Abstract
The death inhibitory proteins, cFLIP and Bcl-2, canonically act at different steps to regulate receptor-mediated apoptosis in cancer cells. Here we report that pharmacological or genetic means to effect an increase in intracellular superoxide result in cFLIP upregulation. Interestingly, Bcl-2 overexpression is associated with a concomitant increase in cFLIP, and reducing superoxide sensitizes Bcl-2 overexpressing cancer cells to receptor-mediated apoptosis via downregulation of cFLIP. Moreover, inhibiting glycolytic flux overcomes apoptosis resistance by superoxide-dependent downregulation of cFLIP. Superoxide-induced upregulation of cFLIP is a function of enhanced transcription, as evidenced by increases in cFLIP promoter activity and mRNA abundance. The positive effect of superoxide on cFLIP is mediated through its reaction with nitric oxide to generate peroxynitrite. Corroborating these findings in cell lines, subjecting primary cells derived from lymphoma patients to glucose deprivation ex vivo, as a means to decrease superoxide, not only reduced cFLIP expression but also significantly enhanced death receptor sensitivity. Based on this novel mechanistic insight into the redox regulation of cancer cell fate, modulation of intracellular superoxide could have potential therapeutic implications in cancers in which these two death inhibitory proteins present a therapeutic challenge.
Keywords: Superoxide, cFLIP, Bcl-2, Apoptosis, Cancer
Graphical abstract
1. Introduction
Cellular redox status plays a critical role in cell survival, growth and death signaling. While an overwhelming increase in intracellular reactive oxygen species (ROS) results in cell and tissue injury and damage, a mild increase in ROS or a ‘pro-oxidant’ intracellular milieu not only blunts execution pathways but also facilitates cell growth/proliferation [[1], [2], [3]]. While most of the earlier findings suggested this dichotomous relationship to be a function of a disruption or deregulation of the tight balance between ROS production and anti-oxidant defense systems [4], our recent work implicated intracellular superoxide (O2•-) as well as an increase in the ratio of O2•- to hydrogen peroxide (H2O2) in the pro-survival activity of ROS [5,6]. In this regard, biochemical or genetic manipulations to tilt the ratio in favor of O2•-, such as activation of small GTPase Rac1 that promotes NADPH oxidase (NOX) assembly and activation, inhibition of superoxide dismutase 1 (SOD1) or increases in mitochondrial oxygen consumption and electron transport shuttling upon overexpression of the anti-apoptotic protein Bcl-2, promoted cell survival that could be rescued by inhibiting or scavenging O2•- [[7], [8], [9]]
The first definitive evidence linking a mild increase in intracellular O2•- to cell survival came from studies involving the classical death receptor signaling triggered upon ligation of the CD95 (Fas/Apo1) receptor [10]. The receptor pathway of apoptosis (i.e., extrinsic apoptosis) is triggered by the binding of ligands such as CD95L (FasL) or TRAIL (Tumor Necrosis Factor Related Apoptosis Inducing Ligand) to members of the TNF receptor family (death receptors). Receptor ligation leads to assembly of the Death-Inducing Signaling Complex (DISC) and activation of initiator caspases such as caspase-8. Caspase-8 causes several downstream effects including activation of executioner caspases (e.g., caspase-3) that bring about disassembly of the cell by inducing DNA fragmentation and proteolytic digestion of a number of cellular proteins [[11], [12], [13], [14], [15]]. Notably, cancer cells were made refractory to CD95-induced apoptosis upon pharmacological inhibition of SOD1, which resulted in an increase in O2•-. In a follow up study, our group corroborated the involvement of O2•- in inhibiting death receptor-induced apoptosis in a model of Type II receptor signaling, whereby the apoptosis inhibitory effect of blocking mitochondrial amplification pathway via overexpression of Bcl-2 was completely rescued upon pharmacological or genetic inhibition of intracellular O2•- production [8]. Interestingly, the restored sensitivity of Bcl-2 overexpressing cells to CD95-induced apoptosis upon reducing intracellular O2•- was not linked to a compromise of the mitochondria-protective function of Bcl-2, but instead to robust activation of caspase 8. These results implicate mechanism(s) and/or factors that regulate the critical early step upon ligation of the CD95 death receptor such as recruitment and activation of caspase 8 [8].
One of the key regulators of caspase 8 activation, downstream of death receptor ligation, is the protein cFLIP (cellular FLICE Inhibitory Protein). cFLIP is a structural homolog of caspase-8 but lacks catalytic activity and is upregulated in a variety of human cancers [11,[16], [17], [18]]. Earlier studies demonstrated that cFLIP protein turnover is affected by ROS-inducing agents, such as menadione, paraquat, or buthionine sulfoximine, via increased T166 phosphorylation and K167 ubiquitination, resulting in cFLIP degradation and increased sensitivity to ligand-induced apoptosis [19]. While these studies linked ROS production to downregulation of cFLIP, there have been no mechanistic studies clearly identifying the nature of ROS involved in cFLIP degradation. As ROS signals are diverse and context-dependent, it is highly desirable to elucidate mechanism(s) underlying the regulation of cFLIP in the context of cancer-relevant redox environment. In this regard, our recent work using a predictive computational modeling approach provides evidence that cFLIP levels fluctuate in correlation with the ratio between intracellular O2•- and H2O2 [20].
On the backdrop of these findings, here we set out to investigate if the inhibitory effect of an increase in intracellular O2•- on death receptor-induced apoptosis is a function of an increase in cFLIP expression. More importantly, we asked if the pro-oxidant effect of Bcl-2 overexpression negatively impacts death receptor signaling via induced expression of cFLIP. Making use of biochemical, genetic and metabolic approaches to modulate intracellular O2•-, we provide evidence that the inhibitory effect of O2•- on death receptor signaling is linked to its ability to induce cFLIP expression. Moreover, results indicate that glucose starvation of cancers cells could be an attractive strategy to overcome drug refractory phenotype via decreasing intracellular O2•- and downregulation of cFLIP.
2. Materials and Methods
2.1. Tumor cell lines and primary lymphoma cells
CEM human leukemia cell lines, stably transfected with the control vector (CEM/Neo) or Bcl-2(CEM/Bcl-2), were generously provided by Dr. Roberta A. Gottlieb (Scripps Cancer Center, La Jolla, CA, USA). Cells were maintained in RPMI 1640 supplemented with 5% fetal bovine serum (Hyclone, Logan, Utah, USA) and 20 μg/ml of G418 (G418 Sulfate, Thermoscientific, Waltham, MA, USA) in a 37 °C incubator with 5% CO2. The expression of Bcl-2 was confirmed by Western blot analysis using a primary anti-Bcl2 antibody (clone Bcl-2/100 at 1:1000 dil.; BD Pharmingen, San Diego, CA, USA) and a secondary HRP-conjugated anti-mouse lgG (1:5000 dil.; Pierce, Rockford, IL, USA). M14TF4, M14 PIRES and M14V12 cells have been previously described [10] and cultured in DMEM, supplemented with 5% FBS in a 37 °C incubator with 5% CO2.
Primary lymphoma tissues were obtained from patients upon informed consent (IRB 2010/00338). Tissues were minced and strained through MACS separation filter (Miltenyi Biotec Asia Pacific, Singapore) to get single-cell suspension and lymphocytes were obtained by Ficoll Hypaque density centrifugation, as decribed previously [46]. Primary cells were kept in culture for 2–3 days with 20% FBS in RPMI at 37 °C in the presence of 5% CO2.
2.2. Intracellular O2•- measurement
Detection of intracellular O2•- was carried our using two different methods as described previously [[10], [21]], a chemiluminescence assay using lucigenin (Sigma Aldrich, St. Louis, MO, USA) and a flow cytometry-based fluorescence assay using Hydroethidine (HE, Molecular Probes, ThermoFisher Scientific, Waltham, MA). For the Chemiluminescence assay, protein concentration in the cell lysates was determined using the Coomasie Plus protein assay reagent from Pierce (Pierce Chemical Company, Rockford, IL, USA), and chemiluminescence was monitored for 60 seconds using a Berthold Sirius Luminometer (Berthold Detection Systems GmbH, Pforzheim, Germany). For HE-based fluorescence detection, cells (1 × 106) were loaded with 2 μM HE for 15 min at 37°C and analyzed (10,000 events) by flow cytometry (Coulter EPICS Elite ESP) using an excitation wavelength of 610 nm. Cells were pre-incubated for 1 hr (unless indicated otherwise) with the various stimuli [PMA (Phorbol 12-myristate 13-acetate, Sigma) 62.5 ng/ml; DPI (Diphenyleneiodonium chloride, Sigma) 5 μM; DDC (Diethyldithiocarbamate, Sigma) 100 μM; Glucose-free RPMI with 2 mM Pyruvate; and 20–40 mM Glucose] before the assessment of intracellular O2•-.
2.3. Induction of death receptor-induced apoptosis and cell survival determination
Apoptosis was induced by exposure of cells (1 × 106/ml) to 0.25 μg/ml of anti-Fas (CD95/Apo-1) lgM (clone CH11 Upstate Biotech., Lake Placid, NY, USA) for 4–18 hrs in glucose-free RPMI supplemented with 5% FBS and 2 mM Na-pyruvate. Cell survival was determined by the MTT assay (Sigma Aldrich, St. Louis, MO, USA) and apoptosis was assessed by propidium iodide (PI) staining for cell cycle (percentage of subG1 for apoptosis), as described previously [1]. For primary lymphoma cells, cell survival was determined by MTT as described previously [46]after exposure of cells (2 × 106/ml) to 50 ng/ml of TRAIL for 24 hrs in normal and glucose-free RPMI supplemented with 5% FBS and 2 mM Na-pyruvate.
