Highlights
-
•
PTSD is associated with lower recognition performance for scene images.
-
•
PTSD is associated with reduced suppression of beta power during encoding in memory.
-
•
Beta power suppression during encoding in memory predicts subsequent recognition.
-
•
Low beta power suppression may reflect the presence of interfering memories in PTSD.
Keywords: Post-traumatic stress disorder, Memory encoding, Beta band oscillations, Magnetoencephalography
Abstract
We studied the relationship between electrophysiological markers of memory encoding, subsequent recognition performance, and severity of PTSD symptoms in service members with combat exposure (n = 40, age: 41.2 ± 7.2 years) and various levels of PTSD symptom severity assessed using the PTSD Check List for DSM V version (PCL-5). Brain activity was recorded using magnetoencephalography during a serial presentation of 86 images of outdoor scenes that were studied by participants for an upcoming recognition test. In a second session, the original images were shown intermixed with an equal number of novel images while participants performed the recognition task. Participants recognized 76.0% ± 12.1% of the original images and correctly categorized as novel 89.9% ± 7.0% of the novel images. A negative correlation was present between PCL-5 scores and discrimination performance (Spearman rs = –0.38, p = 0.016). PCL-5 scores were also negatively correlated with the recognition accuracy for original images (rs = –0.37, p = 0.02). Increases in theta and gamma power and decreases in alpha and beta power were observed over distributed brain networks during memory encoding. Higher PCL-5 scores were associated with less suppression of beta band power in bilateral ventral and medial temporal regions and in the left orbitofrontal cortex. These regions also showed positive correlations between the magnitude of suppression of beta power during encoding and subsequent recognition accuracy. These findings indicate that the lower recognition performance in participants with greater PTSD symptom severity may be due in part to ineffective encoding reflected in altered modulation of beta band oscillatory activity.
1. Introduction
An estimated 5–10% of the general population develop post-traumatic stress disorder (PTSD) symptoms after experiencing psychologically traumatic events (Kessler et al., 2005). These symptoms include re-experiencing of the traumatic event through intrusive thoughts, nightmares or flashbacks, emotional distress and physical reactivity to reminders of the traumatic event, emotional numbing, irritability, and difficulty sleeping and concentrating (Diagnostic and Statistical Manual of Mental Disorders DSM-5, American Psychiatric Association, 2013). The prevalence of PTSD is relatively high among individuals with military combat exposure: approximately 14% of veterans from Operation Enduring Freedom and Operation Iraqi Freedom have been diagnosed with PTSD (Schell and Marshall 2008), and the lifetime prevalence of PTSD among Vietnam War veterans has been estimated at about 19% (Dohrenwend et al., 2006). The PTSD symptoms are accompanied by lower performance on neuropsychological tests of attention, memory and executive function (e.g. Vasterling et al., 1998; Bremner et al., 2004; Yehuda et al., 2005; Aupperle et al., 2012; Scott et al., 2015). A better understanding of the neurophysiological mechanisms responsible for this association is important because some of these cognitive impairments are believed to contribute to or exacerbate the core emotional and arousal symptoms of PTSD (Aupperle et al., 2012) and may reduce the effectiveness of cognitive-behavioral therapy for PTSD (Falconer et al., 2013). In particular, memory impairment in PTSD has been an issue of considerable interest. Besides the phenomenon of vivid intrusive trauma memories that are disabling and difficult to voluntarily suppress, the episodic memory of the index traumatic event is highly fragmented and poorly recalled (Brewin, 2001) and the declarative memory for trauma-unrelated and emotionally neutral information is impaired (Gilbertson et al., 2001; Vasterling et al., 2002; Brewin et al., 2007; Guez et al., 2011). A declarative memory impairment is a prominent component feature of a subgroup of PTSD patients that are treatment resistant (Etkin et al., 2019). The memory deficit in PTSD is often attributed to hippocampal atrophy (Kitayama et al., 2005), as well as to a chronically elevated level of corticotropin releasing factor (CRF), which is detrimental to hippocampus mediated memory encoding (Bangasser and Kawasumi, 2015). However, these structural and neuroendocrine mechanisms do not appear to fully account for the memory impairments seen in PTSD. For example, the declarative memory deficit only modestly correlates with the degree of hippocampal atrophy and children with PTSD do not to exhibit hippocampal atrophy despite having declarative memory deficits (Kitayama et al., 2005). The possible role of altered brain oscillatory dynamics as a mechanistic basis for impaired memory encoding in PTSD has not been investigated, though the important role of such oscillations in memory function in healthy populations has been a topic of intense interest.
Accumulating evidence from electrophysiological studies suggests that brain function relies on a dynamic formation of local neuronal assemblies based on the temporal structure of neuronal spiking and synchronization of oscillatory local field potentials, and on the selection of multiple local assemblies into distributed networks by feedforward and feedback connections (Buzsáki, 2010). Functional cell assemblies oscillating at a broad range of frequencies are continuously forming and dissolving on a timescale of hundreds of milliseconds in order to meet the demands of ongoing brain activity (Breakspear et al., 2004). Electrophysiological studies in healthy individuals and in patients with various pathologies have shown that cognitive processing is associated with measurable modulations of oscillatory activity in specific frequency bands (Axmacher et al., 2006; Düzel et al., 2010; Hanslmayer et al., 2012). Furthermore, impaired cognitive function is related to altered modulation of the oscillatory brain activity in certain neurological disorders (Uhlhaas and Singer, 2006). It is therefore plausible that some alterations in brain oscillatory activity may represent one factor linking PTSD to altered cognitive performance. Evidence in support of this hypothesis comes from studies that reported abnormal oscillatory activity in patients with PTSD in resting-state (Huang et al., 2014; Misić et al., 2016; Popescu et al., 2016) or during cognitive tasks (Khana et al., 2017; Waldhauser et al., 2018; Popescu et al., 2019). When they are due to genetic and developmental factors or to the exposure to psychological trauma (acute stress), such alterations in oscillatory activity may represent a risk factor for PTSD symptoms; they may also be caused or exacerbated by the presence of some PTSD symptoms.
In this study, we use magnetoencephalography (MEG) to investigate the relationship between electrophysiological markers of memory encoding, subsequent recognition performance, and severity of PTSD symptoms in service members with combat exposure. The participants in our study had PTSD symptom severity scores spanning a wide range, from very low scores which would not lead to a diagnosis of PTSD to very high scores which would fulfill the criteria for a PTSD diagnosis; the inclusion of participants with low PTSD symptom severity serve to control for trauma exposure in our analysis. We focused our study on memory encoding because it has been suggested that memory deficits in PTSD may be due, at least in part, to difficulties at the time of the encoding (Jenkins et al., 2000; Brandes et al., 2002; Dickie et al., 2008). We used the subsequent memory paradigm (Sanquist et al., 1980) in which brain activity during encoding in memory is analyzed with respect to performance on a following recognition test. Previous electrophysiological studies that used this paradigm have shown a distinct pattern of modulation of the oscillatory activity in multiple frequency bands during encoding of information that is later remembered compared to information that is forgotten. These studies have typically reported that brain oscillatory activity during encoding of items that are subsequently remembered is characterized by relative increases in power for theta and gamma bands (Sederberg et al., 2003, 2007; Osipova et al., 2006; a review is available in Düzel et al., 2010) and/or decreases in power for alpha and beta bands (Klimesch et al., 1996; Sederberg et al., 2003, 2007; a review is available in Hanslmayer et al., 2012). Most of these studies used visually-presented words as items to be remembered. In our study, we use images of outdoor scenes, which are expected to activate multiple neuronal representations along the ventral visual processing pathway, with posterior representations for attributes of individual scene components and more anterior representations for configural/relational associations between scene elements (Epstein and Kanwisher, 1998; Taylor et al., 2007; Kamps et al., 2016). Notably, some of the brain regions involved in processing associations for scene elements, such as the parahippocampal cortex, may also play a general role in encoding contextual associations in episodic memories (Aminoff et al., 2013). This is relevant for PTSD, since theoretical models have proposed that a poor contextual embedding of the visual memories of the traumatic event can facilitate future intrusive memories of the trauma (Brewin et al., 2010; Meyer et al., 2013, 2017). The associative memory dysfunction in PTSD appears to extend to trauma-unrelated and emotionally neutral information (Guez et al., 2011). It is conceivable that such a deficit in associative/contextual memory in PTSD may be due in part to inefficient encoding, which can be manifested in alterations of oscillatory brain activity in visual processing regions that are also essential for the representation of spatial configuration of scene elements. In our study, we use MEG source estimation methods to determine if PTSD is associated with altered modulation of regional frequency-specific oscillatory activity during memory encoding of scenes, with particular interest in the activity of ventral visual processing regions that are involved in the encoding of associative/contextual memories, and whether this altered modulation is predictive of lower subsequent recognition performance.
