteoblastic differentiation of marrow stromal cells. J. Bone Miner. Res. 2002;17:1997–2003. doi: 10.1359/jbmr.2002.17.11.1997. [DOI] [PubMed] [Google Scholar]
- Ruths S., Bakken M.S., Ranhoff A.H., Hunskaar S., Engesæter L.B., Engeland A. Risk of hip fracture among older people using antihypertensive drugs: a nationwide cohort study. BMC Geriatr. 2015;15:153. doi: 10.1186/s12877-015-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennerby U., Melhus H., Gedeborg R., Byberg L., Garmo H., Ahlbom A., Pedersen N.L., Michaëlsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- Shapses S.A., Sukumar D. Bone metabolism in obesity and weight loss. Annu. Rev. Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivas F., Alemdaroğlu E., Elverici E., Kuluğ T., Ozoran K. Serum lipid profile: its relationship with osteoporotic vertebrae fractures and bone mineral density in Turkish postmenopausal women. Rheumatol. Int. 2009;29:885–890. doi: 10.1007/s00296-008-0784-4. [DOI] [PubMed] [Google Scholar]
- Stocks T., Borena W., Strohmaier S., Bjørge T., Manjer J., Engeland A., Johansen D., Selmer R., Hallmans G., Rapp K., Concin H., Jonsson H., Ulmer H., Stattin P. Cohort profile: the Metabolic syndrome and Cancer project (Me-Can) Int. J. Epidemiol. 2010;39:660–667. doi: 10.1093/ije/dyp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmeyer E.S., Cauley J.A., Schwartz A.V., Nevitt M.C., Resnick H.E., Bauer D.C. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch. Intern. Med. 2005;165:1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- Szulc P., Varennes A., Delmas P.D., Goudable J., Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk—the MINOS study. J. Bone Miner. Res. 2010;25:1446–1454. doi: 10.1002/jbmr.13. [DOI] [PubMed] [Google Scholar]
- Tang X., Liu G., Kang J., Hou Y., Jiang F., Yuan W., Shi J. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpou P., Odén A., Simonsson T., Wilhelmsen L., Landin-Wilhelmsen K. High serum total cholesterol is a long-term cause of osteoporotic fracture. Osteoporos. Int. 2011;22:1615–1620. doi: 10.1007/s00198-010-1367-2. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. Diabetes and its complications and their relationship with risk of fractures in type 1 and 2 diabetes. Calcif. Tissue Int. 2009;84:45–55. doi: 10.1007/s00223-008-9195-5. [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. Hypertension is a risk factor for fractures. Calcif. Tissue Int. 2009;84:103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- Wang Y., Dai J., Zhong W., Hu C., Lu S., Chai Y. Association between serum cholesterol level and osteoporotic fractures. Front. Endocrinol. 2018;9:30. doi: 10.3389/fendo.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Scientific Group on the Prevention and Management of Osteoporosis (2000: Geneva Switzerland) vol 921. World Health Organization; Geneva: 2003. Prevention and management of osteoporosis: report of a WHO scientific group. (WHO Technical Report Series). [Google Scholar]
- Wolbers M., Koller M.T., Stel V.S., Schaer B., Jager K.J., Leffondré K., Heinze G. Competing risks analyses: objectives and approaches. Eur. Heart J. 2014;35:2936–2941. doi: 10.1093/eurheartj/ehu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Yu J.J. Beneath the minerals, a layer of round lipid particles was identified to mediate collagen calcification in compact bone formation. Biophys. J. 2006;91:4221–4229. doi: 10.1529/biophysj.105.075804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Lv X., Wei D., Yue F., Guo J., Zhang T. Metabolic syndrome and the risk of bone fractures: a meta-analysis of prospective cohort studies. Bone. 2016;84:52–56. doi: 10.1016/j.bone.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.