2.4. Analysis of caspases 8, 3 and 9 processing and cFLIP by Western blotting
Activation of caspase 8, 3 and 9 and cFLIP expression were assessed by SDS-PAGE and Western blot analysis. Briefly, lysates were prepared by using 1X RIPA lysis buffer. 50 μg of protein was resolved on 10% SDS-PAGE, transferred to PVDF membrane, and incubated with either polyclonal rabbit anti-caspase-3 or anti-caspase 8 (Pharmingen) or monoclonal mouse anti-caspase-9 (Upstate) or monoclonal Anti-FLIP (1:1000 dil.; Santa-Cruz, TX, USA), followed by the secondary anti-rabbit or anti-mouse IgG-HRP. Membranes were then exposed to Super Signal Substrate Western Blotting Kit (Piece, Rockford, IL, USA). Same membranes were stripped and probed with β-actin or GAPDH (Santa Cruz, Dallas, TX, USA) as an internal control for equal loading.
2.5. Determination of caspase 3, 8 and 9 activity
Caspases 3, 8 and 9 activities were assayed by using AFC-conjugated substrates supplied by Biomol Laboratories (Hercules, CA, USA) as described previously [1]. Cells (1×10 6 cells/ml) were exposed to anti-Fas (0.25 μg/ml) for 4–18 hrs in culture medium or glucose-free medium, washed twice with 1X PBS, resuspended in 50 μl of chilled cell lysis buffer (BD Pharmingen) and incubated on ice for 10 min. Next, 50 μl of 2X reaction buffer (10 mM HEPES, 2 Mm EDTA, 10 mM KCI, 1.5 mM MgCl2, 10 mM DTT) and 1 μl of the fluorogenic caspase-specific substrate (DEVD-AFC for caspase 3, LETD-AFC for caspase 8, and LEHD-AFC for caspase 9) (Applied Biosystem, Thermo Fisher Scientific, Waltham, MA) were added to each sample and incubated at 37 °C for 30 min. Protease activity was determined by measuring the relative fluorescence intensity at 505 nm following excitation at 400 nm using a spectrofluorometer (TECAN Spectrofluor Plus, Mannedorf, Switzerland). Results are shown as fold increase (X increase) in activity relative to the enzymatic activity obtained from untreated control cells (1X).
2.6. Transient transfection with RacV12 mutant, siSOD1 and sicFLIP and β-gal
Transient overexpression in CEM/Neo and CEM/Bcl2 cells was performed using the SuperFect Transfection Reagent (QIAGEN Gmbh, Dusseldorf, Germany). 4 μg of the pIRES (empty vector), pIRESRacV12 or the specific RacV12 mutants (H103A, K166E, H40 and L37) with 1 μg of the pCMVβ plasmid encoding for the β-gal protein were mixed with 20 μl of SuperFect, and transfection was performed as per the manufacturer's protocol. Protein expression following transient transfection was verified by 12% SDS-PAGE Western analysis using 2 μg/ml of a monoclonal anti-human myc epitope antibody (Boehringer Mannheim, Indianapolis, IN, USA). Protein concentration was determined using the Coomasie Plus protein assay reagent from Pierce (Pierce Chemical Company, Rockford, IL, USA).
siRNA-mediated knockdown of SOD1 or cFLIP was performed using RNAiMax (Qiagen Gmbh, Dusseldorf, Germany) according to the manufacturer's instructions. Verification of knockdown was performed by Western blot analysis using, monoclonal anti-FLIP or anti-SOD1 (Santa-Cruz, Dallas, TX, USA). ON-TARGET SOD1 and ON-TARGET plus smartpool cFLIP (Dharmacon cat # J-008364-10 and cFLIP Dharmacon cat # L-003772-00) specific siRNAs were purchased from Dharmacon Technologies (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. RNA isolation and Semi-quantitativePCR
Total RNA was isolated from cells (CEM/Bcl2 cells or M14 stably transfected with RacV12) using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer's instructions after the following treatments: (a) DDC (100 μM) for 2 hrs, (b) DPI (5 μM) for 1hr, (c) DDC (200 μM) or PMA (100 ng/ml) with and without preincubation with cycloheximide (CHX; 5,10 μg/ml) for 2 hrs. Each RT reaction contains 2.5 μg of total RNA, 1X RT buffer, 100U Superscript II Reverse Transcriptase and made up to 20 μl with sterile water. RT reaction was carried out at 25 °C for 10 min followed by 42 °C for 50 min and 70 °C for 15 min cFLIP and β-actin PCR amplifications were performed in the same well using GoScript™ Reverse Transcription system from Promega (Madison, WI, USA). The following primer sequences were used: cFLIP, Forward: 5′-CACCGAGACTACGACAGCTTTGT-3′ and Reverse: 5′-GCCCTGAGTGAGTCTGATCCA-3'; β-actin, Forward: 5′ TCA CCC ACA CTG TGC CCA TCT ACG A 3′ and Reverse: 5′ CAG CGG AAC CGC TCA TTG CCA ATG G 3′. Quantification of RNA content was performed using a Real Time quantitative PCR method with SYBR Master Mix (NEB), cDNA synthesis kit (Abcam, Cambridge, MA) and corresponding primers. β-actin was used as an internal control to normalize CFLAR. Realtime PCR reactions were carried out in triplicate on a LightCycler 480 detection instrument (Roche, Basel, Switzerland). The PCR parameters were as follows: 5 min at 95℃, then 45 amplification cycles of 10 sec at 95℃, 10 sec at 60℃ and 10 sec at 72℃. The relative RNA expression was calculated using 2−ΔΔCT method.
2% agarose gel electrophoresis was used to verify cFLIP expression using β-actin as a control marker. The gel was visualized using BioRAD GelDoc system.
2.8. cFLIP promotor activity
Luciferase tagged plasmids were gifted from Dr David Dicker (University of Pennsylvania, PA, USA) to determine cFLIP promoter activity. Each reporter also harbors the constitutively expressing Renilla luciferase, which serves as an internal control for normalizing transfection efficiencies. The plasmids were transfected into 60% confluent Hela cells by Lipofectamine 2000 reagents (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Cells were treated 24 hrs post-transfection with the intended reagents. The Promega Dual Luciferase Reporter assay system (Promega, Madison, WI, USA) was used for detecting renilla luciferase activities in a single sample as per the manufacturer's instructions. 10 μl of the supernatant was used for each samples and transferred to a 96 well white bottom plate to detect luminescence with the Varioskan LUX Multimode Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA).
3. Results
3.1. Superoxide-induced inhibition of death receptor-mediated apoptosis involves upregulation of cFLIP
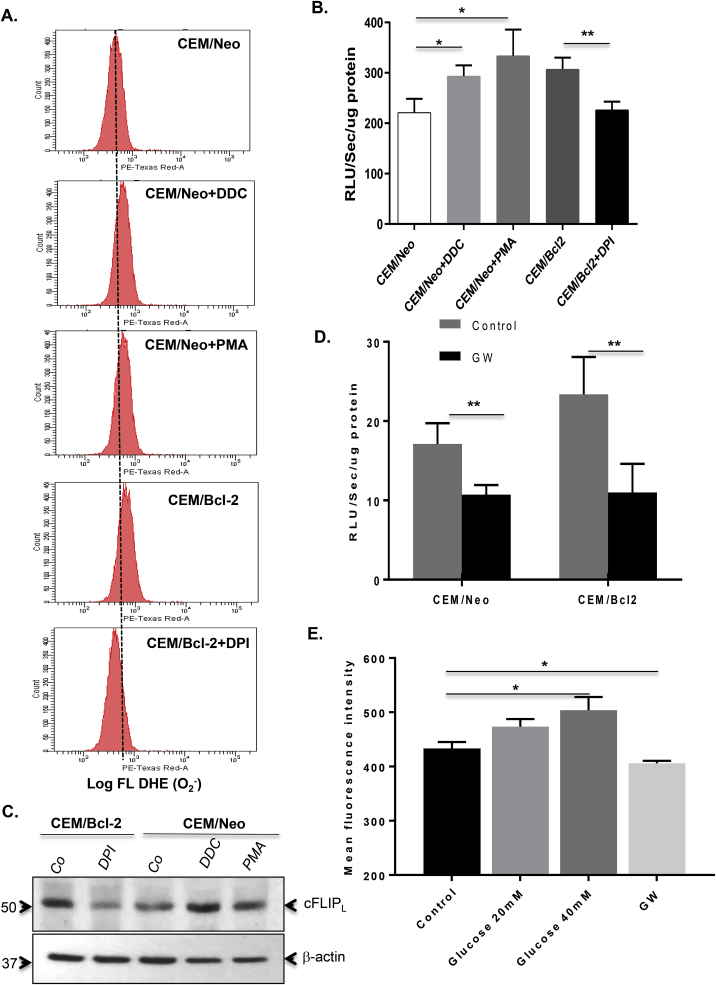
Intrigued by our previous findings that lowering intracellular O2•- restored sensitivity of Bcl-2 overexpressing CEM human leukemia cells to receptor-mediated apoptosis via a significant increase in caspase 8 activity [8], we questioned whether O2•- induced inhibition of death receptor signaling was mediated by increased cFLIP expression. To do so, we first employed a number of biochemical strategies to effect an increase in intracellular O2•-, such as pharmacological inhibition of superoxide dismutase 1 (SOD1) with DDC, PMA-induced activation of NOX and overexpression of Bcl-2. Using two different assays (Flow cytometry following DHE loading and lucigenin-based chemiluminescence assay) [5,21] to measure intracellular O2•-, we show that exposure of CEM cells to DDC or PMA as well as overexpression of Bcl-2 (CEM/Bcl-2) resulted in a significant increase in intracellular O2•- (Fig. 1A and B). In contrast, and as shown previously, pre-incubation of cells with the NOX inhibitor (DPI) neutralized the effect of Bcl-2 overexpression on intracellular O2•- (Fig. 1B) [8]. Having established various conditions to modulate intracellular O2•- we next assessed the expression of cFLIP. Firstly, Bcl-2 overexpression (CEM/Bcl-2) correlated with a higher expression of cFLIP compared to CEM/Neo cells. Secondly, while exposure of CEM/Neo cells to DDC or PMA resulted in a significantly higher cFLIP expression, exposure of CEM/Bcl-2 cells to the NOX inhibitor DPI reduced cFLIP expression to the levels expressed in CEM/Neo cells (Fig. 1C).