2. Methods
2.1. Participants
Study participants (n = 43, all males) were active-duty service members enrolled in a four-week interdisciplinary intensive outpatient program for patients with post-concussive and post-traumatic psychological health symptoms at the National Intrepid Center of Excellence (NICoE), Walter Reed National Military Medical Center, who completed all sessions of this study. Patients were not included in this study if they had a history of moderate or severe TBI or other neurological, developmental or psychiatric disorders such as stroke, epilepsy, bipolar disorder, etc. The study was approved by the Institutional Review Board of the Walter Reed National Military Medical Center in compliance with all applicable federal regulations governing the protection of human subjects. Informed consent was obtained from each participant before participation in the study.
All participants completed the PTSD Check List for DSM V version (PCL-5), which is a 20-item self-report scale used to screen individuals for PTSD symptom severity and to aid in the diagnostic assessment of PTSD (Bovin et al., 2015; Blevins et al., 2015). Individual PCL-5 items ask about symptoms of re-experiencing, avoidance, numbing and hyperarousal elicited by stressful experiences and are rated on a scale from 0 to 4 (the total score ranges from 0 to 80, with higher values indicating higher symptom severity). The psychologically traumatic events experienced by participants in the study included highly emotional combat-related experiences, such as receiving incoming artillery, rocket, or mortar fire, seeing seriously injured bodies, seeing or handling human remains, etc. The exposure to combat-related experiences generally occurred over an extended period of time, during which all participants have also experienced mild traumatic brain injuries (mTBI), defined according to the standard criteria (American Congress of Rehabilitation Medicine, 1993). PTSD is more prevalent in service members or civilians with a history of mTBI (Hoge et al., 2008; Schneiderman et al., 2008; Bryant et al., 2009, 2010; Yurgil et al., 2014; Stein et al., 2015).
Participants completed also the Patient Health Questionnaire PHQ-9, which is a brief 9-item depression severity measure with total score range from 0 to 27 (Spitzer et al., 1999; Kroenke et al., 2001) and the AUDIT-C alcohol consumption screening test (Bush et al., 1998), with total score between 0 and 12. The Wechsler Adult Intelligence Scale (WAIS-IV, Wechsler Adult Intelligence Scale - Fourth Edition, 2008) was administered to the participants by a clinical neuropsychologist as part of their clinical evaluation at the NICoE.
Three participants had to be excluded from the analysis because they had MEG data contaminated by large movement artifacts. Across the remaining participants (n = 40, age 41.2 ± 7.2 years), the mean PCL-5 score was 26.0 ± 18.0, with individual scores ranging from 2 to 71. Thirteen participants (32.5%) had PCL-5 scores lower than 16, fifteen participants (37.5%) had PCL-5 scores in a moderate range between 16 and 32, and twelve participants (30%) had PCL-5 scores equal or higher than 33. Twelve participants met the DSM-V criteria for PTSD, which requires ratings of moderate (2) or above on at least one item in each of the (B) and (C) clusters, and at least two items in each of the (D) and (E) clusters of the PCL-5.
No significant correlations were present between PCL-5 scores and age, education, AUDIT-3 scores and full scale IQ (Table 1). Two participants had PHQ-9 scores in the range of severe depression (PHQ-9 scores greater than 20). The severity of depressive symptoms was not used as an exclusion criterion for this study given that PTSD and depression share a series of symptoms (anhedonia, sleep disturbances, difficulties concentrating) that are probed by both the PCL-5 and PHQ-9 scales. Twenty participants had a history of mTBI without loss of consciousness (LOC) and twenty participants had at least one mTBI with LOC. The difference between the mean PCL-5 scores for the subgroup of participants who had injuries with LOC (mean PCL-5 score = 29.0 ± 17.6) versus the subgroup of participants who had injuries without LOC (mean PCL-5 score = 22.9 ± 18.4) was not statistically significant (Mann–Whitney test: u = 147, z = 1.4, p = 0.16).
Table 1.
Demographic, screening and neuropsychological data (mean values and standard deviations) and their correlation with PCL-5 scores.
| Mean ± std | Correlation with PCL-5 scores | ||
|---|---|---|---|
| Spearman r | p | ||
| Age (years) | 41.2 ± 7.2 | r=−0.12 | p = 0.46 |
| Education (years) | 15.0 ± 2.3 | r=−0.02 | p = 0.9 |
| AUDIT-C | 3.1 ± 1.8 | r=−0.02 | p = 0.9 |
| Full scale IQ* | 114.1 ± 11.9 | r=−0.17 | p = 0.35 |
Full scale IQ information was available for 33 participants (83% of the total).
Participants were not excluded from the study based on their use of medications with central nervous system effects. Eight participants were taking antidepressant medication at the time of the MEG recording (Sertraline-4, Fluoxetine-1, Duloxetine-1, Venlafaxine-1, Bupropion hydrochloride-1). Additionally, thirteen participants were taking anticonvulsant medications for headache prophylaxis (Gabapentin). Four patients were taking Prazosin as a treatment for nightmares. Some participants were taking multiple medications (the total number of participants taking at least one type of medication from the categories described above was nineteen). The distribution of PCL-5 scores was not significantly different between non-medicated versus medicated participants (Mann-Whitney test: u = 253, z=−1.4, p = 0.15). None of the participants in this study, who were all active duty service members, had a history of use or abuse of recreational drugs.
2.2. Experimental paradigm
The experiment consisted of two sessions. In the first (memory encoding) session, MEG recordings were performed while participants were shown a series of 86 color images of outdoor scenes (list 1) presented using the Neuroscan Stim2 software (Compumedics Neuroscan, El Paso, TX, USA) on a screen positioned at 95 cm in front of the participants. Each image had a size of 256 × 256 pixels, subtending a visual angle of 8.1°, and was shown for 3 s in the center of the screen, upon a grey background. The inter-trial interval from the offset of one image to the onset of the next image was 1.5 s. Images were selected from eight different categories included in the urban and natural scene image database developed at the Computational Visual Cognition Laboratory, MIT (Oliva and Torralba, 2001). These categories were: mountain (10 images), forest (11), coast (11), open country (12), inside city (17), highway (4), street (10) and tall buildings (11). Some of the selected scene exemplars within each of these categories contained similar objects and color schemes, while others were more easily identifiable based on the distinctive details of some objects and/or color schemes. Participants were instructed to study each image for a later (recognition) test. The first session lasted for approximately 7 min.
In the second (subsequent recognition) session, participants were shown the images from list 1 (referred to as original images) randomly intermixed with a set of 86 novel images. The number of novel images in each category matched the number of original images in that category. In this second session, participants were asked to respond by pressing one button to indicate whether they recognized an image from list 1 and another button in response to novel images. The recognition session followed approximately 5 min after the encoding session. MEG data were also recorded during the recognition session (the corresponding analysis and results are intended to be presented in a subsequent report focusing on electrophysiological markers of visual recognition and their potential relationship to PTSD).
2.3. MEG data acquisition and pre-processing
MEG signals were recorded inside a magnetically-shielded room using the Elekta VectorView™ whole-head MEG system (Elekta- Neuromag, Helsinki, Finland) with 102 triplet-sensors (each made of one magnetometer and two orthogonal planar gradiometers). The head position relative to the sensors was determined with four localization coils attached to the participant's head. The locations of three fiduciary points (nasion, and left and right auricular points) defining the head-frame coordinate system, together with the location of the four localization coils and of a set of head surface points were digitized with a 3D Fastrak digitizer (Polhemus, Colchester, VT, USA) to co-register the MEG data with T1-weighted MRI data acquired in a separate session with a 3T MRI scanner (General Electric, Milwaukee, WI). MEG data were acquired with 1 kHz sampling rate.
Data recorded in the first session were band-pass filtered off-line between 1 Hz and 100 Hz, with a powerline filter at 60 Hz. Filtering was done using frequency domain, zero-phase and zero-delay finite impulse response (FIR) filters implemented in the MNE software (Gramfort et al., 2014). Data were subsequently processed using the Independent Component Analysis (ICA) Infomax algorithm (EEGLAB, Delorme and Makeig, 2004). Independent components corresponding to cardiac and eye movements/blinks interferences, as well as other sources of external artifacts (if any) were removed. The mean power of the cardiac and eye movements/blinks signals, each determined separately across magnetometer and gradiometer sensors, was not correlated with the PCL-5 scores (all Spearman correlations rs < 0.112, all ps > 0.489). The filtered datasets were divided into epochs from −1200 ms to 4000 ms relative to the onset of the images and band-pass filtered in theta (4–7 Hz), alpha (7.5–13 Hz), beta (14–30 Hz) and gamma (35–100 Hz) bands. At this step, band-pass filtering was done using the frequency domain, zero-phase and zero-delay FIR filters implemented in the Brainstorm software (Tadel et al., 2011). The source reconstruction was performed separately for signals filtered in each of these frequency bands.
2.4. Source reconstruction
The cortical surface was determined from the T1-weighted MR images of each participant using the FreeSurfer image analysis software (http://surfer.nmr.mgh.harvard.edu). The source reconstruction was done using the Brainstorm software (Tadel et al., 2011). Current sources were estimated at 10,000 cortical locations using a minimum norm estimator (Hämäläinen and Ilmoniemi, 1994) and a multiple sphere model of the volume conductor. Cortical currents with unconstrained orientation were estimated using a depth weighting parameter of 0.5 and were subsequently projected on the averaged FreeSurfer template brain. For the analysis in each frequency-band, the inverse projection operator incorporated a diagonal noise-covariance matrix derived from 1 min long empty room noise recordings. The noise-covariance matrix was computed after filtering the empty-room noise recordings in the corresponding frequency band. The power of the reconstructed currents was spatially integrated in each of the 84 cortical regions of a modified Desikan-Killiany anatomical atlas (Desikan et al., 2006). The original Desikan-Killiany atlas with 68 regions was refined by dividing several regions of relatively large area into smaller, functionally more specific sub-regions (details are available in Popescu et al., 2017). The regional power estimated at every time sample in each trial was used to determine the averaged power across trials on the temporal interval from 0.3 s (to exclude the early evoked response) to 3 s. Subsequently, the mean regional power computed across all trials in each frequency band was transformed into percent change relative to a baseline interval from −0.7 s to −0.2 s with respect to the image onset.
2.5. Statistical analysis
The correlation between PCL-5 scores and behavioral performance was evaluated using Spearman rank correlation tests. For the first analysis of the MEG data, we used one-sample t-tests to determine if the relative changes in regional power in each frequency band over the temporal interval from 0.3 s to 3 s with respect to the baseline interval are significantly different than zero across the sample of participants. Statistical significance was determined by controlling the false discovery rate (FDR) at 0.05 (to account for multiple comparisons across 84 brain regions). To investigate the brain activity predictive of subsequent recognition ability, we evaluated the Spearman correlation between recognition performance and regional relative change in power in each frequency band over the temporal interval from 0.3 s to 3 s (with respect to the image onset). We evaluated also the Spearman correlations between the PCL-5 scores and the regional change in power to determine if the PTSD symptom severity is associated with any changes in brain oscillatory activity during memory encoding. Since all participants included in the study had a history of mTBI and combat exposure, for this correlation analysis the participants with low PTSD symptom severity (who would not be diagnosed with PTSD) control for the history of mTBI and combat exposure and any effect these two factors might have on the brain oscillatory activity. For each of these tests, statistical significance was determined by controlling the FDR at 0.05.
3. Results
3.1. Behavioral performance
Participants recognized on average 76.0% ± 12.1% of the original images (range between 46.5% and 95.3%) and correctly categorized as novel 89.9% ± 7.0% of the novel images (range between 69.8% and 100%). A significant negative correlation was present between PCL-5 scores and the discrimination performance as characterized by the cumulative correct responses to original and novel images (rs = −0.38, p = 0.016), indicating lower discriminability for participants with higher PTSD symptom severity. The PCL-5 scores showed also a significant negative correlation with the number of correct responses to original images (rs = −0.37, p = 0.02), indicating lower recognition accuracy for participants with higher PTSD symptom severity. The correlation between PCL-5 scores and the number of correct responses to novel images was not statistically significant (rs = −0.23, p = 0.16).
The mean reaction times were 1145 ms ± 297 ms for correct responses to original images, and 1230 ms ± 333 ms for correct responses to novel images. No significant correlations were present between PCL-5 scores and mean reaction times to original (rs = −0.10, p = 0.52) or novel images (rs = 0.04, p = 0.79).
3.2. Cortical oscillatory activity during memory encoding
Results of the t-tests comparing the relative change in power against baseline are shown for each frequency band in Fig. 1. For the theta band, significant increases in power were present bilaterally in regions of the occipital lobe, medial and lateral temporal lobe, in the insular cortex, anterior middle and superior frontal gyri, frontal pole and orbitofrontal cortex. Increases in theta band power were also present in the posterior part of the right superior parietal region. A decrease in theta band power was present over the right anterior parietal cortex and right postcentral gyrus. Significant reductions in alpha and beta band power were present over extended networks including regions of the occipital, parietal, medial and lateral temporal lobes, cingulate gyrus, insular cortex and orbitofrontal cortex. Dorso-lateral prefrontal regions showed a decrease only in beta power, which was significant in more prefrontal regions of the left hemisphere than of the right hemisphere. Significant increases in gamma power were localized bilaterally in occipital regions and in the right posterior region of the superior parietal cortex.
Fig. 1.
Results of the t-tests comparing the relative change in power during the temporal window from 0.3 s to 3 s against baseline are shown for theta (a), alpha (b), beta (c), and gamma bands (d). Statistical maps (t-values) show only regions with significant results after controlling the FDR. Maps are shown for each frequency band in lateral (upper row) and medial views (lower row) of the two hemispheres.
The variation of the relative change in power over a finer temporal scale (exemplified in Fig. 2 for the lateral occipital and parahippocampal regions of the left hemisphere) allows making several observations. The relative change in power has a generally higher magnitude for posterior regions compared to anterior temporal or frontal regions (reflected in the difference in values on the vertical axis between lateral occipital and parahippocampal regions in Fig. 2). After a transient change immediately following the stimulus onset (during the interval of the early evoked response), the power in each frequency band shows a rather stationary behavior for the duration of the images. After the image offset, the theta power progressively decreases to its baseline level and the alpha power progressively increases to its baseline level. The beta power first rebounds to levels exceeding the baseline level prior to returning to its baseline level. The increase in gamma band power in lateral occipital regions (which was significant compared to its baseline) was very small, in the range of 2%. This suggests that it is possible that increases in gamma power could be present but might be too weak to be detected in some other brain regions more remote from the MEG sensors (such as more anterior temporal and medial temporal regions).
Fig. 2.
Temporal variation of the relative change in power is exemplified for the lateral occipital and parahippocampal regions of the left hemisphere. Power was integrated over 250 ms intervals centered at each time sample. The vertical dotted lines mark the temporal interval from 0.3 s to 3 s used to integrate the power for the primary analysis.
3.3. Correlations between cortical oscillatory activity, PTSD symptom severity and subsequent recognition accuracy
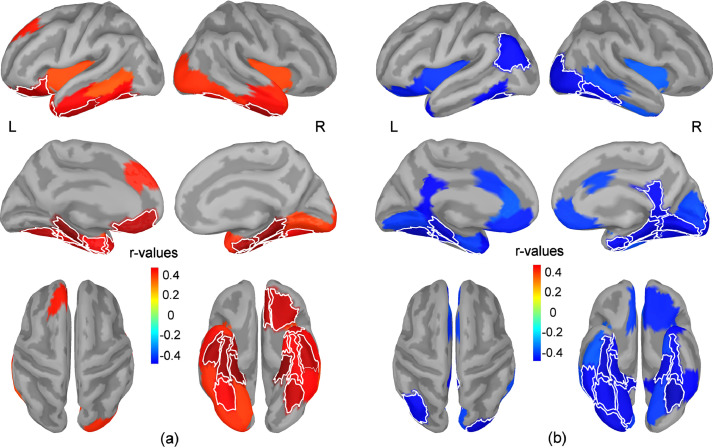
Significant positive correlations were found between PCL-5 scores and regional relative change in beta band power (i.e. higher PCL-5 scores were associated with less suppression of beta band power relative to the baseline interval) bilaterally in the fusiform (anterior and posterior parts) and parahippocampal gyri, entorhinal cortex, and the anterior part of the inferior temporal gyrus (Fig. 3, a). Significant positive correlations were also found in the medial and lateral orbitofrontal cortex and the temporal pole of the left hemisphere. These results indicate an altered suppression of beta-band oscillations in these regions in participants with elevated PTSD symptom severity. No other significant correlations were found between the PCL-5 scores and the regional relative power in theta, alpha and gamma bands.
Fig. 3.