Fig. 1.
Superoxide-induced inhibition of death receptor-mediated apoptosis involves upregulation of cFLIP. Intracellular O2•- was monitored by (A) flow cytometry following labelling of cells with the fluorescent probe HE (Hydroethidine, Molecular Probes Invitrogen) staining and by (B) lucigenin-based chemiluminescence assay (RLU/sec/μg protein) after cells were treated with DDC (200 μM), PMA (62.5 ng/ml) or DPI (5 μM) for 1 hr. (C) cFLIP expression in whole cell lysates from the above treated cells was verified by Western blot analysis using anti-cFLIP. Expression of β-actin served as the loading control. (D) CEM/Neo and CEM/Bcl-2 cells were cultured in normal medium or medium without glucose but containing 2 mM pyruvate (GW) and intracellular O2•- was detected by lucigenin-based chemiluminescence as described above. (E) CEM/Neo cells were cultured in increasing concentrations of glucose (20 mM or 40 mM) for 1 hr and intracellular O2•- was determined by flow cytometer using HE staining and presented as the Mean Fluorescence Intensity. Where applicable, data shown are Mean ± S.D. of three independent experiments and P values (* <0.05; ** <0.01) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
3.2. Death receptor sensitization upon glucose withdrawal is associated with decreases in intracellular O2•- level and cFLIP expression
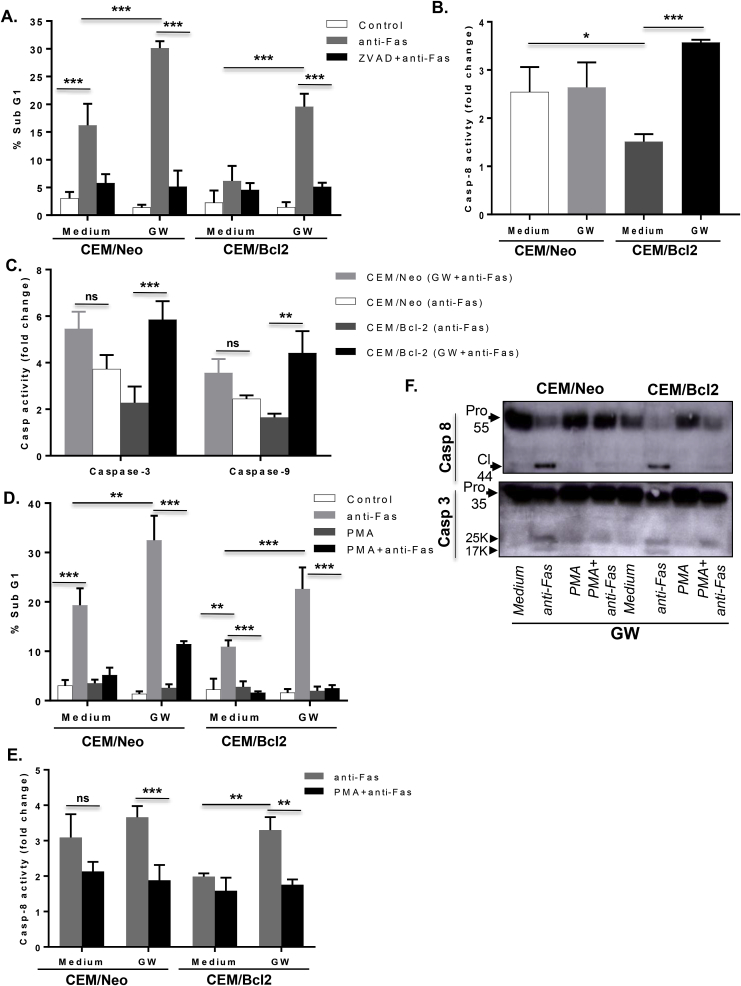
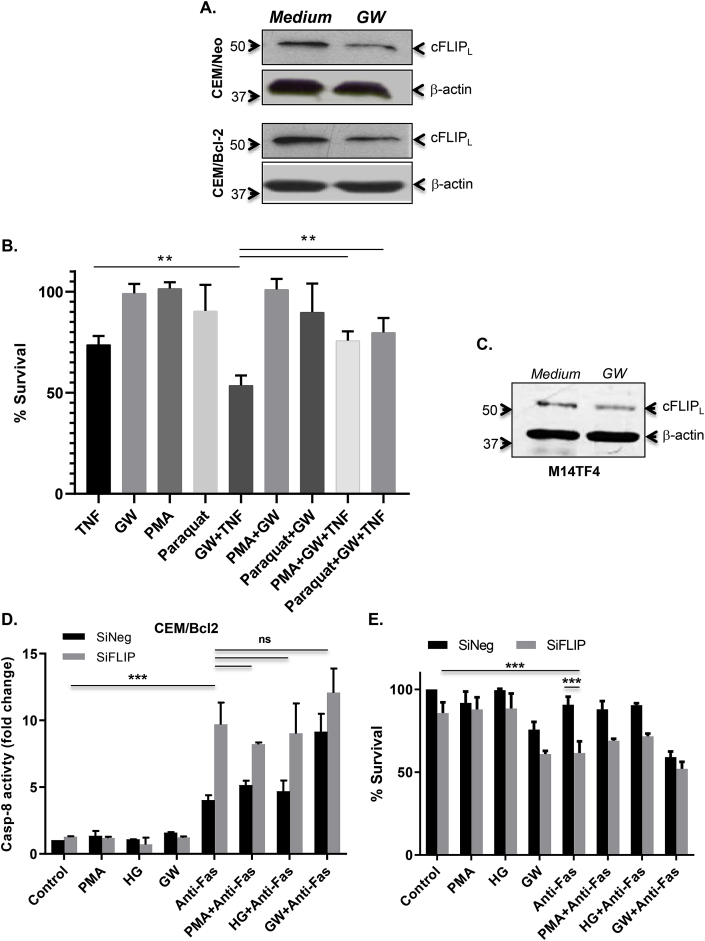
Having shown that modulators of O2•- and overexpression of Bcl-2 correlate with cFLIP expression, we next explored the possibility to modulate intracellular O2•- using a physiological regulator of cells’ metabolism. To that effect, growing CEM/Neo and CEM/Bcl-2 cells in glucose-free medium but in presence of pyruvate, referred to as glucose withdrawal condition (GW), resulted in a significant decrease in O2•- in both cell lines (Fig. 1D), whereas culturing cells in the presence of 20 mM or 40 mM glucose resulted in an increase in intracellular O2•- (Fig. 1E). More importantly, subjecting Bcl-2 overexpressing cells-that are refractory to death receptor-induced apoptosis-to GW resulted in a significant increase in the fraction of sub-G1 population upon ligation of the CD95 receptor, indicating enhanced apoptosis sensitivity (Fig. 2A). This was further corroborated by more than 3-folds increase in the activity of caspase 8 (Fig. 2B), together with a significant induction in the activities of caspase 9 and the executioner, caspase 3 (Fig. 2C). Furthermore, to provide evidence that the apoptosis sensitizing effect of GW was a function of a decrease in intracellular O2•-, cells were incubated with PMA to sustain an increase in O2•- prior to CD95 receptor ligation. PMA treatment neutralized the apoptosis sensitizing effect of GW as indicated by the reduction in sub-G1 fraction (Fig. 2D) and notably inhibition of caspase 8 activity, as well as the processing (activation) of caspases 8 and 3, induced upon withdrawal of glucose (Fig. 2E and F). Notably, PMA treatment negated the effect of GW on intracellular O2•- in CEM/Bcl-2 cells (Fig. S1A) as well as inhibited the CD95 sensitizing effect of GW in both CEM/Neo and CEM/Bcl-2 cells (Fig. S1B). Taken together these results established GW as a valid approach to sensitize Bcl-2 overexpressing cells to receptor-mediated apoptosis through a decrease in intracellular O2•-. Logically, we next assessed the effect of GW on cFLIP expression. Notably, CEM/Neo or CEM/Bcl-2 cells subjected to GW exhibited a significantly reduced expression of cFLIP, compared to the cells cultured in control medium (Fig. 3A). Interestingly, corresponding with the intracellular level of O2•-, the constitutive level of cFLIP was significantly higher in CEM/Bcl-2 cells but could be restored to that of CEM/Neo cells by the decrease in intracellular O2•- upon GW (Fig. 3A). Together, these data show that sensitization of CEM/Bcl-2 (and CEM/Neo) cells to receptor-mediated apoptosis by GW is associated with concomitant decreases in intracellular O2•- level and cFLIP expression.
Fig. 2.
Death receptor sensitization upon GW is associated with decrease in intracellular O2•-. (A) CEM/Neo and CEM/Bcl-2 cells were pretreated for 1hr with 50 μM ZVAD-fmk before triggering apoptosis with anti-Fas (0.25 μg/ml for 18 hrs) in culture medium or in GW conditions and PI staining was used to assess DNA fragmentation (sub-G1 fraction) as described in Materials and Methods. (B) Cells were incubated for 4 hrs with 0.25 μg/ml of anti-Fas in normal medium or GW conditions and caspase 8 and (C) caspase 9 and 3 activities were determined in whole cell lysates using fluorometric assays that detect cleavage of specific substrates, as described in Materials and Methods. Enzyme activity are shown as fold increase over the untreated cells. (D) Cells were pretreated with PMA (62.5 ng/ml) for 1hr before treatment for 4 hrs with anti-Fas (0.25 μg/ml) in normal cell culture medium or GW medium and apoptosis was assessed by PI staining (sub-G1 fraction). (E) Caspase 8 activity was determined in the lysates from cells treated with anti-Fas in the presence and absence of PMA using a fluorometric assay and presented as fold increase over untreated cells. (F) Processing of capase-3 and 8 was determined by Western blot analysis using specific antibodies as described in Materials and Methods. Where applicable, data are Mean ± S.D. of three independent experiments and P values (* <0.05; ** <0.01; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
Fig. 3.