Maps of correlation coefficients between the regional change in beta band power and PCL-5 scores (a), and between the regional relative change in beta band power and recognition performance for original images (b). The regional relative change in beta band power was estimated during the temporal window from 0.3 s to 3 s. Maps show all regions with correlations significant at p<0.05 uncorrected for multiple comparisons (blue colors indicate negative correlations while red colors indicate positive correlations). The thin white lines mark the borders for brain regions with correlations that are significant after adjusting to control the FDR. Maps are shown in lateral (upper row) and medial views (middle row) of the two hemispheres, as well as in top and bottom views of the brain (lower row).
Significant negative correlations were found between the recognition accuracy for original images and the relative change in beta power during encoding in bilateral regions of the fusiform (anterior and posterior parts) and parahippocampal gyri. Significant negative correlations were also present in the inferior parietal cortex of the left hemisphere, and in the lateral occipital cortex, lingual cortex, entorhinal cortex, cingulate isthmus and posterior part of the inferior temporal gyri of the right hemisphere (Fig. 3, b). These results indicate that inefficient suppression of beta-band oscillations in these regions while viewing the images result in lower performance during the subsequent recognition of the original images. No other significant correlations were found between the recognition accuracy for original images and the regional relative power in theta, alpha and gamma bands. Table 2 summarizes the brain regions showing significant correlations between the change in beta band power and both PCL-5 scores and behavioral performance, along with the corresponding correlation coefficients and p-values.
Table 2.
Brain regions showing significant Spearman correlations between the change in beta power and PCL-5 scores, as well as between the change in beta power and recognition accuracy.
| region | Correlation with PCL-5 | Correlation with recognition accuracy | ||
|---|---|---|---|---|
| rs | p | rs | p | |
| Right | ||||
| Parahippocampal | 0.53 | 0.0005 | −0.46 | 0.0027 |
| Posterior fusiform | 0.43 | 0.0061 | −0.44 | 0.0046 |
| Anterior fusiform | 0.52 | 0.0006 | −0.48 | 0.0018 |
| Entorhinal | 0.51 | 0.0008 | −0.43 | 0.0057 |
| Left | ||||
| Parahippocampal | 0.54 | 0.0004 | −0.46 | 0.0027 |
| Posterior fusiform | 0.47 | 0.0021 | −0.49 | 0.0014 |
| Anterior fusiform | 0.43 | 0.0053 | −0.45 | 0.0039 |
The temporal variation in beta band power for regions of the left and right parahippocampal cortex (i.e. two brain regions that showed significant correlations between relative beta band power and both PCL-5 scores and recognition performance) is exemplified in Fig. 4 for subgroups of participants with high and low recognition accuracy, and high and low PCL-5 scores, respectively. For each of these subgroups, the mean beta band power shows a relative decrease throughout the entire time interval when images are on the screen, followed by a transient increase (rebound) after the image offset. Participants with high PTSD symptom severity show less suppression of beta power during the whole interval when images are on the screen. In a subsequent analysis, we investigated if the relative change in beta band power on the temporal window between 3.3 s and 3.8 s (i.e. the temporal interval after the image offset when the beta power rebound is present) is correlated with the subsequent recognition performance or with the PCL-5 scores, respectively, and found no statistically significant correlations in any brain region.
Fig. 4.
The mean change in beta band power for subgroups of patients with high (n = 21) vs. low (n = 19) recognition accuracy, and high (n = 21) vs. low (n = 19) PCL-5 scores, respectively, is exemplified in right and left parahippocampal cortex. The relative power is integrated over 250 ms intervals centered at each time sample. Participants were assigned to subgroups using a median split with respect to recognition accuracy (median value=75.7%) and PCL-5 scores (median value=20), respectively. Thirteen patients with low PCL5 scores were assigned to the high recognition accuracy subgroup.
In the previous analyses, we investigated the change in beta band power relative to the baseline interval to mitigate potential effects due to the inter-subject variability in absolute signal power that may be due to factors unrelated to brain electrophysiology. A follow-up analysis conducted on the absolute beta power within the temporal windows from 0.3 s to 3 s and from 3.3 s to 3.8 s, respectively, showed no significant correlations with PCL-5 scores or behavioral performance.
3.4. Single-trial analysis of beta band power
Our results indicate that a reduced suppression of beta band activity relative to the pre-stimulus interval that occurs during encoding in memory is predictive of lower overall performance for subsequent recognition. The regional beta power measured at macroscopic level by MEG could reflect activity generated in neuronal ensembles involved in the encoding of the current image, in neuronal ensembles involved in the maintenance of memory representations of previous images in the sequence of stimuli, and in neuronal ensembles that perform functions that are not directly related with the task. Taking into account that the recognition performance was estimated over the whole series of images presented in the first session and the neuronal representations of images (particularly those from the same category) are prone to forward-acting and backward-acting interference from each other, the relatively high beta band oscillatory power associated with lower subsequent performance can be interpreted in several possible ways: (1) high beta band activity may reflect an inefficient encoding of images due to endogenous transient (re)activation of stored content representations not directly related to the task (e.g. possibly related with the state of the participants) that may interfere with the active processing of the image to be memorized; (2) high beta band activity may reflect in part a forward-acting interference due to the persistence of activity in neuronal ensembles that were recruited during the encoding of previously presented images in the sequence, impeding the effective encoding of following images and thus decreasing the overall recognition performance; and (3) high beta band activity may reflect an efficient way of encoding an image, but at the cost of overwriting previously stored neuronal representations of other images (e.g. through backward-acting memory interference), leading to a lower overall subsequent recognition performance.
Results from previous studies that investigated subsequent memory effects in beta band (SME, reviewed in Hanslmayer et al., 2012) by comparing the neuronal activity elicited during encoding by subsequently remembered versus subsequently forgotten items provided evidence against scenario 3. To search for additional evidence in our own data, we investigated in a follow-up within-subject analysis whether the rate of correct responses differs between trials (images) with high versus low absolute beta power recorded from the same subject. The trials were first sorted based on the absolute beta power values in each brain region and then the rates of correct responses between the set of 30 trials with the lowest beta power (approx. one third of the total number of trials) and the set of 30 trials with highest beta power were compared across subjects using a Wilcoxon signed-rank test. We note that: 1) this analysis is not affected by inter-subject variability in absolute signal power that is due to factors unrelated to the brain electrophysiology because the regional absolute beta power is used only to sort the set of trials (images) from the same subject, and 2) the sorting of trials is specific for each brain region.
For the temporal window from 0.3 s to 3 s, this analysis showed that higher subsequent recognition rates were present for trials with low absolute beta power in visual processing regions from the bilateral occipital and posterior-temporal lobes, including the lateral occipital, pericalcarine, lingual and posterior part of the fusiform gyrus, and in the left cuneus. Small effects (significant at p<0.05 uncorrected for multiple comparisons) were also observed in the bilateral cingulate isthmus, right cuneus, precuneus, parahippocampal and superior parietal cortex and in the left pars triangularis (Fig. 5, a). These results indicate that a decrease in beta band power in regions of the visual processing network during image encoding predicts a successful subsequent recognition of the image. Furthermore, they show a strong effect of the inter-trials variability in beta–band power on the recognition rate in posterior (occipital) regions, and a smaller effect in more anterior medial temporal regions (parahippocampal gyrus) and in the left inferior frontal gyrus.
Fig. 5.
Results of Wilcoxon signed-rank tests carried out across subjects to compare the rate of correct responses for sets of trials with highest versus lowest regional beta power integrated in temporal windows between 0.3 s to 3 s (a) and 3.3 s to 3.8 s (b), respectively. Maps of z-values show regions that were significant at p<0.05 uncorrected for multiple comparisons. The thin white lines mark the borders for brain regions with z-values that are significant after adjusting to control the FDR. Positive z-values (red colors) indicate a higher rate of correct responses for trials with highest regional beta power, while negative z-values (blue colors) indicate a higher rate of correct responses for trials with lowest regional beta power. Maps are shown in lateral (upper row) and medial views (middle row) of the two hemispheres, as well as in top and bottom views of the brain (lower row).
A similar analysis was also conducted for a temporal window from 3.3 s to 3.8 s to ascertain the possible effects of the rebound in beta power immediately following the image offset on the subsequent recognition of that image. For this temporal window, higher recognition rates for trials with low absolute beta power was present for several prefrontal regions, including the left rostral middle frontal, pars opercularis, pars triangularis and pars orbitalis, and the right frontal pole. An inverse relationship, reflecting higher subsequent recognition rates for trials with high absolute beta power was observed for bilateral cortical regions of the occipital and parietal lobes, including the lateral occipital, pericalcarine, lingual, cuneus, precuneus, and the superior and inferior parietal cortex (Fig. 5, b). These results show that a relatively higher beta power in posterior visual processing regions after an image goes off the screen may facilitate the subsequent recognition of the image, providing support to the hypothesis that a relative increase in beta band power may reflect at least in part the persistence of oscillatory activity in some neuronal ensembles recruited during the encoding of images, serving a short-term memory role in the absence of concurrent visual input.
4. Discussion
Our study characterized the frequency-specific modulation of oscillatory brain activity during memory encoding and its effects on subsequent recognition performance that are related to the presence and severity of PTSD symptoms. In our sample of participants with combat exposure, we primarily observed increases in theta and gamma power and decreases in alpha and beta power over distributed brain networks during memory encoding. These results confirm and also extend the findings of previous studies that investigated subsequent memory effects (SME) in healthy participants or in other categories of patients (reviewed in Düzel et al., 2010; Hanslmayer et al., 2012). SMEs were characterized in those studies by comparing the neuronal activity elicited by subsequently remembered items to that elicited by items that are subsequently forgotten and may be present in a subset of the brain regions that show modulations of oscillatory activity during encoding. We also found a negative correlation between PCL-5 scores and recognition accuracy for original images. Participants with high PTSD symptom severity had a reduced modulation (suppression) of beta band oscillations in bilateral ventral and medial temporal regions and in left orbitofrontal cortex, which was correlated with a lower subsequent recognition performance. Our findings provide support for the hypothesis that memory deficits in patients with PTSD may be due, at least in part, to altered cortical oscillatory physiology at the time of the encoding. In the following, we will discuss the general role of the brain regions with altered modulation of beta band oscillations in memory encoding, the potential mechanisms through which a reduced suppression of beta band oscillations may affect encoding in memory and lower the subsequent recognition accuracy, and how the altered modulation of beta oscillations may be related to core symptoms of PTSD.
The relatively lower modulation of beta band power for participants with high PTSD symptom severity was present in regions of the ventral visual pathway. Many of these regions showed also abnormal activation during a simple picture viewing task in a previous fMRI study on patients with PTSD (Mueller-Pfeiffer et al., 2013). Two regions that showed strong correlations between PTSD scores, modulation of beta band power and behavioral performance were localized bilaterally in the fusiform and parahippocampal gyri. There is evidence for a functional organization of information processing in the ventral visual cortex, with neuronal representations for local features or individual elements of an image in posterior regions and processing of global configurations or relational associations of multiple scene elements (possibly achieved through synchronized oscillations and/or convergence of inputs) in more anterior temporal regions (Epstein and Kanwisher, 1998; Taylor et al., 2007; Kamps et al., 2016). The parahippocampal and fusiform regions presumably incorporate what is known as the parahippocampal place area (PPA) or ventral scene-selective area (VSA) which is centered within the collateral sulcus (which separates the fusiform and parahippocampal gyri) and extends into the medial part of the fusiform gyrus (Nasr et al., 2011; Bastin et al., 2013). PPA/VSA was shown to respond selectively to complex scenes (e.g. landscapes, city streets, rooms) compared to images from other categories (e.g. objects or faces, Aguirre et al., 1998; Epstein and Kanwisher, 1998; Epstein et al., 1999, 2007) and is considered to play a key role in encoding both individual elements and global configurations of scenes (Bastin et al., 2013; Kravitz et al., 2011). Direct electrical stimulation of this region can elicit complex visual percepts including topographic visual hallucinations involving scenes visualized in the past (Bastin et al., 2013) suggesting that it may play a role in re-experiencing of a traumatic event through vivid recollection and flashbacks, which is a core symptom in PTSD (Michael et al., 2005). The parahippocampal cortex, in particular, has been proposed to play a general role in encoding contextual associations, which represents a key element in both visuospatial processing and episodic memory (Aminoff et al., 2013). The parahippocampal gyrus is also among the brain regions that have been characterized by alterations in structure by some studies in patients with PTSD (Liu et al., 2012).
A lower suppression of beta band power in participants with high PTSD symptom severity was also identified in the left medial and lateral orbitofrontal cortex, regions that showed negative correlations between subsequent recognition accuracy and relative beta band power, although of a smaller magnitude compared to the ventral visual processing regions. Lesion studies in monkeys indicated that the orbitofrontal cortex may be critical for the acquisition of new information, including object-in-place scene learning (Meunier et al., 1997; Baxter et al., 2007). Furthermore, PET studies in humans showed that memory encoding for abstract designs (which are less susceptible to memorization strategies based on narratives or verbal associations) activate the orbitofrontal cortex, although this effect was limited to the right hemisphere (Frey, 2000, Frey, 2002). The orbitofrontal cortex may contribute to memory encoding by modulating activity in regions of the ventromedial temporal lobe and hippocampus, e.g. through its connections with the entorhinal cortex (Insausti et al., 1987; Kondo et al., 2005; Insausti and Amaral, 2008).
In addition to the association between the modulation of beta band power and the severity of PTSD symptoms, the single trial analysis showed a within-subject decrease in recognition accuracy for trials with reduced suppression of (absolute) beta band power during the temporal interval when original images were on the screen. This effect was stronger in posterior (occipital) regions that are likely involved in encoding representations for features of the individual elements of the scene (Kamps et al., 2016). Since this analysis involved sorting of the trials based on the beta band power, a possible contribution to this finding could come from the fact that estimates of beta band power in single trials may be more reliable for brain regions that are closer to the MEG sensors, due to the higher signal-to-noise ratio. The sign of this correlation agrees with observations from other electrophysiological studies investigating SMEs (Hanslmayer et al., 2012). The single trial analysis also showed a decrease in recognition accuracy for trials with reduced suppression of beta band power when images were on the screen in left pars triangularis, although this effect was relatively small. A stronger effect (indicative of a negative SME) was observed in prefrontal regions during the temporal interval immediately following the image offset, when occipital and parietal regions show an increase (rebound) in beta band power associated with an increase in recognition rate (indicative of a positive SME). A previous fMRI and EEG study reported negative SME in beta band and a negative correlation between beta power and BOLD signal in left inferior prefrontal cortex, suggesting that this region plays an important role in successful encoding in memory (Hanslmayr et al., 2011). In our study, the strongest correlation between subsequent recognition performance and beta band power suppression in the left inferior and middle frontal gyri was found during the temporal interval immediately following the image offset, when the left prefrontal cortex does not exhibit the relative increase (rebound) seen in early visual processing regions. A possible explanation of this finding is that the activity in these prefrontal regions could be related to semantic processing and elaboration through generation of verbal descriptions of the image which may have persisted after the image offset. Support for this hypothesis comes from studies showing that lower beta band power is associated with successful encoding for verbal memories (Hanslmayr et al., 2009, 2011; Long et al., 2014) and from a meta-analysis study (Kim, 2011) which showed that the left inferior prefrontal cortex exhibited greater SME during encoding of verbal compared to pictorial material. In our study, the beta band power in these prefrontal regions did not show a significant association with the PCL-5 scores.
Although the functional role of the beta band oscillations is not completely understood, evidence from electrophysiological studies supports the general views that they may represent the re-activation of neuronal ensembles that perform specific functions (Spitzer and Haegens, 2017) and may reflect the maintenance of a cognitive, emotional or sensorimotor state or status quo (Engel and Fries, 2010). Some of our findings appear to be consistent with these views. We observed that occipital and temporal brain regions involved in visual processing show a transient rebound of beta band power after the stimulus offset. This finding is consistent with the modulation of beta band power following sensory stimulation that was reported in other studies using visual (Tallon-Baudry et al., 2004) and somatosensory modalities (Spitzer et al., 2010). The higher recognition rate for those images with higher absolute beta band power in occipital and parietal regions during the interval immediately following the image offset is consistent with a role of beta oscillations in the maintenance of recurrent activity within local neuronal assemblies representing short-term memories for visual information that has just been presented. Simulation studies (Kopell et al., 2011) suggested that one key difference between beta and gamma rhythms comes from the fact that local cell assemblies can sustain beta oscillations in the absence of continuing input, whereas gamma oscillations require ongoing input.