GW-induced decrease in intracellular O2•-downregulates cFLIP. (A) cFLIP expression was verified in lysates from CEM/Neo and CEM/Bcl-2 cells cultured in normal growth medium or GW medium using anti-cFLIP by Western blot analysis; anti-β-actin was used as loading control. (B) M14TF4 cells were pretreated with PMA (62.5 ng/ml) or paraquat (50 μM) for 1hr followed by 5 ng/ml of TNFα exposure in culture medium or GW medium for 18 hrs and cell survival was assessed by MTT assay as described in Material and Methods. (C) Western blot analysis for cFLIP expression in M14TF4 cells cultured in normal or GW medium; anti-β-actin was used as loading control. (D) CEM/BCL2 cells were transfected with siNeg or siFLIP using RNAiMAX and 48 hrs post transfection incubated with anti-Fas (0.25 μg/ml for 18 hrs) with or without pre-treatment with PMA (62.5 ng/ml for 1hr) in normal growth medium or GW medium or high glucose medium (HG; 20 mM glucose). Caspase-8 activity was determined using a fluorometric assay as described in Materials and Methods. (E) Cell viability was assayed by the MTT assay and presented as % survival relative to untreated cells. Where applicable, data shown are Mean ± S.D. of three independent experiments and P values (** <0.01; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
Because the cell states exhibiting resistance to apoptosis were also the cell states with increased O2•- levels, we next made use of a complementary O2•--inducing compound, paraquat, to observe whether it too could rescue the cells from the apoptosis sensitizing effect of GW. Paraquat increases intracellular O2•- in conjunction with membrane disruption, and unlike PMA has no known overlap with PKA pathway stimulation. Exogenous O2•- donors such as menadione were not utilized because their effects on intracellular signaling are often dissimilar to the effects of endogenous O2•- [22]. Despite the differences in the mechanisms of O2•-accumulation, pre-incubation with paraquat had the same effect as PMA in neutralizing the apoptosis sensitizing activity of GW in CEM/Neo, CEM/Bcl-2 and Hela cells (Figs. S1B–C).
To support the universality of the effect of GW on the sensitivity to receptor-mediated apoptosis through a decrease in intracellular O2•- level and cFLIP expression, we made use of M14TF4 cells tailored to express a chimeric death receptor consisting of the extracellular domain of the TNFα receptor and cytoplasmic domain of Fas (CD95) receptor. Indeed, the combination of GW and TNFα treatment promoted apoptosis in M14TF4 cells while the addition of PMA or paraquat conferred protection (Fig. 3B). Of note, similar to the effects observed in CEM cells, subjecting M14TF4 cells to GW resulted in a decrease in cFLIP expression (Fig. 3C). Together, these results show that cell sensitivity to receptor-dependent apoptosis was consistently correlated with O2•- levels and cFLIP down-regulation across several different perturbations of the intracellular redox state including glucose deprivation.
To establish a direct link between intracellular O2•- and cFLIP expression in the regulation of death receptor-mediated apoptosis, we next investigated the effect of manipulating intracellular O2•- in CEM/Neo and CEM/Bcl-2 cells in which the expression of cFLIP had been knocked down by siRNA-mediated gene silencing (Fig. S1D). Firstly, knock down of cFLIP robustly increased caspase 8 activity and reduced cell survival in CEM/Bcl-2 cells upon ligation of the CD95 receptor (Fig. 3D and E). More importantly, pharmacological manipulation of O2•- with PMA or high glucose (HG) or GW failed to have any significant effect on death receptor sensitivity, unlike control (siNeg transfected) cells (Fig. 3D and E). These data testify to the critical importance of redox-dependent cFLIP expression in regulating death receptor signaling.
3.3. NOX activating functional mutants of GTPase Rac1 are associated with cFLIP upregulation and apoptosis inhibition
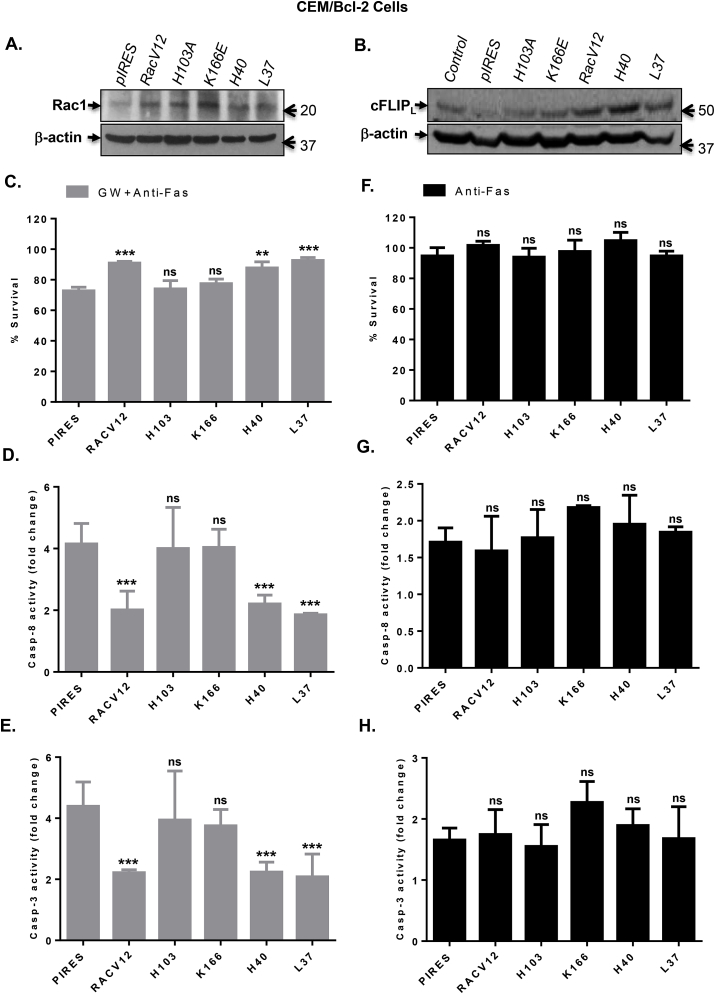
We previously reported that expression of a dominant negative mutant of Rac1 (RacN17) alleviated intracellular O2•- levels and restored sensitivity of Bcl-2 overexpressing cells to apoptosis stimuli [8]. As GTP loaded Rac1 (active) is associated with NOX assembly and activation, we made use of the specific functional mutants [7] to further validate the involvement of intracellular O2•- on cFLIP expression and apoptosis regulation. CEM/Bcl-2 cells were transfected with constitutively active Rac1 (RacV12) or the various mutants (Fig. 4A): H103A and K166E (critical residues for NOX activation; [23], H40 and L37 (mutants that affect other functions of Rac1 while maintaining NOX activating activity). Firstly, expression of RacV12 and the two NOX competent mutants (H40 and L37) resulted in a significant increase in cFLIP expression compared to cells transfected with the vector alone (pIRES) or the two NOX-incompetent mutants, H103 and K166 (Fig. 4B). Next, we assessed the effect of transient expression of the various Rac1 mutants on GW-induced sensitivity to ligation of the CD95 receptor. Interestingly, GW-induced sensitivity of CEM/Bcl-2 cells to death receptor-mediated apoptosis (pIRES) was virtually completely inhibited upon expression of RacV12 and the two NOX-competent mutants H40 and L37, while the other two mutants that lack NOX activating ability (H103A and K166E) had no effect (Fig. 4C). In line with the results shown in the preceding sections, the effect of active RacV12 or the NOX competent mutants (H40 and L37) was strikingly pronounced on caspase 8 and caspase 3 activities (Fig. 4D and E-). As expected, CEM/Bcl-2 cells were resistant to CD95-mediated apoptosis and therefore, no significant differences were observed in cells cultured in growth medium with glucose concentration of 10 mM (Fig. 4F–H). A similar effect was observed in CEM/Neo cells; RacV12 as well as the mutants that retained the ability to produce O2•- increased cFLIP expression and inhibited the death receptor sensitizing effect of GW (Fig. S2).
Fig. 4.