It has been also proposed that the self-sustaining activity in cell assemblies oscillating in beta band does not have a suppressive effect on the activity of other cell assemblies that may be concurrently driven, for example, by sensory inputs (Kopell et al., 2011). This can allow the formation of unified local cell assemblies corresponding to a neuronal representation linking past and present sensory inputs, thereby encoding potentially meaningful relationships between those inputs and forming a coherent representation of a temporally extended episode. One example can be the formation of neuronal representations for configural/relational associations between scene elements explored sequentially during the visual scanning of a scene. This hypothesis has received support from studies of visual perception, including perception of moving objects (Piantoni et al., 2010; Donner et al., 2007). Furthermore, an EEG study of beta band oscillations during encoding of different items reported a category-specific beta band activity that appeared to be related to the preservation of information about categories of recently presented items (Morton and Polyn, 2017). In other cases, however, such integrative processes can be disadvantageous and can degrade task performance, such as in tasks where temporal integration/binding of discrete stimuli is not required. For example, recurrent beta band activations induced by one stimulus in some neuronal assemblies can impede the formation of an accurate independent neuronal representation of another incoming stimulus (unrelated to the first) resulting in forward-acting interference. Studies using multiple trial experiments in which processing of sensory input should remain independent between trials have shown that the power of beta oscillations exhibits an inverse relationship with sensory detection probability (Jones et al., 2010) or decision-making accuracy (Haegens et al., 2014), indicating that high beta power may have negative effects on sensory relay and processing of sequential stimuli that are behaviorally independent of each other. In our study, the regional beta band power measured at the macroscopic level could reflect activity generated in neuronal ensembles that are involved in the encoding of the current image, maintaining representations of previous images in the sequence, or performing functions that are not directly related with the task. The fact that beta power during image presentation was inversely related to the subsequent recognition accuracy for those images suggests that the measured beta band activity may reflect, at least in part, a forward-acting memory interference from recurrent activations in neuronal assemblies recruited during the encoding of previously presented images or interference due to activity in neuronal assemblies that perform functions unrelated to the task.
The possibility that a reduced suppression of beta band activity may reflect persistent neuronal representations of past visual stimuli, sometimes acting as forward-acting interference during the encoding of new stimuli, may be theoretically linked to previous observations of a deficit in associative memory in PTSD (Guez et al., 2011). Participants in that study were asked to memorize pairs of unrelated words or pairs of unrelated pictorial stimuli for a later recognition session. The participants with PTSD showed a tendency to erroneously endorse rearranged pairs during the recognition session, reflected in a higher false-alarm rate compared to healthy control participants. Since item pairs were presented in succession during the encoding session (at a rate of 4 s per pair), a possible contributor to this phenomenon is the fact that the participants with PTSD had longer lasting neuronal representations for visual stimuli (maintained by recurrent activity and beta band oscillations in regions involved in processing of contextual associations), allowing inadvertent associations to be formed between currently presented items and past items. An impaired ability to encode similar experiences into discrete (non-overlapping) memory representations has been linked to intrusive memories and overgeneralization of fear in PTSD (Meyer et al., 2013, 2017).
The reduced suppression of beta band activity during encoding may also reflect a possible interference from recurrent activity in neuronal assemblies that perform functions unrelated to the task. A previous study of resting state oscillatory brain activity reported higher beta band power in patients with PTSD in multiple brain regions including the medial and lateral temporal lobe, orbitofrontal cortex and lingual gyri (Huang et al., 2014), some of which showing also altered suppression of the beta band oscillations during memory encoding in participants with high PTSD symptom severity in our study. The overlap between the sets of brain regions showing increased power or altered modulation of beta band activity in the two studies may be explained by the fact that at rest thoughts typically do not cease, and a train of thoughts engages some of the areas involved in processing associative relations during the encoding of scene images or episodic memory, such as the parahippocampal gyrus (Aminoff et al., 2013). Mind-wandering may be present during task performance as well, and it is conceivable that the activity in neuronal assemblies that perform such functions may be influenced during both rest and task performance by factors with some impact on the general state. Sleep disturbances that include, for example, nightmares, distressed awakenings, nocturnal panic attacks and insomnia, are common symptoms of PTSD (Ohayon and Shapiro, 2000; Spoormaker and Montgomery, 2008; Germain, 2013), and sleep problems following psychological trauma are a strong predictor for future development of PTSD (Koren et al., 2002). EEG studies in patients with primary insomnia reported an increase in beta band EEG power during wakefulness or at sleep onset (Freedman, 1986; Merica and Gaillard, 1992; Lamarche and Ogilvie, 1997; Wolynczyk-Gmaj and Szelenberger, 2011; Corsi-Cabrera et al., 2012; Colombo et al., 2016). The increase in beta band power in insomnia was also interpreted as being indicative of a general state of hyperarousal (Rieman et al., 2010; Kay and Buysse, 2017), which is also characteristic to PTSD. Hence, insomnia and hyperarousal may be linked to higher beta band power during rest or to the altered modulation of beta band power during task performance in participants with high PTSD symptom severity. Although the exact mechanisms underlying such an association are not elucidated and a definitive cause-effect relationship cannot be established, this possible link suggests that therapies designed to alleviate hyperarousal and insomnia, which may include adjuvant therapies that promote a relaxation response, may also be effective at decreasing beta band power during encoding in memory. Since impairments in attention and memory may prevent an effective engagement in cognitive-behavioral therapy (CBT) for a substantial proportion of patients with PTSD, lowering their treatment response (Bradley et al., 2005; Falconer et al., 2013), such therapies may be also considered as “add-on” interventions that can potentially improve the effectiveness of CBT.
To conclude, while beta band activity is normally seen with MEG and EEG during sensory and cognitive processing in the healthy brain, a reduced suppression of beta band activity during encoding of visual stimuli in patients with PTSD symptoms may promote (or decrease the ability to disengage from) certain cognitive states. This may have a negative impact when required to encode a significant number of serially presented items. The beta band activity recorded with MEG and EEG may partly arise from recurrent activations of neuronal assemblies recruited during encoding of previous percepts in a sequence of stimuli and may reflect a forward-acting interference that prevents the efficient processing of sequential sensory inputs. This suggests that an altered suppression of beta band activity may be associated to more general difficulties in processing of continuous or rapidly unfolding streams of sensory inputs in PTSD, a hypothesis that can be addressed in future studies. Additionally, our findings raise the potential of a pathologically increased propensity to reactivate memory representations that may not be salient to the current task or thought process but are of general emotional salience to the PTSD patient. These may represent pathological attractor states in neuronal networks. Such “parasitic attractors” have been described in neural network models of psychiatric disorders (Hoffman and McGlashan, 2001), and enhanced beta band activity may represent a mechanistic basis for such phenomena. These attractor states may have an exceedingly low threshold for activation due to a pathologically robust Hebbian strengthening of connections within the cell assembly as well as to other networks. Because of the presence of overgeneralization of fear in PTSD, the perception of otherwise neutral stimuli may invoke a pathological associative reactivation of fear-related stimuli. These may be activated to the level of conscious awareness in the case of intrusive memories or below the level of awareness and contribute to symptoms of anxiety.
One limitation of this study arises from the fact that all participants had a history of TBI, which may contribute to alterations in brain activity. Across our sample of participants, PCL-5 scores were not significantly different for subgroups of participants with different injury severities (characterized by the presence versus absence of injuries with LOC). Nevertheless, future studies would be helpful to determine if the observed correlations between the PTSD symptom severity, altered modulation of beta band power during encoding in memory, and subsequent recognition performance are also present in PTSD patients without a history of TBI. All participants in our study had a history of combat exposure (acute stress), which helped us to determine that the severity of present PTSD symptoms rather than the history of acute stress is associated with distinct patterns of brain electrophysiology. Future studies using healthy control participants with no combat exposure may help to elucidate if some alterations in beta band oscillations are also present in participants with combat exposure (i.e. who experienced psychologically traumatic events) without PTSD.
Disclaimers
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
The identification of specific products, scientific instrumentation or organizations is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency.
The study protocol was approved by the Walter Reed National Military Medical Center in compliance with all applicable Federal regulations governing the protection of human subjects.
CRediT authorship contribution statement
Mihai Popescu: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft. Elena-Anda Popescu: Methodology, Software, Formal analysis, Writing - review & editing. Thomas J. DeGraba: Conceptualization, Writing - review & editing. John D. Hughes: Conceptualization, Methodology, Writing - original draft, Supervision.
Declaration of Competing Interest
None.