Rac1 functional mutants that retain O2•-producing activity inhibit GW-induced sensitization to anti-Fas by upregulating cFLIP. (A) Transient transfection of CEM/Bcl-2 cells with the empty pIRES vector or various Rac1 mutants (RacV12, H103A, K166E, H40, and L37) was performed as described in Material and Methods. After 48 hrs of transfection, the expression of transiently expressed proteins was detected by Western blot using anti-Rac1 with β-actin expression as the loading control. (B) cFLIP expression in lysates from CEM/Bcl-2 cells transfected with the various Rac1 mutants was assessed by Western blot analysis using anti-cFLIP; anti-β-actin was used as loading control. (C) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 18 hrs in GW medium and cell survival was determined by the β-gal survival assay as described in Materials and Methods. (D–E) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 4 hrs in GW medium and activities of capase-8 and 3 were determined in lysates using fluorometric assays as described in Materials and Methods. Results are shown as fold increase in enzyme activity over the untreated control cells. (F) Cells expressing the various Rac1 mutants were treated with anti-Fas (0.25 μg/ml) for 18 hrs in normal growth medium and cell survival was determined by the β-gal survival assay as described in Materials and Methods. (G–H) Cells were treated with anti-Fas (0.25 μg/ml) for 4 hrs in normal growth medium and activities of capase-8 and 3 were determined in lysates using fluorometric assays. Results are shown as fold increase in enzyme activity over the untreated control cells. Where applicable, data shown are Means ± S.D. of three independent experiments and P values (** <0.01; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
3.4. Increase in O2•-Upregulates cFLIP via enhanced gene transcription
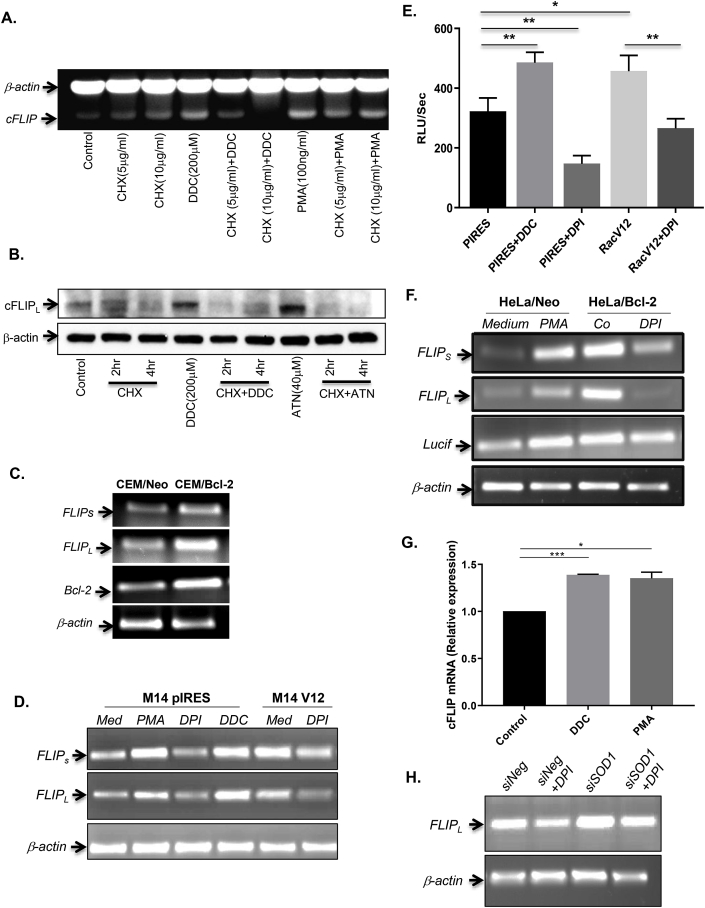
It is interesting to note that the basal level of cFLIP was significantly higher in cells overexpressing Bcl-2, and incubation with the NOX inhibitor DPI reduced cFLIP protein levels (Fig. 1C). Having established a link between intracellular O2•- and cFLIP expression, we next queried whether the effect of redox manipulation was at the level of cFLIP gene transcription or at the post-translational level via enhanced protein stability/half-life. To do so, cells were co-treated with the protein synthesis inhibitor cycloheximide (CHX; 5–10 μg/ml for 2–8 hrs) and DDC (200 μM) or PMA (100 ng/ml) and mRNA levels of cFLIP were assessed by RT-PCR. Results show that exposure of cells to DDC or PMA upregulated cFLIP, however, this effect was virtually completely neutralized in the presence of CHX (Fig. 5A). Furthermore, Western blot analysis of lysates following CHX treatment (2 hrs and 4 hrs) in the presence or absence of O2•- inducing stimuli, DDC and ATN (40 μM), not only confirmed the relatively short half-life of cFLIPL (~4 hrs), but also provided clear evidence linking O2•- increase to a significant upregulation of cFLIPL that was completely blocked in the presence of CHX (Fig. 5B). These data indicate that elevated levels of O2•- did not affect cFLIP protein stability/half-life, but that the increased expression was a function of upregulation of cFLIP transcription. Indeed, analysis of cFLIP mRNA by RT-PCR clearly revealed a significant upregulation of cFLIPL and cFLIPs in cells overexpressing Bcl-2 (Fig. 5C), which also harbor an increase in intracellular O2•-, as shown consistently in this report and in our earlier findings [8,9].
Fig. 5.
Superoxide anion regulates cFLIP gene expression. (A) CEM/Neo cells were pretreated with cyclohexamide (CHX; 5, 10 μg/ml) for 2 hrs followed by DDC (200 μM) or PMA (100 ng/ml) for 4 hrs and mRNA level of cFLIP was assessed by RT-PCR as described in Materials and Methods using β-actin as the internal loading control. (B) Cells were treated with 10 μg/ml of CHX for 2 hrs or 4 hrs before the addition of DDC (200 μM) for 2 hrs or ATN (40 μM) for 4 hrs and cFLIP expression was determined by Western blot analysis as described in Materials and Methods; β-actin was used as the loading control. (C) Basal mRNA level of cFLIP (L and S isoforms) and Bcl-2 in CEM/Neo and CEM/Bcl-2 cells were determined by RT-PCR as described above with β-actin serving as control. (D) M14 cells stably transfected with RacV12 were treated for 1hr with either DDC (200 μM), PMA (62.5 ng/ml), or DPI (5 μM) and mRNA levels of cFLIP (L and S) were determined by RT-PCR with luciferase and β-actin as internal controls, as described in Materials and Methods. (E) Intracellular O2•- was measured by lucigenin-based chemiluminescence assay following transient expression in CEM/Neo cells transiently expressing RacV12 and treatment with DDC or DPI. (F) HeLa/Neo and HeLa/Bcl-2 cells were treated with PMA (62.5 ng/ml) or DPI (5 μM) for 1hr and mRNA level of cFLIP (and luciferase) was determined by RT-PCR; β-actin amplification was used as internal control. (G) CEM/NEO cells were treated with DDC (200 μM) or PMA (100ng/ml) for 2 hrs and cFLIP mRNA was assessed by Real Time PCR as described in Materials and Methods. (H) HeLa/Neo cells were transfected with siNeg or siSOD1 using RNAiMAX for 48 hrs and treated with DPI (5 μM) for 1hr and extracted RNA was analyzed for cFLIP expression by RT-PCR as described in Materials and Methods. Data shown in E and G are Means ± S.D. of three independent experiments and P values (* <0.05; ** <0.01; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
To validate that the upregulation of cFLIP was indeed a function of an increase in intracellular O2•-, we made use of M14 cells expressing a constitutively active form of the small GTPase Rac1 (M14V12). Assessment of cFLIP mRNA by RT-PCR confirmed that, compared to M14pIRES cells, the expression of cFLIP was significantly higher in M14V12 cells (Fig. 5D). Moreover, incubation of M14pIRES cells with PMA or DDC (to affect an increase in intracellular O2•-) resulted in a significant upregulation of cFLIPL and cFLIPs mRNAs, while incubation of M14V12 cells with the NOX inhibitor, DPI, resulted in a significant downregulation of cFLIP mRNAs (Fig. 5D). As expected, the intracellular O2•- levels were significantly higher in M14V12 cells and treatment with DPI almost completely inhibited the increase associated with V12 expression (vs pIRES vector alone), while DDC resulted in a significant increase in O2•- in M14pIRES cells (Fig. 5E). Also, we confirmed that M14V12 cells exhibited significantly higher proteins levels of cFLIP, compared to M14pIRES cells, and that pharmacological manipulation of O2•- regulated cFLIP similarly to that observed with CEM/Bcl-2 cells (Fig. S3A).
These results were further corroborated in HeLa cells overexpressing Bcl-2 (Hela/Bcl-2). Firstly, similar to the results obtained with CEM/Bcl-2 cells, overexpression of Bcl-2 resulted in a significant upregulation of cFLIPL and cFLIPs mRNAs (two isoforms of cFLIP), compared to the vector alone transfected (Hela/Neo) cells (Fig. 5F). In addition, PMA significantly amplified cFLIPL and cFLIPs mRNAs in Hela/Neo cells, while exposure of Hela/Bcl-2 cells to DPI resulted in a significant downregulation of cFLIP mRNAs (Fig. 5F). To further confirm that the effect of an increase in O2•- was on cFLIP transcription, we performed Real Time PCR following treatment with DDC or PMA. Treatment with DDC or PMA resulted in a significant increase in cFLIP mRNA (Fig. 5G).
Finally, in addition to using pharmacological inhibitors to manipulate intracellular O2•-, we employed RNAi-mediated silencing of SOD1 and assessed its effect on cFLIP at the mRNA and protein levels. Notably, cFLIP mRNA was significantly upregulated in cells upon knockdown of SOD1 (siSOD1), which could be rescued by DPI (Fig. 5H). The effect of siSOD1 on the protein levels of cFLIP and SOD1 was confirmed by SDS-PAGE Western analysis (Fig. S3B).
3.5. Increase in O2•- amplifies cFLIP promoter activity distinctly in contrast to H2O2
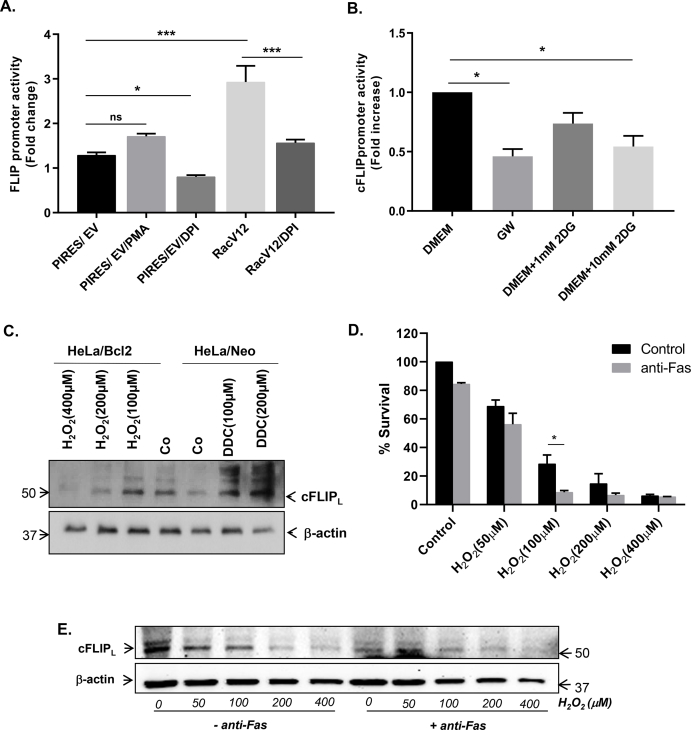
The results presented so far provide strong evidence that the effect of manipulating intracellular O2•- on cFLIP expression is at the level of transcription. To further explore that, we made use of a luciferase-tagged vector carrying the full-length promoter region of cFLIP. M14pIRES or M14V12 cells were transfected with the vector carrying the cFLIP promoter and activity was assessed by luciferase reporter assay. Firstly, the cFLIP promoter activity was significant amplified in M14V12 cells, compared to M14pIRES cells (Fig. 6A). Secondly, incubation of cells with DPI virtually completely reversed the increase observed in M14V12 cells (Fig. 6A). Similarly, cells subjected to GW or exposed to the glycolysis inhibitor, 2 Deoxy-d-glucose (2-DG; 10 mM), also exhibited a significant decrease in the basal cFLIP promoter activity (Fig. 6B).
Fig. 6.
Increase in O2•-amplifies cFLIP promoter activity distinctly in contrast to H2O2. (A) M14PIRES and M14RacV12 cells were transfected with a luciferase-tagged plasmid containing the cFLIP promoter for 24 hrs and then treated with PMA (62.5 ng/ml) or DPI (5 μM) for 1hr and promoter activity was measured by luciferase reporter assay. Results are expressed as fold change in luciferase activity. (B) HeLa cells carrying the cFLIP promoter construct were cultured in normal growth medium, GW medium or in the presence of 2DG and cFLIP promoter activity was assessed by the luciferase reporter assay. (C) HeLa/Neo cells were treated with DDC (100 μM and 200 μM) for 2 hrs while HeLa/Bcl-2 cells were treated with H2O2 (100 μM–400 μM) for 2 hrs and whole cell lysates were subjected to Western blot analysis using anti-cFLIP and anti-β-actin. (D) CEM/Neo cells were pretreated with H2O2 (50 μM–400 μM) for 1hr followed by anti-Fas (0.25 μg/ml) for 18 hrs and cell survival was determined by the MTT assay. (E) cFLIP expression in whole cell lysates from the cells in D was determined by Western blot analysis using anti-cFLIP as described in Materials and Methods; anti-β-actin was used as loading control. Data in A, B and D are shown as Mean ± S.D. of three independent experiments and P values (* <0.05; *** <0.005) were calculated by Ordinary one-way ANOVA using GraphPad Prism.
Earlier reports have linked H2O2 with the downregulation of cFLIP [20,24]. Based on our findings linking the increase in intracellular O2•- with cFLIP upregulation, we next set out to confirm these contrasting effects of the two ROS. Firstly, we confirmed that exposure of Hela/Bcl-2 cells-which express significantly higher levels of cFLIP-to increasing concentrations of H2O2 resulted in a dose-dependent decrease in cFLIP expression, while DDC induced a significant increase in cFLIP in Hela/Neo cells (Fig. 6C). Secondly, H2O2 was also able to inhibit the promoter activity of cFLIP at concentrations as low as 50 μM (Fig. S4A). To further validate the latter, cells were transfected with either SOD1 or the H2O2 scavenger, CAT (catalase), and cFLIP promoter activity was determined. Notably, while SOD1 overexpression significantly decreased cFLIP promoter activity, induced expression of CAT significantly amplified the promoter activity (Fig. S4B). Analysis of intracellular H2O2 using DCFH-DA loading and flow cytometry confirmed the contrasting effects of transfection with SOD1 (increase) and CAT (decrease) on DCF fluorescence (Fig. S4C). Most importantly, and in stark contrast to O2•-, addition of exogenous H2O2 resulted in a dose-dependent increase in sensitivity to CD95/Fas mediated apoptosis as well as downregulation of cFLIP (Fig. 6D and E). These results provide evidence that O2•-. and H2O2 exhibit contrasting effects on the expression of the apoptosis inhibitory protein cFLIP and on the sensitivity to death receptor-induced apoptosis.
3.6. Peroxynitrite is implicated in O2•--induced cFLIP amplification
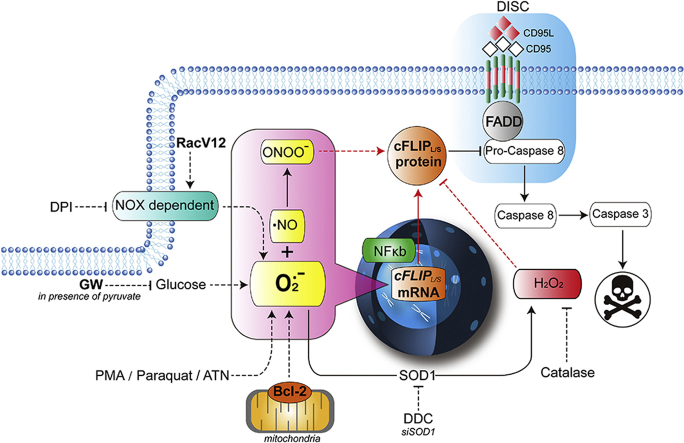
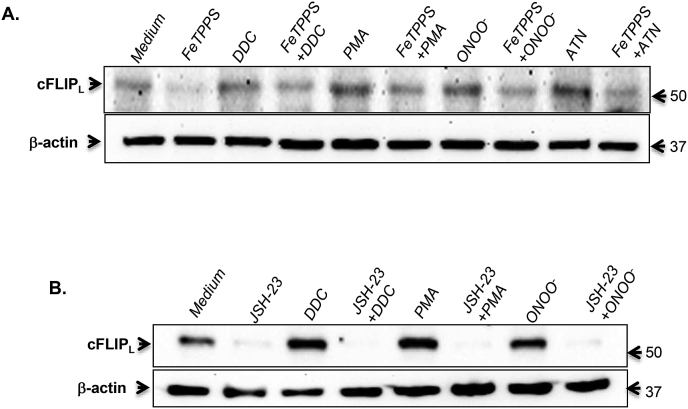
The natural fate of intracellular O2•- is a function of its dismutation to H2O2 by SODs or its reaction with nitric oxide (.NO) to generate the relatively more stable and reactive peroxynitrite (ONOO−) anion [25]. Notably, the rate constant for the reaction between O2•- and .NO (KNO) to generate ONOO− is an order of magnitude higher than that for SOD (KSOD) [25]. Therefore, we exposed cells to: (a) the same triggers used to amplify intracellular O2•- (DDC, PMA, and ATN) in the presence and absence of the ONOO− catalytic decomposer, FeTPPS and (b) purified ONOO−. Results show that a priori treatment with FeTPPS nullified the increase in cFLIP expression induced by DDC, PMA or ATN (Fig. 7A). Furthermore, exogenously added ONOO− had the same effect on cFLIP as other inducers of O2•-, which was inhibited upon its decomposition by FeTPPS (Fig. 7A). Finally, a strong inhibitory effect of the NF-kB inhibitor, JSH-23, was observed on O2•- (DDC and PMA) or ONOO− -induced upregulation of cFLIP (Fig. 7B). Together, the effect of an increase in O2•- on cFLIP is a function of intracellular ONOO− generation and implicates the transcriptional activity of NF-kB.
Fig. 7.
Peroxynitrite is implicated in cFLIP amplification by increased intracellular O2•-. (A & B) CEM/Neo cells were pretreated with FeTPPS (50 μM for 4 hrs) or JSH-23 (25 μM for 1hr) followed by incubation with DDC (200μΜ), PMA (62.5 ng/ml), ATN (40μΜ) or ONOO−(20 μM). Whole cell lysates were then subjected to Western blot analysis using anti-cFLIP and β-actin expression was used as the loading control.
3.7. GW downregulates cFLIP and sensitizes primary cells from lymphoma patients to TRAIL mediated apoptosis
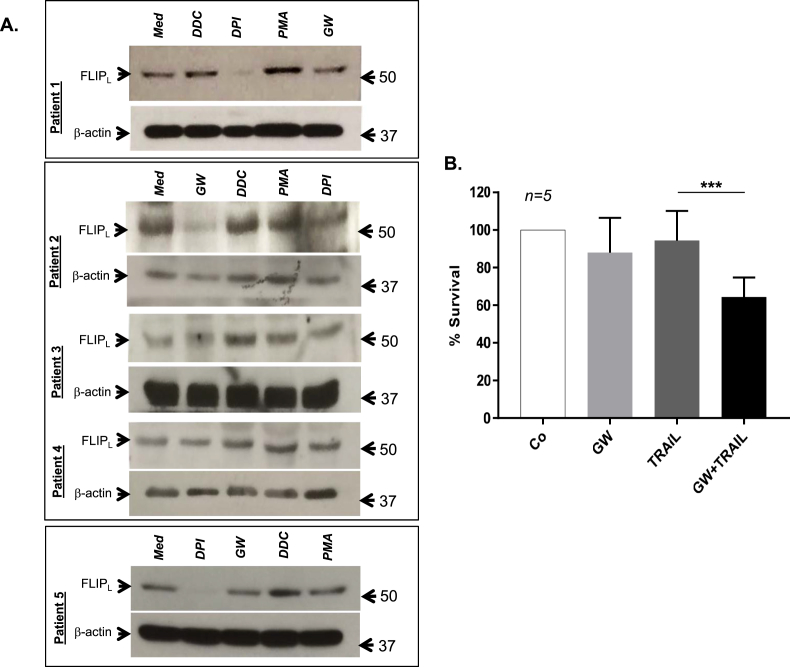
Intrigued by our findings, we next set out to explore the translational relevance of these results using primary cells derived from patients with lymphoma. Primary cells derived from 5 different lymphoma biopsies were treated with DPI, DDC, PMA or subjected to GW for 1 hr, before subjecting to SDS-PAGE and Western blot analysis using anti-cFLIP. Corroborating the results obtained in established cell lines, exposure of primary lymphoma cells to DDC or PMA (that increases O2•-) resulted in an increase in cFLIP expression, while treatment with DPI or GW (that reduces O2•-) significantly downregulated cFLIP (Fig. 8A). Importantly, subjecting primary cells from lymphoma biopsies to GW significantly enhanced their sensitivity to death receptor ligation as evidenced by TRAIL (TNF-α Related Apoptosis Inducing Ligand) induced execution (Fig. 8B).
Fig. 8.