Acknowledgment
Authors would like to thank the participants who volunteered for this study. They also want to thank Jacqueline Dyer for study coordination, David Fernandez-Fidalgo and Andrew Bryant for MEG data acquisition, and Adam Cliffton, Joe Hindinger and the neuroimaging team led by Dr. Grant Bonavia for technical and logistical support with MRI acquisition.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Aguirre G.K., Zarahn E., D'Esposito M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders; pp. 271–276. [Google Scholar]
- American Congress of Rehabilitation Medicine Definition of mild traumatic brain injury. J. Head Trauma Rehab. 1993;8:86–87. [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62(2):686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N., Mormann F., Fernández G., Elger C.E., Fell J. Memory formation by neuronal synchronization. Brain Res. Rev. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bangasser D.A., Kawasumi Y. Cognitive disruptions in stress-related psychiatric disorders: a role for corticotropin releasing factor (CRF) Horm. Behav. 2015;76:125–135. doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin J., Vidal J.R., Bouvier S., Perrone-Bertolotti M., Bénis D., Kahane P., David O., Lachaux J.P., Epstein R.A. Temporal components in the parahippocampal place area revealed by human intracerebral recordings. J. Neurosci. 2013;33(24):10123–10131. doi: 10.1523/JNEUROSCI.4646-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Vermetten E., Afzal N., Vythilingam M. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2004;192(10):643–649. doi: 10.1097/01.nmd.0000142027.52893.c8. [DOI] [PubMed] [Google Scholar]
- Baxter M.G., Gaffan D., Kyriazis D.A., Mitchell A.S. Orbital prefrontal cortex is required for object-in-place scene memory but not performance of a strategy implementation task. J Neuroscience. 2007;27(42):11327–11333. doi: 10.1523/JNEUROSCI.3369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma. Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bovin M.J., Marx B.P., Weathers F.W., Gallagher M.W., Rodriguez P., Schnurr P.P., Keane T.M. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol. Assess. 2015;28:1379–1391. doi: 10.1037/pas0000254. [DOI] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., Dutra L., Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162(2):214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- Brandes D., Ben-Schachar G., Gilboa A., Bonne O., Freedman S., Shalev A.Y. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002;110(3):231–238. doi: 10.1016/s0165-1781(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Breakspear M., Williams L.M., Stam C.J. A novel method for the topographic analysis of neural activity reveals formation and dissolution of ‘dynamic cell assemblies’. J. Comput. Neurosci. 2004;16(1):49–68. doi: 10.1023/b:jcns.0000004841.66897.7d. [DOI] [PubMed] [Google Scholar]
- Brewin C.R. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behav. Res. Ther. 2001;39(4):373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Brewin C.R., Kleiner J.K., Vasterling J.J., Field A.P. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. J. Abnorm. Psychol. 2007;116:448–463. doi: 10.1037/0021-843X.116.3.448. [DOI] [PubMed] [Google Scholar]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010;117:210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Creamer M., O'donnell M., Silove D., Clark R., Mcfarlane A.C. Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2009;15:862–867. doi: 10.1017/S1355617709990671. [DOI] [PubMed] [Google Scholar]
- Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., Silove D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry. 2010;167:312–320. doi: 10.1176/appi.ajp.2009.09050617. [DOI] [PubMed] [Google Scholar]
- Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M.A., Ramautar J.R., Wei Y., Gomez-Herrero G., Stoffers D., Wassing R., Benjamins J.S., Tagliazucchi E., van der Werf Y.D., Cajochen C., Van Someren E.J.W. Wake high-density electroencephalographic spatiospectral signatures of insomnia. Sleep. 2016;39:1015–1027. doi: 10.5665/sleep.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi-Cabrera M., Figueredo-Rodriguez P., del Rio-Portilla Y., Sanchez-Romero J., Galan L., Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35:501–511. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-755 trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickie E.W., Brunet A., Akerib V., Armony J.L. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia. 2008;46:1522–1531. doi: 10.1016/j.neuropsychologia.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Dohrenwend B.P., Turner J.B., Turse N.A., Adams B.G., Koenen K.C., Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313(5789):979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner T.H., Siegel M., Oostenveld R., Fries P., Bauer M., Engel A.K. Population activity in the human dorsal pathway predicts the accuracy of visual motion detection. J. Neurophysiol. 2007;98:345–359. doi: 10.1152/jn.01141.2006. [DOI] [PubMed] [Google Scholar]
- Düzel E., Penny W.D., Burgess N. Brain oscillations and memory. Curr. Opin. Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Epstein R., Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Epstein R., Harris A., Stanley D., Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R.A., Parker W.E., Feiler A.M. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J. Neurosci. 2007;27(23):6141–6614. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Maron-Katz A., Wu W., Fonzo G.A., Huemer J., Vertes P.E., Patenaude B. Using fMRI connectivity to identify a treatment-resistant form of post-traumatic stress disorder. Sci. Transl. Med. 2019;11:486. doi: 10.1126/scitranslmed.aal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E., Allen A., Felmingham K.L., Williams L.M., Bryant R.A. Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder. J. Clin. Psychiatry. 2013;74(9):895–901. doi: 10.4088/JCP.12m08020. [DOI] [PubMed] [Google Scholar]
- Freedman R.R. EEG power spectra in sleep-onset insomnia. Electroencephalogr. Clin. Neurophysiol. 1986;63:408–413. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- Frey S., Petrides M. Orbitofrontal cortex: A key prefrontal region for encoding information. Proc Natl Acad Sci USA. 2000;97(15):8723–8727. doi: 10.1073/pnas.140543497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Petrides M. Orbitofrontal cortex and memory formation. Neuron. 2002;36(1):171–176. doi: 10.1016/s0896-6273(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am. J. Psychiatry. 2013;170:372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson M.W., Gurvits T.V., Lasko N.B., Orr S.P., Pitman R.K. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J. Trauma. Stress. 2001;14:413–432. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- Gramfort A., Luessi M., Larson E., Engemann D., Strohmeier D., Brodbeck C., Parkkonen L., Hämäläinen M.S. MNE software for processing MEG and EEG data. NeuroImage. 2014;86:446–460. doi: 10.1016/j.neuroimage.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guez J., Naveh-Benjamin M., Yankovsky Y., Cohen J., Shiber A., Shalev H. Traumatic stress is linked to a deficit in associative episodic memory. J. Trauma. Stress. 2011;24(3):260–267. doi: 10.1002/jts.20635. [DOI] [PubMed] [Google Scholar]
- Haegens S.1., Vázquez Y., Zainos A., Alvarez M., Jensen O., Romo R. Thalamocortical rhythms during a vibrotactile detection task. Proc. Natl. Acad. Sci. USA. 2014;111(17):E1797–E1805. doi: 10.1073/pnas.1405516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Spitzer B., Bauml K.H. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Staudigl T., Fellner M.C. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci. 2012;6:74. doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S., Volberg G., Wimber M., Raabe M., Greenlee M.W., Bauml K.H. The relationship between brain oscillations and bold signal during memory formation: a combined EEG-FMRI study. J. Neurosci. 2011;31:15674–15680. doi: 10.1523/JNEUROSCI.3140-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen M.S., Ilmoniemi R.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., McGlashan T.H. Neural network models of Schizophrenia. The Neuroscientist. 2001;7:441–454. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Huang M., Yurgil K.A., Robb A., Angeles A., Diwakar M., Risbrough V.B., Nichols S.L., McLay R., Theilmann R.J., Song T., Huang C.W., Lee R.R., Baker D.G. Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. NeuroImage: Clin. 2014;5:408–419. doi: 10.1016/j.nicl.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G., Cowan W.M. The entorhinal cortex of the monkey: II. Cortical afferents. J. Comp. Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G. The entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. J. Comp. Neurol. 2008;509(6):608–641. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.A., Langlais P.J., Delis D.A., Cohen R.A. Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clin. Neuropsychol. 2000;14(1):7–12. doi: 10.1076/1385-4046(200002)14:1;1-8;FT007. [DOI] [PubMed] [Google Scholar]