GW downregulates cFLIP and sensitizes primary lymphoma cells to TRAIL mediated cell death. (A) Primary lymphoma cells were exposed to GW or treated with DDC (200μΜ), DPI (5μΜ) and PMA (62.5 ng/ml) for 1 hr and whole cell lysates were analyzed for cFLIP expression by Western blot analysis with β-actin as the loading control. (B) Primary lymphoma cells (2 × 106) were treated with TRAIL (25 ng/ml) for 18 hrs in culture medium or GW medium and cell viability was determined by the MTT assay. Data shown are Mean ± S.D. from primary cells derived from 5 different patients and P value (*** <0.005) was calculated by Ordinary one-way ANOVA using GraphPad Prism.
4. Discussion
4.1. Redox regulation of the receptor inhibitory factor cFLIP
Induced expression of cFLIP has been associated with a variety of disease states, most noticeably observed in a host of human cancers [[26], [27], [28]]. While the most significant survival promoting effect of cFLIP is associated with its ability to interfere with death receptor-induced activation of caspase 8, there is also evidence to implicate cFLIP expression with the regulation of other forms of execution, such as necroptosis, and even chemotherapy-induced execution [29]. Therefore, an understanding of cellular mechanisms and/or signaling networks involved in modulating cFLIP levels could be of paramount importance in the therapeutic management of refractory cancers. Results presented in this report provide evidence that cFLIP expression is influenced by the redox milieu of the cell. Interestingly, pharmacological or genetic approaches to attain an increase in intracellular O2•-, such as the use of SOD1 and NOX inhibitors, functional mutants of Rac1 GTPase, PKC activator (PMA), chemical inducers like paraquat and ATN, resulted in a significant increase in cFLIP expression, which correlated with inhibition of CD95-mediated apoptosis. Interestingly, this effect on cFLIP expression seems not to involve events that trigger post-translational modifications of the protein, but instead are mediated at the transcription level. Redox-dependent changes in the expression of cFLIP have previously been reported with experimental evidence for both, upregulation or downregulation, in response to oxidative stress [19,24,30]. A number of these studies point to ROS-induced phosphorylation and ubiquitination, resulting in proteasomal degradation of cFLIP [19], such as also seen upon ligation of TRAIL receptors [30]. In a majority of these scenarios, the evidence points to an effect of an increase in intracellular H2O2, endogenously or exogenously. Contrasting evidence has been reported with nitric oxide (NO) by way of demonstrating nitrosylation-mediated stabilization of cFLIP protein [24]. While the data presented here corroborate the effect of exogenously added H2O2 on the degradation of cFLIP, more importantly they highlight a distinctly opposing effect of increased O2•- on cFLIP, i.e. upregulation of cFLIP transcription. The latter lends credence to our earlier findings linking O2•- to inhibition of apoptotic signaling as well as the critical role that the ratio of intracellular O2•- to H2O2 plays in the context of cancer cell survival and apoptosis sensitivity. It should be pointed out that similarly opposing effects of the two ROS have been reported on the Na+/H+ exchanger, NHE-1 [31] with distinctly different functional outcomes. It is highly plausible that the effect on gene transcription elicited by cellular redox environment influenced by an increase in O2•- (unlike H2O2) is a function of regulatory elements in the upstream non-coding regions that might be shared by functionally unrelated genes. To that end, the large full-length promoter region of cFLIP is a potential binding site for a plethora of transcription factors (TFs), including NF-kB, AP-1, c-Myc, p53, p63, SP1, CREB, Androgen receptor (AR), FOXO3a and NF-AT [[32], [33], [34], [35], [36], [37]]. As these TFs regulate cFLIP expression differently (inducers vs repressors) [[32], [33], [34], [35], [36], [37]], it is highly likely that changes in cellular O2•- affect cFLIP expression by promoting/facilitating the binding of TFs that positively regulate cFLIP transcription (such as NF-kB, AP-1, p63, CREB and AR). Our results demonstrating amplified promoter activity of cFLIP upon an increase in O2•- provide some evidence to that effect; however, a more detailed study is required to delineate the putative TFs or regulatory elements involved in the induction of cFLIP transcription by O2•-. Another possibility could be the methylation of non-coding RNA (miRNA) that regulates cFLIP expression, as shown previously by the ability of the DNMT inhibitor, DZNep, to suppress cFLIP expression by targeting miRNA [38].
4.2. Glucose deprivation reduces cFLIP levels by regulating O2•-
It is almost a century ago that Otto Warburg put forward the hypothesis that tumor cells switch from mitochondrial OXPHOS to aerobic glycolysis to meet their enhanced energy requirements [39]. This is associated with increased glucose influx as well as enhanced glycolytic flux, resulting in the shuttling of pyruvate to lactate. As such, there has been a heightened interest in designing strategies to interfere with glycolysis in an effort to starve cancer cells to execution. To that end, non-metabolizable form of glucose, 2DG, has shown promise in enhancing sensitivity of cancer cells to chemotherapy [40]. We show that exogenous addition of glucose to the culture medium resulted in a sustained increase in intracellular O2•-, while GW (in the presence of pyruvate) was associated with a significant decrease in O2•-. Interestingly, GW resulted in a significant downregulation of cFLIP in O2•--dependent manner and significantly enhanced the sensitivity of cells to apoptosis, irrespective of the expression of Bcl-2. It should be pointed out that other reports have demonstrated the ability of GW to downregulate cFLIP in a redox dependent manner [[41], [42], [43]]; however, these studies implicated intracellular H2O2 as the trigger. Our experimental set up was designed to dissect the role of O2•- and H2O2, and therefore, experiments with GW were conducted in the presence of pyruvate that can scavenge H2O2. We show consistently that GW results in a decrease in O2•- as well as downregulation of cFLIP both in established cell lines and in primary cells derived from patients with lymphomas.
4.3. Concordant expression of cFLIP and Bcl-2 negatively correlates with intracellular O2•- levels
Our previous work has also implicated an increase in intracellular O2•- to receptor- and drug-induced apoptosis resistance upon overexpression of Bcl-2. Alleviating intracellular O2•- restored apoptosis sensitivity of Bcl-2 expression cells via robust activation of caspase 8, thereby suggesting involvement of pathways directly downstream of death receptor ligation [8]. Indeed, we provide evidence that the expression of cFLIP is concordantly upregulated in cells overexpressing Bcl-2. This effect seems also to be at the level of gene transcription as evidenced by mRNA analysis by RT-PCR. Furthermore, down modulating cellular levels of O2•- not only repressed cFLIP expression but also enhanced sensitivity of Bcl-2 expressing cells to death receptor signaling. Similarly, targeting SOD1 by RNAi-mediated gene silencing (to increase O2•-) resulted in an increase in cFLIP expression and apoptosis resistance, while enforced expression of SOD1 (to increase H2O2) had a directly opposite effect on cFLIP promoter activity and expression. These data provide further evidence for the distinctly different effects of O2•- and H2O2 in cell fate determination. Notably, the positive effect of an increase in intracellular O2•- on cFLIP expression appears to involve the intermediacy of OONO−, as evidenced by its negation by OONO- catalytic decomposer, FeTPPS (schematic summary in Fig. 9). The latter is further supported by the ability of exogenously added OONO− to increase cFLIP expression. These data testify to the involvement of signaling network(s) that employ the ready interaction between O2•- and .NO in the face of stimuli that affect an increase in intracellular O2•-. This scenario is highly likely considering the stronger probability of O2•- reacting with .NO rather than its dismutation to H2O2.
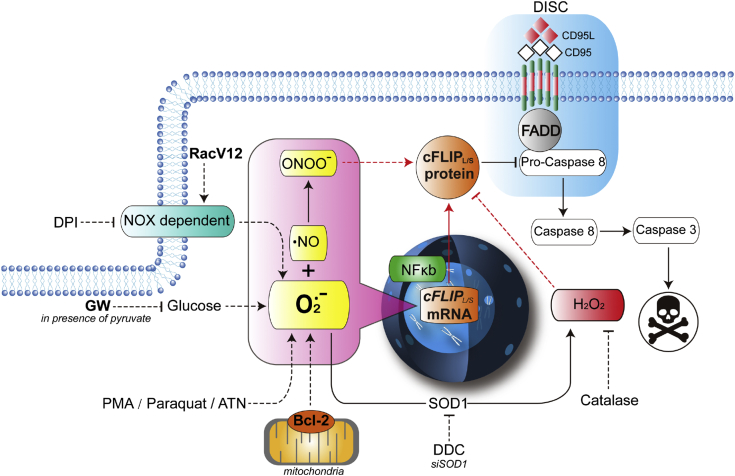
Fig. 9.
A schematic diagram summarizing the results. Ligation of the CD95 (or TRAIL) death receptor triggers DISC assembly and activation of caspase 8 dependent apoptosis, which is blocked by the caspase 8 inhibitory protein cFLIP. Overexpression of Bcl-2 inhibits receptor-mediated apoptosis via its canonical effect on mitochondrial permeability as well as non-canonically by affecting an increase in intracellular O2•-, which is associated with increased transcriptional of cFLIP. Similarly, pharmacological (DDC, PMA, high glucose, paraquat, ATN) or genetic (SOD1 knockdown, active RacV12) means to increase intracellular O2•- induce cFLIP expression, resulting in inhibition of death receptor signaling. Corroborating this, treatment with the NOX inhibitor DPI or GW (in presence of pyruvate) reversed the effect on cFLIP via a significant decrease in O2•-. The positive effect of an increase in O2•- on cFLIP is mediated via its reaction with NO to generate OONO− (rescued by FeTPPS) and is starkly opposite to the effect of H2O2, which downregulates cFLIP.
From the therapeutic standpoint, the significantly enhanced sensitivity to death receptor apoptosis lends support to the idea of tailoring cancer cells’ redox metabolism to significantly improve outcomes. It is relevant to mention here that glycolysis inhibitors, such as metformin, have shown great promise as anti-cancer agents [44]. Similar effects of SOD inducers could be envisioned, given the observations linking an increase in O2•-: H2O2 to apoptosis inhibition; SOD expression would tilt the balance in favor of H2O2 that sensitizes to apoptosis as well as promotes the degradation of cFLIP. To that end, in a recent report we provided evidence to that increase mitochondrial SOD (MnSOD; SOD2) resulted in an increase in the sensitivity of cells to apoptosis [45].
Authorship contribution
SP and MVC conceptualized the study. JH, KS, SS and JQ performed the experiments. SP and MVC interpreted the data and GB performed statistical analyses of the data. TL provided the clinical samples. SP, MVC, LTK, JH and GB wrote the manuscript. This study was funded and supervised by SP.
Declaration of competing interest
The Authors do not have any potential conflict of interest to disclose.
Acknowledgements
This work was supported by a grant from the National Medical Research Council, Singapore (NMRC/1214/2009) to SP.JH is supported by the Experimental Therapeutics program of the Cancer Science Institute (CSI), NUS, Singapore to Dr Goh Boon Cher. The authors would like to thank Dr Goh Boon Cher for supporting JH and Dr. Greg Tucker-Kellogg for valuable suggestions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101403.
Contributor Information
Marie-Veronique Clement, Email: bchmvc@nus.edu.sg.
Shazib Pervaiz, Email: phssp@nus.edu.sg.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pervaiz S. Superoxide anion inhibits drug-induced tumor cell death. FEBS Lett. 1999;459(3):343–348. doi: 10.1016/s0014-5793(99)01258-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad K.A., Clement M.V., Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hydrogen peroxide-induced apoptosis. Ann. N. Y. Acad. Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad K.A. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004;64(4):1452–1459. doi: 10.1158/0008-5472.can-03-2414. [DOI] [PubMed] [Google Scholar]
- 4.Clement M.V., Pervaiz S. Intracellular superoxide and hydrogen peroxide concentrations: a critical balance that determines survival or death. Redox Rep. 2001;6(4):211–214. doi: 10.1179/135100001101536346. [DOI] [PubMed] [Google Scholar]
- 5.Clement M.V., Ponton A., Pervaiz S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Lett. 1998;440(1–2):13–18. doi: 10.1016/s0014-5793(98)01410-0. [DOI] [PubMed] [Google Scholar]
- 6.Pervaiz S., Clement M.V. A permissive apoptotic environment: function of a decrease in intracellular superoxide anion and cytosolic acidification. Biochem. Biophys. Res. Commun. 2002;290(4):1145–1150. doi: 10.1006/bbrc.2001.6274. [DOI] [PubMed] [Google Scholar]
- 7.Pervaiz S. Activation of the RacGTPase inhibits apoptosis in human tumor cells. Oncogene. 2001;20(43):6263–6268. doi: 10.1038/sj.onc.1204840. [DOI] [PubMed] [Google Scholar]
- 8.Clément M.V., Hirpara J.L., Pervaiz S. Decrease in intracellular superoxide sensitizes Bcl-2-overexpressing tumor cells to receptor and drug-induced apoptosis independent of the mitochondria. Cell Death Differ. 2003;10:1273–1285. doi: 10.1038/sj.cdd.4401302. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z.X., Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007;14(9):1617–1627. doi: 10.1038/sj.cdd.4402165. [DOI] [PubMed] [Google Scholar]
- 10.Clement M.V., Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996;15(2):216–225. [PMC free article] [PubMed] [Google Scholar]
- 11.Mellier G., Pervaiz S. The three Rs along the TRAIL: resistance, re-sensitization and reactive oxygen species (ROS) Free Radic. Res. 2012;46(8):996–1003. doi: 10.3109/10715762.2012.690514. [DOI] [PubMed] [Google Scholar]
- 12.Suda T. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 13.Wiley S.R. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 14.Kischkel F.C. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14(22):5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kischkel F.C. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12(6):611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya Y., Nakabayashi O., Nakano H. FLIP the switch: regulation of apoptosis and necroptosis by cFLIP. Int. J. Mol. Sci. 2015;16(12):30321–30341. doi: 10.3390/ijms161226232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thome M. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386(6624):517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 18.Safa A.R. Roles of c-FLIP in apoptosis, necroptosis, and autophagy. J. Carcinog. Mutagen. 2013;(S6:003) doi: 10.4172/2157-2518.S6-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkie-Grantham R.P., Matsuzawa S., Reed J.C. Novel phosphorylation and ubiquitination sites regulate reactive oxygen species-dependent degradation of anti-apoptotic c-FLIP protein. J. Biol. Chem. 2013;288(18):12777–12790. doi: 10.1074/jbc.M112.431320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y. Computational modelling of LY303511 and TRAIL-induced apoptosis suggests dynamic regulation of cFLIP. Bioinformatics. 2013;29(3):347–354. doi: 10.1093/bioinformatics/bts702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad K.A. Hydrogen peroxide-mediated cytosolic acidification is a signal for mitochondrial translocation of Bax during drug-induced apoptosis of tumor cells. Cancer Res. 2004;64(21):7867–7878. doi: 10.1158/0008-5472.CAN-04-0648. [DOI] [PubMed] [Google Scholar]
- 22.Loor G. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010;49(12):1925–1936. doi: 10.1016/j.freeradbiomed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toporik A. Mutational analysis of novel effector domains in Rac1 involved in the activation of nicotinamide adenine dinucleotide phosphate (reduced) oxidase. Biochemistry. 1998;37(20):7147–7156. doi: 10.1021/bi9800404. [DOI] [PubMed] [Google Scholar]
- 24.Wang L. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J. Immunol. 2008;180(5):3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U. S. A. 2018;115(23):5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLornan D. Prognostic and therapeutic relevance of c-FLIP in acute myeloid leukaemia. Br. J. Haematol. 2013;160(2):188–198. doi: 10.1111/bjh.12108. [DOI] [PubMed] [Google Scholar]
- 27.Valnet-Rabier M.B. c-Flip protein expression in Burkitt's lymphomas is associated with a poor clinical outcome. Br. J. Haematol. 2005;128(6):767–773. doi: 10.1111/j.1365-2141.2005.05378.x. [DOI] [PubMed] [Google Scholar]
- 28.Yao Q. Prognostic significance of TRAIL signalling molecules in cervical squamous cell carcinoma. J. Clin. Pathol. 2016;69(2):122–127. doi: 10.1136/jclinpath-2014-202811. [DOI] [PubMed] [Google Scholar]
- 29.Chang D.W. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21(14):3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo B.R. Inhibition of cathepsin S induces mitochondrial ROS that sensitizes TRAIL-mediated apoptosis through p53-mediated downregulation of Bcl-2 and c-FLIP. Antioxidants Redox Signal. 2017;27(4):215–233. doi: 10.1089/ars.2016.6749. [DOI] [PubMed] [Google Scholar]
- 31.Akram S. Reactive oxygen species-mediated regulation of the Na+-H+ exchanger 1 gene expression connects intracellular redox status with cells' sensitivity to death triggers. Cell Death Differ. 2006;13(4):628–641. doi: 10.1038/sj.cdd.4401775. [DOI] [PubMed] [Google Scholar]
- 32.Micheau O. NF-kappaB signals induce the expression of c-FLIP. Mol. Cell. Biol. 2001;21(16):5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun N.K. Damaged DNA-binding protein 2 (DDB2) protects against UV irradiation in human cells and Drosophila. J. Biomed. Sci. 2010;17:27. doi: 10.1186/1423-0127-17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangert A. Histone deacetylase inhibitors sensitize glioblastoma cells to TRAIL-induced apoptosis by c-myc-mediated downregulation of cFLIP. Oncogene. 2012;31(44):4677–4688. doi: 10.1038/onc.2011.614. [DOI] [PubMed] [Google Scholar]
- 35.Gates L.T., Shisler J.L. cFLIPL interrupts IRF3-CBP-DNA interactions to inhibit IRF3-driven transcription. J. Immunol. 2016;197(3):923–933. doi: 10.4049/jimmunol.1502611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safa A.R., c-FLIP A master anti-apoptotic regulator. Exp. Oncol. 2012;34(3):176–184. [PMC free article] [PubMed] [Google Scholar]
- 37.Ueffing N. Up-regulation of c-FLIP short by NFAT contributes to apoptosis resistance of short-term activated T cells. Blood. 2008;112(3):690–698. doi: 10.1182/blood-2008-02-141382. [DOI] [PubMed] [Google Scholar]
- 38.Braun F.K. Inhibition of methyltransferases accelerates degradation of cFLIP and sensitizes B-cell lymphoma cells to TRAIL-induced apoptosis. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.W., Dang C.V. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66(18):8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 40.Halicka H.D. 2-Deoxy-D-glucose enhances sensitivity of human histiocytic lymphoma U937 cells to apoptosis induced by tumor necrosis factor. Cancer Res. 1995;55(2):444–449. [PubMed] [Google Scholar]
- 41.Munoz-Pinedo C. Inhibition of glucose metabolism sensitizes tumor cells to death receptor-triggered apoptosis through enhancement of death-inducing signaling complex formation and apical procaspase-8 processing. J. Biol. Chem. 2003;278(15):12759–12768. doi: 10.1074/jbc.M212392200. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y. Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J. Cell Mol. Med. 2003;7(1):49–56. doi: 10.1111/j.1582-4934.2003.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y.J. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J. Biol. Chem. 1998;273(9):5294–5299. doi: 10.1074/jbc.273.9.5294. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang Y. The novel function of tumor protein D54 in regulating pyruvate dehydrogenase and metformin cytotoxicity in breast cancer. Cancer Metabol. 2019;7:1. doi: 10.1186/s40170-018-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loo S.Y. Manganese superoxide dismutase expression regulates the switch between an epithelial and a mesenchymal-like phenotype in breast carcinoma. Antioxidants Redox Signal. 2016;25(6):283–299. doi: 10.1089/ars.2015.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirpara J. Aberrant localization of apoptosis protease activating factor-1 in lipid raft sub-domains of diffuse large B cell lymphomas. Oncotarget. 2016;7(51):83964–83975. doi: 10.18632/oncotarget.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.