- Jones S.R., Kerr C.E., Wan Q., Pritchett D.L., Hämäläinen M., Moore C.I. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci. 2010;30(41):13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps F.S., Julian J.B., Kubilius J., Kanwisher N., Dilks D.D. The occipital place area represents the local elements of scenes. Neuroimage. 2016;132:417–424. doi: 10.1016/j.neuroimage.2016.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D.B., Buysse D.J. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7:23. doi: 10.3390/brainsci7030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khanna M.M., Badura-Brack A.S., McDermott T.J., Embury C.M., Wiesman A.I., Shepherd A., Ryan T.J., Heinrichs-Graham E., Wilson T.W. Veterans with post-traumatic stress disorder exhibit altered emotional processing and attentional control during an emotional Stroop task. Psychol. Med. 2017;47(11):2017–2027. doi: 10.1017/S0033291717000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kitayama N., Vaccarino V., Kutner M., Weiss P., Bremner J.D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Doppelmayr M., Ripper B., Schwaiger J., Pfurtscheller G. Event-related desynchro- nization (ERD) and the DM effect: does alpha desynchronization during encoding predict later recall performance? Int. J. Psychophysiol. 1996;24:47–60. doi: 10.1016/s0167-8760(96)00054-2. [DOI] [PubMed] [Google Scholar]
- Kondo H., Saleem K.S., Price J.L. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kopell N., Whittington M.A., Kramer M.A. Neuronal assembly dynamics in the beta1 frequency range permits short-term memory. Proc Natl Acad Sci USA. 2011;108(9):3779–3784. doi: 10.1073/pnas.1019676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D., Arnon I., Lavie P., Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am. J. Psychiatry. 2002;159:855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Kravitz D.J., Saleem K.S., Baker C.I., Mishkin M. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche C.H., Ogilvie R.D. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20:724–733. [PubMed] [Google Scholar]
- Liu Y., Li Y.J., Luo E.P., Lu H.B., Yin H. Cortical thinning in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. PLoS One. 2012;7(6):e39025. doi: 10.1371/journal.pone.0039025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long N.M., Burke J.F., Kahana M.J. Subsequent memory effect in intracranial and scalp EEG. NeuroImage. 2014;84:488–494. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merica H., Gaillard J.M. The eeg of the sleep onset period in insomnia: a discriminant analysis. Physiol. Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Meyer T., Smeets T., Giesbrecht T., Quaedflieg C.W.E.M., Girardelli M.M., Mackay G.R.N. Individual differences in spatial configuration learning predict the occurrence of intrusive memories. Cogn. Affect. Behav. Neurosci. 2013;13:186–196. doi: 10.3758/s13415-012-0123-9. [DOI] [PubMed] [Google Scholar]
- Meyer T., Krans J., van Ast V., Smeets T. Visuospatial context learning and configuration learning is associated with analogue traumatic intrusions. J. Behav. Ther. Exp. Psychiatry. 2017;54:120–127. doi: 10.1016/j.jbtep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Michael T., Ehlers A., Halligan S.L., Clark D.M. Unwanted memories of assault: what intrusion characteristics are associated with PTSD? Behav. Res. Ther. 2005;43:613–628. doi: 10.1016/j.brat.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Mišić B., Dunkley B.T., Sedge P.A., Da Costa L., Fatima Z., Berman M.G., Doesburg S.M., McIntosh A.R., Grodecki R., Jetly R., Pang E.W., Taylor M.J. Post-Traumatic stress constrains the dynamic repertoire of neural activity. J. Neurosci. 2016;36(2):419–431. doi: 10.1523/JNEUROSCI.1506-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton N.W., Polyn S.M. Beta-band activity represents the recent past during episodic encoding. NeuroImage. 2017;147:692–702. doi: 10.1016/j.neuroimage.2016.12.049. [DOI] [PubMed] [Google Scholar]
- Mueller-Pfeiffer C., Schick M., Schulte-Vels T., O'Gorman R., Michels L., Martin-Soelch C., Blair J.R., Rufer M., Schnider U., Zeffiro T., Hasler G. Atypical visual processing in posttraumatic stress disorder. NeuroImage: Clin. 2013;3:531–538. doi: 10.1016/j.nicl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr S., Liu N., Devaney K.J., Yue X., Rajimehr R., Ungerleider L.G., Tootell R.B. Scene-selective cortical regions in human and nonhuman primates. J. Neurosci. 2011;31:13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M.M., Shapiro C.M. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr. Psychiatry. 2000;41:469–478. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- Oliva A., Torralba A. Modeling the shape of the scene: a holistic representation of the spatial envelope. Int. J. Comput. Vis. 2001;42(3):145–175. [Google Scholar]
- Osipova D., Takashima A., Oostenveld R., Fernandez G., Maris E., Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantoni G., Kline K.A., Eagleman D.M. Beta oscillations correlate with the probability of perceiving rivalrous visual stimuli. J. Vis. 2010;10:18. doi: 10.1167/10.13.18. [DOI] [PubMed] [Google Scholar]
- Popescu M., Hughes J.D., Popescu E.A., Reidy G., DeGraba T.J. Reduced prefrontal MEG alpha-band power in mild traumatic brain injury with associated posttraumatic stress disorder symptoms. Clin. Neurophysiol. 2016;127(9):3075–3085. doi: 10.1016/j.clinph.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Popescu M., Hughes J.D., Popescu E.A., Mikola J., Merrifield W., DeGraba M., Riedy G., DeGraba T.J. Activation of dominant hemisphere association cortex during naming as a function of cognitive performance in mild traumatic brain injury: insights into mechanisms of lexical access. NeuroImage: Clin. 2017;15:741–752. doi: 10.1016/j.nicl.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M., Popescu E.A., DeGraba T.J., Fernandez-Fidalgo D., Riedy G., Hughes J.D. Post-traumatic stress disorder is associated with altered modulation of prefrontal alpha band oscillations during working memory. Clin. Neurophysiol. 2019 doi: 10.1016/j.clinph.2019.06.227. in press. [DOI] [PubMed] [Google Scholar]
- Riemann D., Spiegelhalder K., Feige B., Voderholzer U., Berger M., Perlis M., Nissen C. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med. Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Sanquist T.F., Rohrbaugh J.W., Syndulko K., Lindsley D.B. Electrocortical signs of levels of processing: perceptual analysis and recognition memory. Psychophysiology. 1980;17:568–576. doi: 10.1111/j.1469-8986.1980.tb02299.x. [DOI] [PubMed] [Google Scholar]
- Schell T.L., Marshall G.N. Survey of individuals previously deployed for OEF/OIF. In: Tanielian T, Jaycox LH, editors. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; Santa Monica, CA: 2008. pp. 87–117. [Google Scholar]
- Schneiderman A.I., Braver E.R., Kang H.K. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am. J. Epidemiol. 2008;167:1446–1452. doi: 10.1093/aje/kwn068. [DOI] [PubMed] [Google Scholar]
- Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015;141(1):105–140. doi: 10.1037/a0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg P.B., Kahana M.J., Howard M.W., Donner E.J., Madsen J.R. The theta and gamma oscillations during encoding predict subsequent recall. J. Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg P.B., Schulze-Bonhage A., Madsen J.R., Bromfield E.B., McCarthy D.C., Brandt A., Tully M.S., Kahana M.J. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb. Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Spitzer B., Wacker E., Blankenburg F. Oscillatory correlates of vibrotactile frequency processing in human working memory. J. Neurosci. 2010;30:4496–4502. doi: 10.1523/JNEUROSCI.6041-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of the PRIME-MD: the PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Spitzer B., Haegens S. Beyond the status quo: a role for beta oscillations in endogenous content (re)activation. eNeuro. 2017;4(4) doi: 10.1523/ENEURO.0170-17.2017. ENEURO.0170-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker V.I., Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med. Rev. 2008;12:169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Kessler R.C., Heeringa S.G., Jain S., Campbell-Sills L., Colpe L.J., Fullerton C.S., Nock M.K., Sampson N.A., Schoenbaum M., Sun X., Thomas M.L., Ursano R.J. Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: results from the army study to assess risk and resilience in service members (Army STARRS) Am. J. Psychiatry. 2015;172(11):1101–1111. doi: 10.1176/appi.ajp.2015.14121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011;2011 doi: 10.1155/2011/879716. ID 879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C., Mandon S., Freiwald W.A., Kreiter A.K. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb. Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- Taylor J.C., Wiggett A.J., Downing P.E. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J. Neurophysiol. 2007;98:1626–1633. doi: 10.1152/jn.00012.2007. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52(1):155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12(1):125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Duke L.M., Constans J.I., Allain A.N., Sutker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Waldhauser G.T., Dahl M.J., Ruf-Leuschner M., Müller-Bamouh V., Schauer M., Axmacher N., Elbert T., Hanslmayr S. The neural dynamics of deficient memory control in heavily traumatized refugees. Sci. Rep. 2018;8(1):13132. doi: 10.1038/s41598-018-31400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolynczyk-Gmaj D., Szelenberger W. Waking EEG in primary insomnia. Acta Neurobiol. Exp. (Wars) 2011;71:387–392. doi: 10.55782/ane-2011-1860. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Golier J.A., Tischler L., Stavitsky K., Harvey P.D. Learning and memory in aging combat veterans with PTSD. J. Clin. Exp. Neuropsychol. 2005;27(4):504–515. doi: 10.1080/138033990520223. [DOI] [PubMed] [Google Scholar]
- Yurgil K.A., Barkauskas D.A., Vasterling J.J., Nievergelt C.M., Larson G.E., Schork N.J., Litz B.T., Nash W.P., Baker D.G. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiatry. 2014;71(2):149–157. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]